Abstract
Introduction and Objectives
Coronavirus disease-2019 (COVID-19)-related severe acute respiratory distress syndrome (ARDS) differs pathophysiological from other pulmonary septic shock-related ARDS. Thus, we assessed whether all-cause in-hospital mortality differs for severe COVID-19-related and classical severe ARDS and which inflammatory biomarkers can predict mortality among these patients.
Material and Methods
This single-center, retrospective, observational cohort study included pulmonary septic shock patients (n = 114) with COVID-19-related and classical severe ARDS admitted in the Intensive Care Unit.
Results
Patients with a mean age of 73 (IQR 62–82), predominantly male (63%), were divided into two groups based on outcomes: survivors (n = 50) and non-survivors (n = 64). COVID-19-related severe ARDS (n = 48) accounts for 75% of deaths. Present comorbidities like heart disease (p = 0.043), neurologic disorders (p = 0.018), and liver disease (p = 0.038) were associated with in-hospital mortality, as well. Regarding inflammatory biomarkers, the AUC/c-statistic was 0.656 (95% CI: 0.53–0.759) for leukocytes, 0.613 (95% CI: 0.509–0.717) C-reactive protein (CRP) and 0.651 (95% CI: 0.548–0.753) for procalcitonin in predicting all-cause in-hospital mortality among patients with pulmonary septic shock and severe ARDS.
Conclusion
Patients with pulmonary septic shock and with COVID-19-related severe ARDS had a higher incidence of in-hospital mortality than those with classical severe ARDS. The high value of leukocytes, C-reactive protein, and procalcitonin were predictive for all-cause in-hospital mortality in patients with pulmonary septic shock and ARDS. Infection with COVID-19 was an independent predictor of in-hospital mortality in the presence of ARDS.
Introduction
Septic shock, as a severe medical problem, has placed a substantial socioeconomic burden on intensive care units (ICUs), affecting both patients and physicians. Several pathophysiological indicators, including hypotension in the absence of hypovolemia, leukocytosis/leukopenia, elevated serum lactate, and hyper/hypothermia, define this life-threatening organ dysfunction caused by a dysregulated host response to infection.Citation1 Acute respiratory distress syndrome (ARDS) is a severe complication of septic shock. Both septic shock and ARDS share similar mechanisms, characterized by inflammation and dysfunction of the endothelium. Furthermore, septic shock is the leading cause of ARDS, and individuals with sepsis-induced ARDS have a higher fatality rate compared to those with other risk factors for ARDS.Citation2
Moreover, the pulmonary origin of septic shock (ie, pneumonia) is the most common cause of ARDS.Citation3 Since the first documentation of ARDS, viral pneumonia has also been identified as one of its causes. However, certain strains of viruses that have a higher propensity to induce ARDS surface intermittently. Notable examples include SARS-CoV (2003), H1N1 influenza (2009), MERS-CoV (2012), and most significantly, the SARS-CoV-2 virus (2019) that sparked the global COVID-19 pandemic.Citation4 As of the time of this report, the pandemic has tragically claimed the lives of at least 6 million individuals worldwide, with the majority succumbing to ARDS.Citation5
The precise prevalence and mortality rate of ARDS in pulmonary septic shock is still uncertain. Disparities on a significant scale have been proposed, with ARDS in Europe purportedly being ten times lower compared to the United States.Citation6 A study that included ICUs from fifty different countries discovered that the occurrence of ARDS among ICU admissions was 10.4%.Citation3 It became clear that this syndrome was not receiving the attention it deserved, leading to a significant mortality rate. These findings highlight the necessity for enhancing the care and treatment of patients suffering from ARDS.
Procalcitonin (PCT) levels, one of the inflammatory markers, can be evaluated to predict survival depending on the pathogen in sepsis patients.Citation7 Additionally, procalcitonin levels of sepsis patients who do not survive are higher than those who survive.Citation8 On the other hand, inflammatory findings such as procalcitonin, C-reactive protein (CRP), and ferritin are also associated with the severity of the disease in COVID-19 patients. Monitoring these inflammatory findings may help predict prognosis and treat COVID-19.Citation9,Citation10
Therefore, we aimed to investigate several more crucial aspects of ARDS. The main goal was to assess all-cause in-hospital mortality in patients with pulmonary septic shock and severe ARDS, considering the presence or absence of SARS-CoV-2 infection and the second one sought to determine the ability of these factors to predict in-hospital mortality.
Material and Methods
Study Population, Inclusion, and Exclusion Criteria
For the present study, we reviewed all electronic and paper medical records covering admission to the ICU hospitalization, including administered treatments of consecutive patients aged 18 years or older with pulmonary septic shock and ARDS (n = 229), to analyze the medical reports, ventilation modes, and outcomes. All patients from this study were hospitalized at the County Emergency Hospital Resita, Romania, between 1 November 2021 and 31 October 2022.
Patients included in the study were those with septic shock resulting from pulmonary infections. The diagnosis of septic shockCitation11 was determined by the need for a vasopressor to maintain a mean arterial pressure (MAP) of ≥65 mmHg, an elevated serum lactate level above two mmol/L, and procalcitonin levels higher than 0.5 ng/dl.
One hundred fourteen patients admitted with ARDS in the ICU from our hospital who met the inclusion criteria were selected. The patient’s choice were made based on the following inclusion criteria even from the ICU admission: age 18 years or older, diagnosis of pulmonary septic shock and severe ARDS supported by Berlin-definition criteriaCitation12 (PaO2/FiO2 < 100 mmHg), and severe lung lesions (>60% on chest computed tomography or radiography). Patients were excluded for insufficient data available regarding analyzed data, therapy, and outcome, as well as the patients with septic shock caused by another source than the lungs, severe renal (Creatinine clearance <30 mL/min), and liver failure (>5X normal transaminase levels) and major COVID-19 complications as acute myocardial infarction (AMI), pulmonary embolism and ICU length of stay. The baseline data of all patients was collected first at admission in the ICU and second at 30 days, following up in-hospital mortality. The pulmonary septic shock patients were divided into two groups based on death outcome in survivors (n = 50, 56% of patients with COVID-19-related severe ARDS patients) and non-survivors (n = 64, 75% of patients with COVID-19-related severe ARDS).
SARS‐CoV‐2 infection was confirmed by positive detection of viral RNA in nasopharyngeal secretions using a specific real-time reverse transcriptase–polymerase chain reaction (PCR) test. A consistent clinical history, epidemiological contact, and a positive SARS‐CoV‐2 test confirmed COVID‐19 illness.Citation13 COVID-19-related severe ARDS was diagnosed when someone with confirmed COVID‐19 infection meets the Berlin 2012 ARDS diagnostic criteria.Citation12 These criteria use the arterial oxygen partial pressure ratio to inspired oxygen fraction (PaO2/FiO2) to categorize the severity of the condition.
For comparisons with COVID-19 ARDS, we included patients admitted to our department who, during hospitalization, met the diagnostic criteria for ARDS. To ensure the homogeneity of comparison groups about ARDS, we excluded patients with ARDS attributable to coinfection with viral and bacterial pulmonary pathogens. Based on available clinical and microbiological studies, we included only patients with ARDS secondary to direct lung injury from viral, bacterial, or culture-negative pneumonia.
Lung protective mechanical ventilation was part of the management of patients with ARDS. The patients were intubated with endotracheal intubation (ETI) in case of severe hypoxemia with PaO2/FiO2 <150 mmHg and a breathing rate above 25–30 per min despite maximal noninvasive support.Citation14
Data Collection
The following patient data were collected, including their demographics (age, sex, smoking status), length of ICU stay, use of anticoagulant or antiplatelet therapy, presence of comorbidities (COVID-19, heart failure, chronic obstructive pulmonary disease, chronic venous insufficiency, chronic kidney disease, liver cirrhosis, neurological disorders, active cancer, hematological diseases), laboratory analysis results (complete blood count, C-reactive protein, serum creatinine, procalcitonin, international standard ratio, fibrinogen), vital signs (SpO2, heart rate, blood pressure) and arterial blood gases (pH, PaO2, FiO2, PaCO2, lactate).
Statistical Analysis
All statistical analysis was performed using Statistical Package for Social Sciences software (SPSS v28.0.1.1., IBM Corporation, Armonk, NY, USA). Patients were divided, according to their evolution, into survivors and non-survivors. They were characterized using descriptive statistics [percentage, median, range of quarters]. The analytical Kolmogorov–Smirnov test was performed to evaluate the normality of distribution. All numerical variables displayed non-normal distribution, plotted as median (25th and 75th IQR), and the Kruskal–Wallis test was used for intergroup comparison. Categorical variables were plotted as numerical and percentile values. They were compared using the Chi-squared test. The Spearman rank correlation coefficient (r) was used to evaluate the relationships between several clinical and biological parameters and mortality. In addition, binary logistic regression was performed to assess factors associated with mortality among the study population. Furthermore, a receiver operating characteristic (ROC) curve was plotted to evaluate the predictive value of inflammatory biomarkers about patient mortality across the entire study population. A two-tailed p-value <0.05 was considered statistically significant.
Ethics
This study follows the Declaration of Helsinki and was performed according to the Ethics Committees of the Resita County Emergency Hospital (1424/30.01.19).
Results
Descriptive Statistics
The entire study formed 114 patients, who, according to their evolution, were divided into two groups: survivors (n = 50) and non-survivors (n = 64). These patients’ demographic, clinical, and biological parameters are summarized in . Fifty-six percent (n = 28) of survivors and 75% (n = 48) of non-survivors were positive for SARS-COV-2. There were no significant differences about age and gender between the two groups. ()
Table 1 Descriptive Statistics for the Studied Groups (n = 114)
Regarding blood analyses, we noticed a trend toward lower blood pH and hemoglobin among non-survivors, although not reaching statistical significance (p = 0.120 and p = 0.889, respectively). Lactic acid was also higher in non-survivors (median 3.30 mmol/L, IQR: 1.95–5.10), although not reaching statistical significance (p = 0.074).
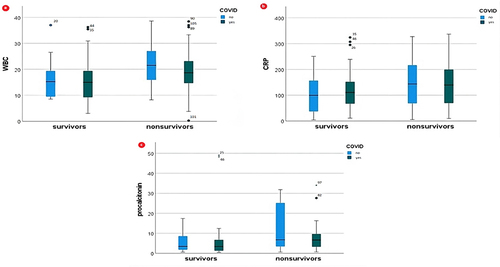
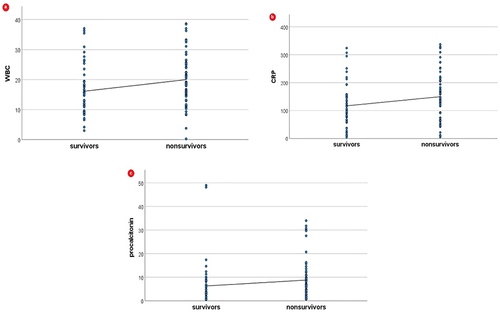
Furthermore, non-survivors displayed higher levels of inflammatory biomarkers: WBC (p = 0.004), C-reactive protein (p = 0.038), and procalcitonin (p = 0.006). These differences are also depicted in .
Factors Correlated with Mortality Across the Entire Lot
As can be seen in , there was a significant positive correlation between mortality and COVID-19 infection, heart failure, chronic venous insufficiency, neurologic disorders, and hepatic disorders.
Table 2 Correlation Analysis of Factors Associated with Mortality Across the Entire Lot
In addition, inflammatory biomarkers, WBC, CRP, and procalcitonin, were also positively correlated with mortality (, ).
Factors Associated with Mortality Across the Study Population
In addition, we performed binary logistic regression analysis to verify the association of COVID-19 infection, inflammatory biomarkers, and ventilation modes with patient mortality in severe ARDS patients. As seen in , COVID-19 infection, WBC, and plasma exchange appeared as independent factors associated with mortality of ARDS patients (p = 0.026, p = 0.027, and p = 0.015, respectively).
Table 3 Binary Logistic Regression Analysis of Risk Factors Associated with Mortality in Severe ARDS
Moreover, using ROC analysis, we evaluated the discriminatory performance of several inflammatory biomarkers in predicting mortality among severe ARDS patients, as summarized in and .
Figure 3 Discriminatory performance of various inflammatory biomarkers in predicting mortality among patients with pulmonary septic shock and severe ARDS; ROC, receiver operating characteristic.
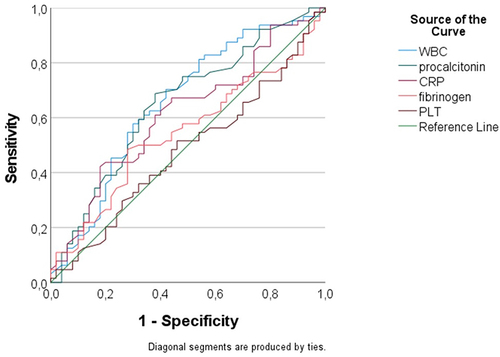
Table 4 Discriminative Ability Comparison of Analysis in Predicting Death Outcomes in Patients with Pulmonary Septic Shock and Severe ARDS
Among the evaluated biomarkers, WBC had an AUC/c-statistic of 0.656 (95% CI: 0.53–0.759), CPR had an AUC/c-statistic of 0.613 (95% CI: 0.509–0.717) in predicting mortality and procalcitonin had an AUC/c-statistic of 0.651 (95% CI: 0.548–0.753) in predicting mortality among those with pulmonary septic shock and severe ARDS.
Discussion
Our study found that the prevalence of non-surviving patients with pulmonary septic shock and ARDS was 56.14% (n = 64). These findings are different from another previous study conducted in America, which included the VALID cohort (Validating Acute Lung Injury markers for Diagnosis; n = 337) and Berlin criteria defined the ARDS, where the attributable mortality of ARDS was 37% (CI 10%, 51%). It should be mentioned that this study included hospitalized patients before the onset of the COVID-19 pandemic.Citation15 However, another study, Field 12 from China, with a similar design to ours, was employed to examine the mortality rates of COVID-19 and non-COVID-19 ARDS in the Asian population in 2021. This study focused on patients who needed invasive mechanical ventilation. The findings revealed that the all-cause in-hospital mortality rate for all patients was 38 (38%), and there were no significant differences between COVID-19 and non-COVID-19-related ARDS (17 [34%] vs 21 [42%], p = 0.410). It is important to note that this study included only unvaccinated patients and highlighted that the two potential risk factors for patients with ARDS are immunocompromised status and progression to severe ARDS. COVID-19 itself was not identified as a risk factor for mortality. Similar to our study, high in-hospital mortality was also reported by other studies that included patients with ARDS. The one conducted by Khandelwal et alCitation16 reported a mortality of 68% higher among patients who received rescue therapy compared with patients treated conventionally, or the one performed by Schuijt et al, which had a primary end-point 28-day mortality and which observed a mortality of 44% in patients with severe ARDS and COVID-19. Differences in these studies’ results may be from differences in disease severity. For example, in our study, we included patients with pulmonary septic shock and severe ARDS. In addition, variations in study populations (eg, comorbidities, age, sex) may also affect study results.
To assess the significance of risk factors in 60-day mortality, a study examined the interaction between disease groups (Sepsis, ARDS, or COVID-19) using logistic regression models. The study included a total of 32,501 adult ICU patients. Notably, the model analyzing 60-day mortality in sepsis and COVID-19 revealed significant interactions with disease groups for age, sex, and asthma. Similarly, the model investigating 60-day mortality in ARDS and COVID-19 identified significant interactions with cohorts for acute disease severity, age, and chronic renal failure.Citation17 In our study, the risk factors for in-hospital mortality were the presence of COVID-19 infection, heart failure, chronic venous insufficiency, neurologic disorders, and hepatic disorders. When all-cause in-hospital mortality was analyzed in a recent study where a 1:1 propensity score matching was performed to correct potential confounders by age, obesity or not, and ARDS severity for all included patients (n = 100), this was 38 (38%), with no significant differences found between COVID-19 (n = 50) and non-COVID-19 (n = 50) ARDS (p = 0.410). Both groups analyzed had no statistically significant differences regarding length of hospitalization (30.0 [20.0–46.0] vs 27.0 [13.0–45.0] days, p = 0.312) and length of ICU hospitalization (19.0 [13.0–35.0] vs 16.0 [10.0–32.0] days, p = 0.312). Immunocompromised status (Hazard ratio: 3.63; 95% CI: 1.51–8.74, p = 0.004) and progression to severe ARDS (Hazard ratio: 2.92; 95% CI: 1.18–7.22, p = 0.020) were significant predictors related to in-hospital mortality.Citation18 Another study enrolled 63 patients with moderate to severe primary ARDS, including 38% (n = 24) of patients with confirmed SARS-CoV-2 infection and 62% (n = 39) of patients with other causes of ARDS (including six cases associated with a flu diagnosis). The median age was 61 years (IQR, 51–69). There were statistically significant differences in the COVID group, as they were older (P = 0.02) and had a higher incidence of obesity (P = 0.04) and diabetes (P = 0.03). The prevalence of immunodeficiency was significantly higher in the non-COVID-19 group (P = 0.004). The median time (IQR) between symptom onset and orotracheal intubation was longer in the COVID-19 group (ten vs five days; P = 0.0001). On discharge from the ICU, the survival rate was 46% in the non-COVID-19 group and 42% in the COVID-19 group (p = 0.80).Citation19 In a similar study, overall hospital mortality was 59% (n = 108), with high in-hospital mortality for patients with severe ARDS. Still, over 80% of study patients discharged alive survived the mid-term observation period. Non-survivors were at a median of 68 years (IQR 63–75) and significantly older than survivors (IQR 58–70).Citation20
In our study, most patients were between 50 and 80 years old, with 35% from the 70–80 age decade. Two young patients, ages 21 and 30 years, were also included, of whom one died. This is concordant with other studies that concluded that elderly patients were at the highest risk of developing a severe form of COVID–19; however, although rarely seen, young patients can also be affected.Citation21
Gender distribution displayed a male prevalence (63% male (n = 72)). This is concordant with general observations and studies, which state higher risks for males regarding severe forms of COVID–19.Citation22 The mortality for male patients was slightly higher (61% vs 59%) than female patients. The smaller total number of female patients can explain this insignificant statistical difference. It is already known that the number of chronic comorbidities is increasing the risk of severe disease in COVID-19,Citation23 which was also observed in this study. Patients from the survivor’s group (n = 50) had a mean of 3.2 ±0.6 comorbidities, while the non-survivor patients’ group (n = 64) had a mean of 3.33 ±1.49 comorbidities. Only one patient from the non-survivor’s group did not present any comorbidity, showing a significant risk for a severe form of COVID–19 due to comorbidities. In terms of the gender comparison, we observed that male patients had more often hepatic, cardiac, and pulmonary diseases, which could be because men in Romania drink alcohol more frequently and in higher quantities than women and, therefore, have a higher risk for chronic diseases.Citation24,Citation25 The same is shown for smoking and working in hazardous conditions as etiological factors for pulmonary diseases, from which men are more often affected.Citation26 Unlike males, female patients in this study have a BMI > 30 kg/m2 than male patients (56% vs 37%). This is also concordant with the PREDATOR study of Popa et al on 2681 subjects aged 20–79 years from Romania, where they observed that women were more likely to be obese than men.Citation27 This would also explain why female patients in this study have a higher prevalence of diabetes mellitus, as obesity is a significant risk factor for it.
To evaluate which factors are associated with mortality across the patients with pulmonary septic shock and severe ARDS included in our study, we performed binary logistic regression analysis. Concerning the inflammatory biomarkers analyzed (CRP, lactic acid, procalcitonin, and leukocytes), significantly more significant increases were found for routine markers (CRP, WBC, and procalcitonin) in patients with worse evolution. This test revealed that in our study population, the inflammatory biomarkers upon admission, such as WBC, CRP, and procalcitonin, were the most critical factors associated with patients’ mortality, as opposed to age, gender, oxygenation at admission/during the first hour in the ICU and ventilation modes, which were similar between the two groups. A single-center prospective cohort study of patients diagnosed with COVID-19 (n = 60) showed a link to mortality in these patients for CRP, lactate dehydrogenase, ferritin, and IL-6, not just COVID-19 severity. In fatal patients, these parameters were statistically increased.Citation28
As our results show, the discriminatory performance of different inflammatory biomarkers in predicting mortality among patients with pulmonary septic shock and severe ARDS was as follows: the WBC had an AUC/c statistic of 0.656 (95% CI: 0.53–0. 759), CPR had an AUC/c-statistic of 0.613 (95% CI: 0.509–0.717) in predicting mortality and procalcitonin had an AUC/c-statistic of 0.651 (95% CI: 0.548–0.753). These biomarkers are still a diagnostic tool for differentiating between favorable outcomes and mortality in patients with severe ARDS. Contrary to our results, the study of Schupp et al that investigates the diagnostic and prognostic value of C-reactive protein and procalcitonin in patients with sepsis and septic shock shows that CRP and procalcitonin have a poor predictive value about 30-day all-cause mortality.Citation29 Therefore, the present study highlights the need to further evaluate inflammatory biomarkers in patients with pulmonary septic shock and severe ARDS.
Limits of Study
There are a few limitations to consider in our study. Firstly, being a retrospective observational cohort study, selection bias is possible due to other prognostic variables. However, we took measures to minimize this bias by including participants from both groups who were diagnosed with pulmonary septic shock and severe ARDS and admitted to the same hospital simultaneously, thus reducing potential confounders. Secondly, the sample size in our study was small due to inclusion criteria (severe ARDS), which means that the clinical results only represent patients with pulmonary septic shock and severe ARDS from a single regional hospital. Although we used propensity score matching to address extreme values and adjusted for known confounders, we may have missed other variables. Thirdly, it is essential to note that only a tiny part of our study’s patients with COVID-19-related severe ARDS were vaccinated. This is due to several factors, such as vaccination policies, incomplete information, and individual choices in 2021. Therefore, the study primarily shows the results of unvaccinated patients. Fourthly, our study mainly included patients with an average PaO2/FiO2 ratio of 55 mmHg, as we had limited ICU beds available. This limitation hindered our ability to fully assess the effectiveness of noninvasive mechanical ventilation, as these patients would have benefited more from endotracheal intubation. Furthermore, it made it challenging to compare our results with other studies that used different criteria for defining ARDS or did not analyze patients based on ARDS severity. Additionally, the absence of specialized equipment such as ECMO restricted the extent of therapy we could provide.
Conclusion
Patients with pulmonary septic shock and with COVID-19-related severe ARDS had a higher incidence of in-hospital mortality than those with classical severe ARDS. The high value of leukocytes, C-reactive protein, and procalcitonin were predictive for all-cause in-hospital mortality in patients with pulmonary septic shock and ARDS. Infection with COVID-19 was an independent predictor of in-hospital mortality in the presence of ARDS. Furthermore, the importance of these inflammatory biomarkers in predicting mortality needs to be noted and further investigated. Therefore, routine laboratory markers CRP, WBC, and procalcitonin can distinguish patients who develop severe ARDS in terms of an unfavorable outcome.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and approved by the Ethics Committee of Emergency Clinical Hospital Resita (approval number: 1424/30.01.2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Data Sharing Statement
The datasets are private, but de-identified data may be provided upon request from Popa Daian.
Additional information
Funding
References
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
- Kim WY, Hong SB. Sepsis and acute respiratory distress syndrome: recent update. Tuberc Respir Dis. 2016;79(2):53–57. doi:10.4046/trd.2016.79.2.53
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi:10.1001/jama.2016.0291
- Bos, L.D.J, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet. 2022;400(10358):1145–1156. doi:10.1016/S0140-6736(22)01485-4
- Martin TR, Zemans RL, Ware LB, et al. New insights into clinical and mechanistic heterogeneity of the acute respiratory distress syndrome: summary of the aspen lung conference 2021. Am. J. Respir. Cell Mol. Biol. 2022;67(3):284–308. doi:10.1165/rcmb.2022-0089WS
- Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med. 2017;5(14):282. doi:10.21037/atm.2017.06.62
- Bilgin M, Aci R, Keskin A, et al. Evaluation of the relationship between procalcitonin level and the causative pathogen in intensive care patients with sepsis. Future Microbiol. 2023;18(13):875–883. doi:10.2217/fmb-2023-0010
- Keskin A, Aci R. Procalcitonin to albumin ratio as a biomarker for predicting mortality in Sepsis. J Coll Physicians Surg Pak. 2024;34(3):360–363.
- Acİ R, Erdem M, Ünlügüzel Üstün G, et al. The relationship between inflammatory indicators and the severity of the disease in coronavirus disease. Meandros Med Dental j. 2022;23(2):208–213. doi:10.4274/meandros.galenos.2021.65477
- Unluguzel Ustun G, Keskin A, Aci R, et al. Association between Hb A 1c and severity of covid-19 patients. Hemoglobin. 2021;45(2):124–128. doi:10.1080/03630269.2021.1926278
- Gavelli F, Castello LM, Avanzi GC. Management of sepsis and septic shock in the emergency department. Intern Emerg Med. 2021;16(6):1649–1661. doi:10.1007/s11739-021-02735-7
- Ranieri VM, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307(23):2526–2533. doi:10.1001/jama.2012.5669
- Kevadiya BD, Machhi J, Herskovitz J, et al. Diagnostics for SARS-CoV-2 infections. Nat Mater. 2021;20(5):593–605. doi:10.1038/s41563-020-00906-z
- Liaqat A, Mason M, Foster BJ, et al. Evidence-based mechanical ventilatory strategies in ARDS. J Clin Med. 2022;11(2):319. doi:10.3390/jcm11020319
- Auriemma CL, Zhuo H, Delucchi K, et al. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 2020;46(6):1222–1231. doi:10.1007/s00134-020-06010-9
- Khandelwal N, Hough CL, Bansal A, et al. Long-term survival in patients with severe acute respiratory distress syndrome and rescue therapies for refractory hypoxemia*. Crit Care Med. 2014;42(7):1610–1618. doi:10.1097/CCM.0000000000000322
- Ahlström B, Frithiof R, Larsson I-M, et al. A comparison of impact of comorbidities and demographics on 60-day mortality in ICU patients with COVID-19, sepsis and acute respiratory distress syndrome. Sci Rep. 2022;12(1):15703. doi:10.1038/s41598-022-19539-0
- Hsieh YH, Chang H-T, Wang P-H, et al. Mortality in patients with COVID-19 versus non-COVID-19- related acute respiratory distress syndrome: a single center retrospective observational cohort study. PLoS One. 2023;18(6):e0286564. doi:10.1371/journal.pone.0286564
- Brault C, Zerbib Y, Kontar L, et al. COVID-19- versus non-COVID-19-related acute respiratory distress syndrome: differences and similarities. Am J Respir Crit Care Med. 2020;202(9):1301–1304. doi:10.1164/rccm.202005-2025LE
- Heubner L, Petrick PL, Güldner A, et al. Extreme obesity is a strong predictor for in-hospital mortality and the prevalence of long-COVID in severe COVID-19 patients with acute respiratory distress syndrome. Sci Rep. 2022;12(1):18418. doi:10.1038/s41598-022-22107-1
- Selickman J, Vrettou CS, Mentzelopoulos SD, et al. COVID-19-related ards: key mechanistic features and treatments. J Clin Med. 2022;11(16):4896. doi:10.3390/jcm11164896
- Chaturvedi R, Lui B, Aaronson JA, et al. COVID-19 complications in males and females: recent developments. J Comp Eff Res. 2022;11(9):689–698. doi:10.2217/cer-2022-0027
- Anesi GL, Kerlin MP. The impact of resource limitations on care delivery and outcomes: routine variation, the coronavirus disease 2019 pandemic, and persistent shortage. Curr Opin Crit Care. 2021;27(5):513–519. doi:10.1097/MCC.0000000000000859
- Andreescu O, Leaşu F, Rogozea L. Alcoholism in Romania in the late nineteenth century and at the beginning of the twentieth century. Clujul Med. 2014;87(4):288–292. doi:10.15386/cjmed-369
- Pop GN, Christodorescu R, Velimirovici DE, et al. Assessment of the impact of alcohol consumption patterns on heart rate variability by machine learning in healthy young adults. Medicina. 2021;57(9):956. doi:10.3390/medicina57090956
- Gan H, Hou X, Zhu Z, et al. Smoking: a leading factor for the death of chronic respiratory diseases derived from Global Burden of Disease Study 2019. BMC Pulm Med. 2022;22(1):149. doi:10.1186/s12890-022-01944-w
- Popa S, Moţa M, Popa A, et al. Prevalence of overweight/obesity, abdominal obesity and metabolic syndrome and atypical cardiometabolic phenotypes in the adult Romanian population: PREDATORR study. J Endocrinol Invest. 2016;39(9):1045–1053. doi:10.1007/s40618-016-0470-4
- Martinez mesa A, Cabrera César E, Martín-Montañez E, et al. Acute lung injury biomarkers in the prediction of covid-19 severity: total thiol, ferritin and lactate dehydrogenase. Antioxidants. 2021;10(8):1221. doi:10.3390/antiox10081221
- Schupp T, Weidner K, Rusnak J, et al. C-reactive protein and procalcitonin during course of sepsis and septic shock. Ir J Med Sci. 2024;193(1):457–468. doi:10.1007/s11845-023-03385-8