?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study assessed the gastric acid antisecretory effect of DLBS2411 fractionated from Cinnamomum burmannii. Hydrogen potassium adenosine triphosphatase (H+/K+ ATPase) activity and its gene expression were observed, and the antioxidant activity of DLBS2411 was also investigated. Treatment of DLBS2411 decreased the level of H+/K+ ATPase messenger RNA expression on human embryonic kidney 293 cells and rat gastric parietal cells in a dose-dependent manner, in vitro and ex vivo. DLBS2411 also acted as a competitive inhibitor by showing inhibition in gastric H+/K+ ATPase activity at various pHs. In gastric ulcer animal models induced with indomethacin and ethanol, DLBS2411showed a reduction in the number of petechiae, suggesting that the fraction also confers gastroprotective activity. Moreover, DLBS2411 was also found to have potent antioxidant activity. Taken together, DLBS2411 is a promising novel agent for the management of dyspepsia, a condition of hyperacidity and diseases in the stomach requiring gastroprotection.
Introduction
Gastrointestinal disorders such as dyspepsia and gastric and duodenal ulcers are a common diagnosis in the medical world. The diseases significantly affect millions of people worldwide and cause significant economic impact in terms of health care costs and work absenteeism, in addition to patients’ decreased quality of life. Generally, these disorders are caused by a hypersecretion of gastric acid from parietal cells of gastric mucosa. This hyperacidic condition might be stimulated by caffeine, alcohol, such drugs as nonsteroidal anti-inflammatory drugs (NSAIDS), stress, and such bacteria as Helicobacter pylori.Citation1–Citation3
The gastric enzyme hydrogen potassium adenosine triphosphatase (H+/K+ ATPase), also known as the proton pump, plays an important role in the acidification process in the stomach. This enzyme is found in parietal, kidney, and heart tissues.Citation4,Citation5 The inhibition of H+/K+ ATPase in gastric parietal cells reduces the overproduction of gastric acid.Citation6 Therefore, a H+/K+ ATPase inhibitor (proton-pump inhibitor) could be used as a pharmacological target for drug development against the disturbances of gastric acid production.Citation6,Citation7 In addition to the disturbance of acid production, reactive oxygen species (ROS), especially the hydroxyl radical, may also play a major role in causing oxidative damage of mucosa.Citation3,Citation8 Excellent correlations exist between the production of ROS and tissue damage of gastric and intestinal mucosa.Citation9
The current approach for gastrointestinal disorder therapy involves multiple mechanisms of pharmacological activities, including a gastric acid reducer, a gastroprotector, and an ROS scavenger. Currently, several pharmaceutical products, eg, histamine blockers and proton-pump inhibitors, are being used to manage gastric hyperacidity; however, these drugs also have limitations because of their side effects, such as headache, nausea, vomiting, and even nose inflammation.Citation3,Citation10–Citation12 Development of a new drug, preferably that which comes from natural sources, with multiple pharmacological activities targeted toward alleviation of gastrointestinal disorders will therefore serve the unmet medical need.
We introduce a new natural bioactive fraction from Cinnamomum burmannii that can potentially be used as a pharmacological agent against gastric intestinal disorders, especially those related to hyperacidity. Our laboratory has in the past and is currently investigating the actions of natural products, some of which have subsequently been developed into agents to treat diseases related to internal medicine.Citation13–Citation15 We report here the activities of DLBS2411, a bioactive fraction of C. burmannii, as an H+/K+ ATPase downregulator and inhibitor as well as a gastroprotector.
Materials and methods
Chemicals
Minimum essential medium (MEM), Roswell Park Memorial Institute (RPMI) medium, fetal calf serum, and penicillin/streptomycin were purchased from Life Technologies (Carlsbad, CA, USA). Trizol reagent was also purchased from Life Technologies. Reverse-transcription reagents were purchased from Promega (Fitchburg, WI, USA). An EnzCheck® phosphate assay kit was bought from Life Technologies. Indomethacin was supplied by CSPC Ouyi Pharmaceutical (batch 2175080208; Shijiazhuang, People’s Republic of China). Ethanol, ethidium bromide, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, trichloroacetic acid, potassium ferricyanide, and ferric chloride were obtained from Sigma-Aldrich (St Louis, MO, USA).
Plant material and preparation of bioactive fraction DLBS2411
Bark of C. burmannii was purchased from Padang, West Sumatra, Indonesia. This plant has been identified by the Herbarium Bogoriense, Research Center of Biology, Indonesian Institute of Sciences with certificate 1261/IPH. 1.02/If.8/XII/2009. The extraction process was started by maceration of the bark in different solvents. Maceration of the material in hot water at temperatures of 60°C–90°C for 1–2 hours was seen to be giving the most biological activity. Miscella was collected during the filtration process and evaporated by vacuum using a rotary evaporator at a temperature of 60°C–80°C to obtain concentrate. The concentrate was further processed through liquid-liquid extraction using dichloromethane at a ratio of 1:2 to separate from organic components. Subsequently the water phase was collected and then evaporated using the rotary evaporator at temperatures of 50°C–120°C to obtain the dry extract. This dry extract was referred to as bioactive fraction DLBS2411 and then subjected to further molecular and biochemical analysis.
Tissue culture
Gastric parietal cells were isolated from the stomach of Wistar strain rats by collagenase digestion on fundic mucosa followed by enrichment of cells, as described by Chew et al.Citation16 The parietal cell preparation contained approximately 1 × 107 cells/well in six-well plates. Human embryonic kidney (HEK)293 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). This cell line was also shown to express H+/K+ ATPase gene. HEK293 cells were cultured in MEM and gastric parietal cells in RPMI medium supplemented with 10% serum and 1% antibiotic penicillin/streptomycin in six-well plates. The media were incubated at 37°C, 5% CO2 for 24 hours. Cell medium was refreshed every 2–3 days. A subconfluent monolayer of cells was used in all experiments. Prior to experimentation, the cell medium was changed to that without serum and incubated for 18–24 hours before treatment. HEK293 and gastric parietal cells were treated with DLBS2411 in various concentrations: 10 μg/mL, 25 μg/mL, and 50 μg/mL. Each cell in the medium grown without serum was treated with DLBS2411 for 24 hours.
RNA isolation and reverse-transcription polymerase chain reaction
Total RNA was extracted from cells using Trizol reagent according to the manufacturer’s instructions. RNA was determined for concentration and purity using a spectrophotometer at 260 and 280 nm (Bio-Rad Hercules, CA, USA). The integrity of the RNA was verified using gel electrophoresis to detect the 18S and 28S ribosomal bands.
Before reverse-transcription (RT) reaction, RNA was incubated at 65°C for 10 minutes. One microgram aliquot of total RNA was reverse-transcribed with 20 U RNasin® (Progmega, Fitchburg, WI, USA), 25 mM deoxyribonucleotide triphosphate mix, 0.5 ng Oligo dT, and 5 U avian myeloblastosis virus reverse transcriptase (RT). The reaction mixture was incubated at 30°C for 10 minutes, 45°C for 45 minutes, 99°C for 5 minutes, and 6°C for 5 minutes. Polymerase chain reaction (PCR) was performed using specific primers for H+/K+ ATPase (forward 5′ GCT GCA GCT CCA TCC TTA TC 3′; reverse 5′ AGG CGG GTA GTC CTT CTC AT 3′). PCR products were visualized by ethidium bromide staining after agarose gel electrophoresis, and the result was quantified using ChemiDoc™ (Bio-Rad).
In vitro H+/K+ ATPase activity assay
The effect of DLBS2411 as inhibitor was observed on H+/K+ ATPase enzyme activity wherein the assay was based on the assessment of the inorganic phosphate released from the hydrolysis of ATP. This assay was done using the Enzcheck phosphate assay kit (Life Technologies) according to the manual available from the kit. The enzyme assay was done in gastric parietal cells that had been isolated from Wistar rats and cultured with addition of 30 μg/mL DLBS2411, and the pH levels during the assay were varied: 7.4, 4, and 2. This enzyme-activity study was done with and without the addition of DLBS2411.
Free radical scavenging activity
The antioxidant activity of DLBS2411 on the basis of the scavenging activity of the stable DPPH free radical was determined using the method described by Brand-Williams et al.Citation17 DPPH solution (0.1 mM) in methanol was prepared and 1.0 mL of this solution was added to 3.0 mL of DLBS2411 solution at different concentrations (0–50 μg/mL). Thirty minutes later, the absorbance was measured at 517 nm. A blank was prepared without addition of DLBS2411. Ascorbic acid at various concentrations (0–50 μg/mL) was used as standard. The lower absorbance of the reaction mixture indicates higher free radical scavenging activity. The capability to scavenge the DPPH radical was calculated using the following equation:
where Acontrol is the absorbance of the control reaction and Atest is the absorbance in the presence of the sample of the fractions. The antioxidant activity of DLBS2411 was expressed as DPPH scavenged (%).
Reducing-power ability
The reducing power of DLBS2411 was determined according to the method of Oyaizu.Citation18 Various concentrations of DLBS2411 (0–50 μg/mL) in 1.0 mL of deionized water were mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 minutes. Aliquots of trichloroacetic acid were added to 2.5 mL of the mixture and then centrifuged at 3,000 rpm for 10 minutes. Aliquots of the supernatant were mixed with 2.5 mL distilled water and 0.5 mL of a freshly prepared 0.1% ferric chloride solution. Ascorbic acid in various concentrations (0–50 μg/mL) was used as positive control. The absorbance was measured at 700 nm using a spectrophotometer. Increase in absorbance of the reaction mixture indicated an increase in reducing power.
Animal experiments
Experimental animals used were Wistar rats (Rattus norvegicus, Wistar strain), 2-month-old males with weight ranging from 200 to 210 g, obtained from D’Wistar (Bandung, Indonesia). They were housed individually and had access to food and water ad libitum. Experimental animals were maintained at a temperature of 22°C–23°C in regular light cycles of 12/12 hours’ light/dark in accordance with the guidelines for the use and maintenance of experimental animals that have been published by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Rats were acclimated for 1 week before treatment. The animal facility has received AAALAC accreditation. All experiments were reviewed for ethical treatment by the independent Institutional Animal Care and Use Committee members of Dexa Medica.
Gastric ulcer induction using indomethacin in rats
Rats were allocated into four groups, consisting of a negative control, a positive control, and two treatment groups. The negative-control group received normal saline, while the positive-control group received normal saline and indomethacin orally. Two treatment groups were treated with DLBS2411 at doses of 20 and 40 mg/kg body weight, respectively, for 7 consecutive days. At the end of the treatment period, the experimental animals were fasted overnight and surgeries to tie up the pylorus were performed following a fasting period. Prior to surgery, the animals were sedated using the anesthetizing drugs ketamine at a concentration of 75 mg/kg body weight and xylazine at a concentration of 10 mg/kg body weight. Their pylori were identified and then tied up using a piece of silk yarn with size of 3/0. Subsequently, test animals received indomethacin 30 mg/kg body weight and water. After treatment for 6 hours, experimental animals were killed by decapitation. The gaster of the animals was then examined to check ulcer development. The final results are shown based on mean lesion formation. The data were analyzed statistically using Student’s t-test.
Gastric ulcer induction using ethanol in rats
Rats were allocated into four groups, consisting of a negative control, a positive control, and two treatment groups. All groups received initial oral treatment with absolute ethanol and then received ethanol 15% for 1 week. After 1 week, the positive control group was treated with normal saline, while the two treatment groups were treated with DLBS2411 at doses of 20 and 40 mg/kg body weight. At the end of the treatment period, the experimental animals were fasted overnight. The animals were then killed by cervical decapitation, and their gaster was then examined for ulcer development.
Ex vivo H+/K+ ATPase assay
Enzyme assays were performed using gastric parietal cells isolated from the gaster of Wistar rats from the in vivo study. The rats were previously induced with ethanol and indomethacin. Enzyme-activity assays were carried out using the EnzCheck phosphate assay kit.
Results
Expression of H+/K+ ATPase on HEK293 and gastric parietal cells
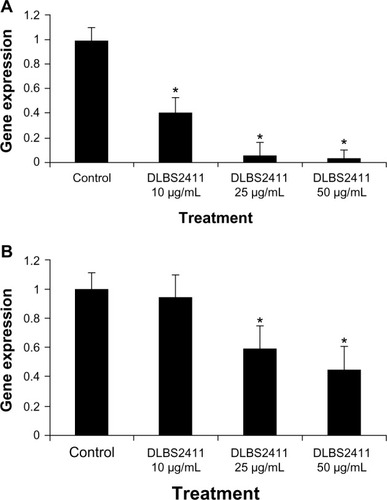
The present in vitro study demonstrated that treatment with DLBS2411 decreased the level of H+/K+ ATPase messenger RNA (mRNA) expression of HEK293 and gastric parietal cells in a dose-dependent manner. The downregulation of H+/K+ ATPase mRNA expression of HEK293 cells was seen as high as 90% at a dose of 50 μg/mL (), a level which was greater than that seen in the gastric parietal cells (50%) ().
Inhibitory effect of DLBS2411 on H+/K+ ATPase gastric parietal cells in vitro
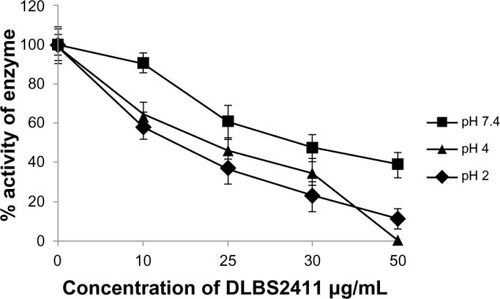
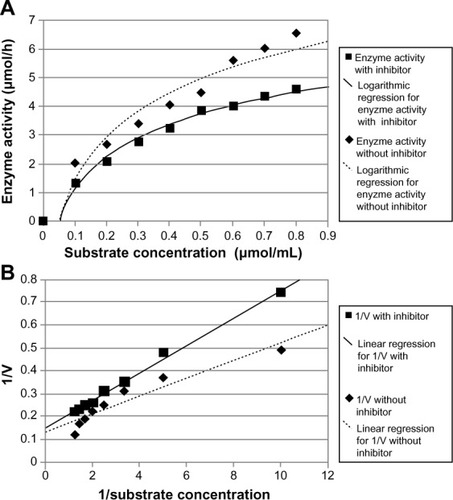
The effect of DLBS2411 on enzyme activity was investigated in various pH conditions. The result showed that DLBS2411 inhibited H+/K+ ATPase activity in a dose-dependent manner. DLBS2411 was shown to be most effective at pH 2 (). Specifically, the half-maximal inhibitory concentration of DLBS2411 at pH 7.4 was 29.20 μg/mL, pH 4 was 18.70 μg/mL, and pH 2 was 9.20 μg/mL. In addition, the analysis of enzyme kinetics revealed that in the absence of DLBS2411, the Michaelis constant (Km) and maximum velocity (Vmax) were 0.3076 μmol/mL and 7.69 mmol Pi/hour, respectively. However, treatment with DLBS2411 yielded Km and Vmax values of 0.4002 μmol/mL and 6.67 mmol Pi/hour, respectively ( and ). These data suggested that DLBS2411 served as a competitive inhibitor of the enzyme, having an inhibition constant (Ki) of 0.1153 μmol/mL.
Inhibitory effect of DLBS2411 on ulceration in vivo
Indomethacin as a gastric ulcer inducer in rats
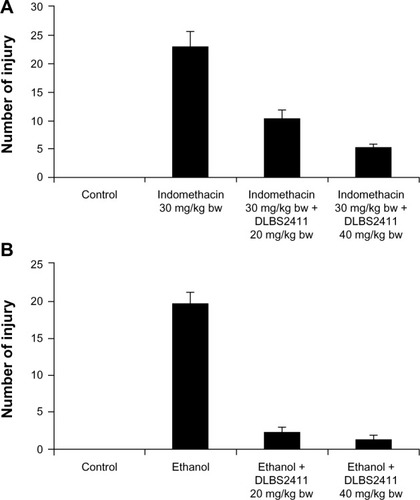
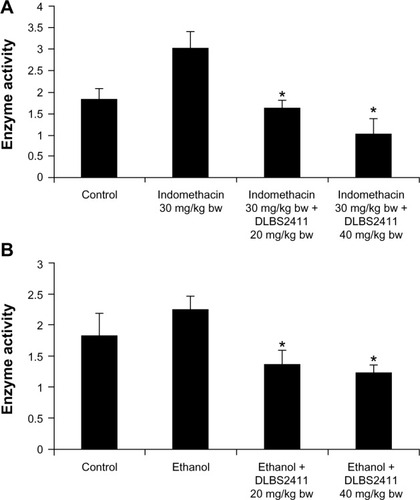
The activity of DLBS2411 in reducing gastric ulcers was also shown in the animals treated with indomethacin. Under this condition, the animals developed petechiae as a result of the treatment. The number of petechiae in the positive group that received indomethacin only was 23 ± 2.65, while the number receiving DLBS2411 with doses of 20 and 40 mg/kg body weight, prior to addition of indomethacin, was 10.33 ± 1.53 and 5.33 ± 0.58, respectively (). The fact that the number of petechiae was significantly different when DLBS2411 was given prior to indomethacin compared to indomethacin alone suggested that DLBS2411 conferred its gastroprotection activity to the microenvironment of the gaster.
Ethanol induction of gastric ulcers in rats
Experimentation on gastric ulceration was performed on rats using ethanol treatment. The number of petechiae in experimental animals that received only absolute and 15% ethanol was 19.67 ± 1.53, while animals that were treated with DLBS2411 at doses of 20 and 40 mg/kg body weight had a significantly lower number of petechiae of 2.33 ± 0.58 and 1.33 ± 0.58, respectively (). As in the case of indomethacin treatment, these data suggested that DLBS2411 also conferred protection to the gaster, which led to reduced gastric injuries.
Inhibitory effect of DLBS2411 on H+/K+ ATPase gastric parietal cells ex vivo
H+/K+ ATPase enzyme-activity assays were conducted on gastric parietal cells isolated from the rats from the in vivo study. When the animals were administered indomethacin or ethanol, the enzyme activity increased ( and ). However, simultaneous treatment of indomethacin or ethanol with DLBS2411 caused the enzyme activity to be decreased in a dose-dependent manner. It can be inferred from these data that DLBS2411 confers gastroprotection partly through inhibition of H+/K+ ATPase activity.
Free radical scavenging activity and reducing-power activities
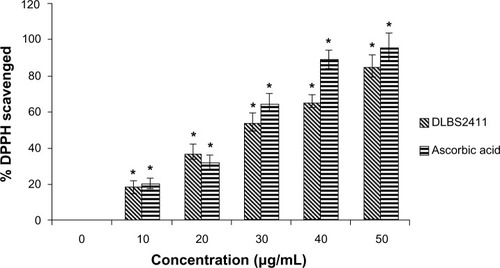
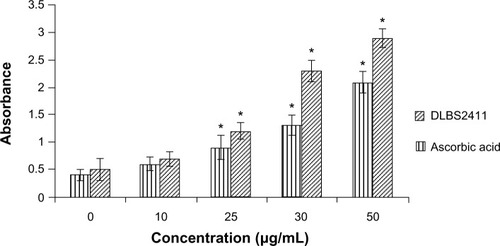
The free radical scavenging activity of DLBS2411 was observed and compared to the activity of ascorbic acid (). At a concentration of 10 μg/mL, DLBS2411 was seen to yield similar free radical scavenging activity to that of ascorbic acid. An experiment was conducted to investigate the reducing power of DLBS2411, compared to an active control – ascorbic acid. As shown in , DLBS2411 was able to increase its reducing power in a dose-dependent manner. Taken together, the data suggested DLBS2411 might have an antioxidant activity, although the activity is lower than that of ascorbic acid.
Discussion
This study was carried out to elucidate gastroprotective and gastric acid antisecretory effects of DLBS2411, a bioactive fraction of C. burmannii. DLBS2411 was particularly investigated for its effect on the gene expression and activity of the proton-pump H+/K+ ATPase enzyme. This enzyme has a significant role in the regulation of acid secretion.Citation4,Citation5
In this study, it was shown that treatment of DLBS2411 downregulated H+/K+ ATPase mRNA expression ( and ) as well as inhibited H+/K+ ATPase activity observed in the genes isolated from the HEK293 and gastric parietal cells (). The results reflected a positive correlation between gene expression and protein translations. The reduction in H+/K+ ATPase enzyme activity might have been partly due to reduced expression of the gene. In addition, we also studied H+/K+ ATPase mRNA expression of the gastric parietal cells with epinephrine as inducer (data not shown). Epinephrine has been shown to stimulate gastric acid secretion in humans and animals.Citation19,Citation20 Under this condition, expression was also seen to decrease (data not shown). As we know that epinephrine binds through the beta-adrenergic receptor and uses cyclic adenosine monophosphate and protein kinase A as signal-transduction molecules, the data suggested that DLBS2411 may have used a cyclic adenosine monophosphate-dependent mechanism through which it regulated the expression of H+/K+ ATPase.
Several factors have been shown to affect enzyme activity. These may include temperature and pH as well as the presence of an inhibitor. It was found that DLBS2411 was able to exert its function in inhibiting H+/K+ ATPase enzyme activity at various gastric pH levels. The inhibition was most pronounced at the lowest gastric pH of 2 (). Ulceration occurs when the gaster becomes hyperacidic, where the level of gastric pH is very low. The root cause of this condition is partly related to the activity of H+/K+ ATPase in the acid-secretion process. The fact that DLBS2411 is able to work more effectively in low gastric pH suggests that DLBS2411 could serve as a potent pharmacological agent to suppress hyperacidity in the clinical setting and reduce gastric ulceration. The result of chemical identification using thin-layer chromatography (data not shown) showed that the major compound of DLBS2411 is a molecule currently being investigated, but belonging to the class of polyphenol. Reyes-Chilpa et al reported that phenolic compounds are important in the interaction with H+/K+ ATPase, as they are potent in blocking the activity of this enzyme.Citation7 This suggested that as-yet-unidentified phenolic compounds may be present in DLBS2411 and may also contribute to the inhibition of H+/K+ ATPase.
In order to investigate further the activity of DLBS2411 in inhibiting H+/K+ ATPase, an enzyme kinetic experiment was performed. It was found that the Km of enzyme activity treated with DLBS2411 was higher than the Km of control ( and ). This indicates that DLBS2411 has a characteristic as a competitive inhibitor for the enzyme. The competitive inhibitor mimics the action of an enzyme substrate and binds to the active site of the enzyme. The substrate is thereby prevented from binding to the same active site. A competitive inhibitor diminishes the rate of catalysis by reducing the proportion of enzyme molecules bound to a substrate.Citation21 Km values used as a measure of affinity-enzyme substrate are also linked to a complex dissociation balance constant. In this regard, DLBS2411 competitively inhibits H+/K+ ATPase, an action which leads to the inhibition of enzyme activity in the acid-secretion process.
In addition to the in vitro studies, we also conducted two animal studies with indomethacin and ethanol as ulcer inducers. The ability of NSAIDS such as indomethacin to cause ulceration and bleeding in the upper gastrointestinal tract has been shown in many studies, including one reference study.Citation22 Indomethacin has the ability to suppress gastric prostaglandin synthesis. A study by Yoshikawa et al suggested that the enhanced gastric motility induced by indomethacin caused microcirculatory disturbances that led to an increase of microvascular permeability and cellular damage.Citation23 The gastric ulceration in the indomethacin-induced group was also induced by pylorus ligation treatment. This procedure caused the accumulation of gastric acid, which then led to the development of gastric ulcers. Both treatments – indomethacin and pylorus ligation – were chosen as the method to stimulate the development of gastric ulcers. In the other experiment, in vivo ulceration was also induced with ethanol. Previous data have suggested acute damage of gastric mucosa can develop at a higher rate in ethanol-treated experimental animals.Citation24 This gaster-damaging agent induced mucosal lesions that were associated with the production of oxygen free radicals and lipid peroxidation.Citation25,Citation26 In the case of gastric injury by these gaster-damaging treatments, the injuries can be shown to cause the development of petechiae in the stomach. DLBS2411 in doses of 20 and 40 mg/kg reduced the number of petechiae significantly in a dose-dependent manner in both treatment groups ( and ). This finding correlated with the in vitro antioxidant activity of DLBS2411, where DLBS2411 also reduced the numbers of free radicals that might cause ulceration. These data also suggested that DLBS2411 not only confers activity in proton-pump inhibition but also in protecting mucosa and in minimizing gastric injury.
In order to confrm the mechanism of the DLBS2411 toward the H+/K+ ATPase enzyme, gastric parietal cells were isolated from the animals in the in vivo study. It was found that H+/K+ ATPase enzyme activity was increased with the addition of indomethacin and 15% ethanol ( and ), suggesting that those agents induced gastric wounds that could lead to the induction of ulceration. DLBS2411 was able to decrease the activity of H+/K+ ATPase. These data may also suggest the interaction of compounds contained in the DLBS2411 with H+/K+ ATPase, which then inhibited the acid-secreting enzyme.
In addition to its activity as enzyme inhibitor, DLBS2411 was also found to possess free radical scavenging activity and reducing-power capability. These data suggested that this agent is also acting as a potent antioxidant. DPPH free radical scavenging is a common method used to evaluate the antioxidant potential of an agent. The free radical chain reaction is widely accepted as a mechanism of lipid peroxidation. For measurements of the reducing power of DLBS2411, the concept of transformation of Fe3+–Fe2+ adopted from Oyaizu was applied.Citation18 This study revealed the free radical scavenging activity and reducing power of DLBS2411, which were increased in a dose-dependent manner ( and ). This is in line with the finding that DLBS2411 works in inhibiting enzyme activity that further reduced ulceration. Ulceration often happens due to a hyperoxidation process in the gaster.Citation3,Citation8,Citation9 With a highly effective reductive ability, it is expected that DLBS2411 could reduce the hyperoxidation process. This oxygen radical reductive activity indicated that DLBS2411 has the potential to be an antioxidant agent that contributes to the alleviation of gastric ulcer problems.
In conclusion, we have shown in this report some pharmacological data that collectively indicate that DLBS2411 possesses an efficacy to inhibit and downregulate gastric H+/K+ ATPase, as well as act as an antioxidant. This agent can potentially be used for treating gastric ulcers from various etiologies, such as alcohol, drug, and stress. These findings provide evidence for DLBS2411 to potentially be administered as a natural alternative for gastric acid regulation. Currently, a number of clinical trials are ongoing to prove this concept in subjects suffering from this condition in several hospitals in Asia.
Acknowledgments
The authors would like to thank Dr James Sinambela for preparing DLBS2411, and Audrey Clarissa, Venni Carolina, and Yemima Septiany Puraja for their assistance in editing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- MillerTAMechanisms of stress-related mucosal damageAm J Med1987838143321980
- KonturekPCBrzozowskiTKonturekSJApoptosis in gastric mucosa with stress-induced gastric ulcersJ Physiol Pharmacol19995021122510424718
- BandyopadhyayUBiswasKChatterjeeRGastroprotective effect of Neem (Azadirachta indica) bark extract: possible involvement of H(+)-K(+)-ATPase inhibition and scavenging of hydroxyl radicalLife Sci2002712845286512377267
- SachsGShinJMBrivingCWallmarkBHerseySThe pharmacology of the gastric acid pump: the H+, K+ ATPaseAnnu Rev Pharmacol Toxicol1995352773057598495
- DuBoseTDJrCodinaJBurgesAPressleyTARegulation of H(+)-K(+)-ATPase expression in kidneyAm J Physiol1995269F500F5077485534
- OnoKSawadaTMurataYPentagoloylglucose an antisecretory component of Paeoniae radix inhibits gastric H+/K+ ATPaseClin Chim Acta200029015916710660806
- Reyes-ChilpaRBaggioCHAlavez-SolanoDInhibition of gastric H+, K+-ATPase activity by flavonoids, coumarins and xanthones isolated from Mexican medicinal plantsJ Ethnopharmacol200610516717216314059
- MaityPBiswasKChattopadhyayIBanerjeeRKBandyopadhyayUThe use of neem for controlling gastric hyperacidity and ulcerPhytother Res20092374775519140119
- BagchiDCarrylORTranMXStress, diet and alcohol-induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylateJ Appl Toxicol1998183139526828
- FortFLMiyajimaHAndoTMechanism for species-specific induction of Leydig cell tumors in rats by lansoprazoleFundam Appl Toxicol1995261912027589908
- PowersRELawtonGPModlinIMGenotoxicity, carcinogenicity and acid-suppressing medicationsPharmacol Ther1995653033177644566
- MartelliAMattioliFMeretoEEvaluation of omeprazole genotoxicity in a battery of in vitro and in vivo assaysToxicology19983029419846994
- TrisinaJSunardiFSuhartonoMTTjandrawinataRRDLBS1033, a protein extract from Lumbricus rubellus, possesses antithrombotic and thrombolytic activitiesJ Biomed Biotech20112011519652
- TandrasasmitaOMWulanDDNailufarFSinambelaJTjandrawinataRRGlucose-lowering effect of DLBS3233 is mediated through phosphorylation of tyrosine and upregulation of PPARγ and GLUT4 expressionInt J Gen Med2011434535721674027
- TjandrawinataRRArifinPFTandrasasmitaOMDLBS1425, a Phaleria macrocarpa (Scheff.) Boerl. Extract confers anti proliferative and proapoptosis effects via eicosanoid pathwayJ Exp Ther Oncol2010818720120734918
- ChewCSNakamuraKLjungströmMCalcium signaling mechanisms in the gastric parietal cellYale J Biol Med1992655616231341064
- Brand-WilliamsWCuvelierMEBersetCUse of a free radical method to evaluate antioxidant activityFood Sci Technol1995282530
- OyaizuMStudies on products of browning reaction: antioxidant activities of products of browning reaction prepared from glucosamineJpn J Nutr198644307315
- ChristensenKCStadilFEffect of epinephrine and norepinephrine on gastrin release and gastric secretion of acid in manScand J Gastroenterol Suppl19763787921064147
- MarolfCJSchultzBDClemensETEpinephrine effects on gastrin and gastric secretions in normal and stress-susceptible pigs and in dogsComp Biochem Physiol C19931063673707904912
- BergJMTymoczkoJLStryerLBiochemistry5th edNew YorkWH Freeman2002
- WallaceJLHow do NSAIDs cause ulcer disease?Baillieres Best Pract Res Clin Gastroenterol20001414715910749095
- YoshikawaTNaitoYKishiARole of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in ratsGut1993347327378314503
- BieniaASodolskiWLuchowskaEThe effect of chronic alcohol abuse on gastric and duodenal mucosaAnn Univ Mariae Curie Sklodowska Med20025757058212898897
- PatilPBMahadikVJPatilSBNaikwadeNSEvaluation of antiulcer activity of aqueous and ethanolic extract of Oxalis corniculata leaf in experimental ratsInt J Pharm Res Dev2011398104
- ChenSHLiangYCChaoJCProtective effects of Ginkgo biloba extract on the ethanol-induced gastric ulcer ratsWorld J Gastroenterol2005113746375015968732