Abstract
Objective
To report the case of a 28-year-old woman who presented with hypercalcemia (total calcium =4.11 mmol/L), elevated parathyroid hormone (PTH) 24.6 pmol/L, normal parathyroid hormone-related peptide 7.8 pg/mL, and a 63 mm × 57 mm, poorly differentiated neuroendocrine carcinoma (small-cell type) pancreatic mass with liver metastases.
Investigations and treatment
Hypercalcemia was acutely managed with intravenous fluids, pamidronate and calcitonin. Investigations for multiple endocrine neoplasia type 1 and parathyroid adenoma were initiated. The identified neuroendocrine tumor was treated with cisplatinum/etoposide chemotherapy.
Results
The pancreatic mass (56 mm × 49 mm) and metastases decreased in size with chemotherapy and calcium levels normalized. Eight months later, calcium increased to 3.23 mmol/L, PTH increased to 48.2 pmol/L, and the pancreatic mass increased in size to 67 mm × 58 mm. The patient was given a trial of cinacalcet but was unable to tolerate it. Chemotherapy was restarted and resulted in a decrease in the pancreatic mass (49 mm × 42 mm), a reduction in PTH levels (16.6 pmol/L), and calcium levels (2.34 mmol/L).
Conclusion
Ectopic PTH secreting tumors should be considered when there is no parathyroid related cause for an elevated PTH. Recognizing the association between PTH and hypercalcemia of malignancy may lead to an earlier detection of an undiagnosed malignancy.
Introduction
Hypercalcemia is a common metabolic disorder with multiple etiologies, of which primary hyperparathyroidism is the most common cause.Citation1 Hypercalcemia can occur at any age but occurs most often in patients over the age of 50.Citation2 It is most commonly due to either a single adenoma or hyperplasia of the parathyroid gland. Hypercalcemia may also be part of a hereditary syndrome (multiple endocrine neoplasia [MEN1] or multiple endocrine neoplasia type 2A [MEN2A]) particularly when identified in children or young adults.Citation2,Citation3 Parathyroid lesions are routinely identified with 99 mTc-Sestamibi scintigraphy scans and often successfully treated with surgical resection.
Hypercalcemia is also a well-established paraneoplastic condition that is associated with many malignancies and may occur through a number of different mechanisms (). In 1941, AlbrightCitation4 was first to suggest that humoral factors secreted by cancer cells caused bone resorption and impaired renal calcium excretion.Citation4 Parathyroid hormone (PTH) was originally thought to be the humoral factor that caused hypercalcemia of malignancy, however, in 1987 PTH-related peptide (PTHrP) was found to be the primary mediator associated with malignancy-induced hypercalcemia.Citation5–Citation8 In such cases, patients often have suppressed PTH levels, metabolic alkalosis and low 1, 25 dihydroxyvitamin D levels.Citation9
Table 1 A summary of the humoral causes and frequency of malignancy-related hypercalcemia with the associated biochemical findings
Since the discovery of PTHrP, there have been only rare cases of ectopic production of PTH by neuroendocrine tumors reported in the literature.Citation10–Citation26 In contrast to PTHrP-secreting tumors, patients with elevated PTH levels are often found to have normal PTHrP levels, hyperchloremic acidosis and elevated 1,25 dihydroxyvitamin D levels.Citation9
We present a rare case of malignancy associated hypercalcemia secondary to ectopic PTH. We will review previously reported cases of PTH-secreting tumors, discuss the differences in biochemical findings between humoral causes of hypercalcemia,Citation27–Citation31 and possible treatment options for management of hypercalcemia caused by PTH secreting tumors.
Case report
Background
A previously healthy 28-year-old presented to hospital with a 2-week history of nausea, fatigue, abdominal pain, and weight loss. Initial laboratory investigations showed severe hypercalcemia (Ca =4.11 mmol/L, N=2.20–2.52 mmol/L) and acute renal failure (creatinine =215 μmol/L, N=35–88 μmol/L). Other laboratory investigations are summarized in . An electrocardiography (ECG) showed a normal sinus rhythm of 74 beats per minute and a corrected QT interval of 395.
Table 2 Investigations and results for 28-year-old previously healthy patient found to have hypercalcemia on presentation to hospital
Treatment and investigations
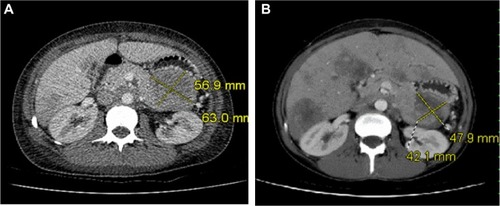
Following therapy with intravenous (IV) fluids, pamidronate disodium (60 mg IV), and calcitonin (200 units twice daily), her serum calcium improved to 2.82 mmol/L within 3 days. Further investigations revealed an elevated serum PTH level at 24.6 pmol/L (1.6–9.3 pmol/L, intact PTH immunometric assay) and a high circulating 1,25 dihydroxyvitamin D level at 229 pmol/L (29–193 pmol/L) ( and ). This raised the possible diagnoses of primary hyperparathyroidism or MEN1. There was no family history of either of these disorders. Ongoing abdominal discomfort despite improvements in calcium levels resulted in a diagnostic abdominal ultrasonography being performed, which showed a 6.0 × 6.7 × 8.0 cm solid mass between the stomach and tail of the pancreas with multiple liver metastases. A computed tomography scan (CT-scan) showed a large 63 × 57 mm mass that was inseparable from the pancreas in the lesser sac infiltrating the splenic artery (). An ultrasonography-guided core biopsy of the liver metastases revealed a poorly differentiated neuroendocrine carcinoma of the small-cell type consistent with the pancreas as the primary site of malignancy. Investigations to identify the source of PTH secretion including a CT-scan of the neck and 99mTc-sestamibi scintigraphy scan failed to find evidence of a parathyroid adenoma. Further staging investigations, including pituitary magnetic resonance imaging (MRI) and genetic testing for MEN1, were negative. A skeletal survey did not show any bony abnormalities and bone mineralization was normal throughout. A bone scan did not show any evidence of metastatic disease. The patient did not suffer any pathologic fractures. A bone density scan was not performed.
Figure 1 PTH, PO4, and calcium levels for case study.
Abbreviations: PTH, parathyroid hormone; PO4, phosphate; Ca, calcium.

Figure 2 CT scan of case study patient.
Abbreviation: CT, computed tomography.
Treatment course
After one round of chemotherapy treatment with cisplatinum (40 mg IV) and etoposide (160 mg IV), there was a decrease in the size of the pancreatic mass (56 mm × 49 mm) and liver metastases. With shrinkage of the tumor, calcium and PTH levels also decreased (Ca 2.02 mmol/L, PTH 21.2 pmol/L) (). At the request of the patient, chemotherapy was stopped after six cycles.
Eight months later, she was readmitted with recurrent hypercalcemia (Ca 3.23 mmol/L). An abdominal CT-scan showed an increase in the size of the pancreatic mass (67 mm × 58 mm). Her serum PTH level was increased (48.2 pmol/L), whereas PTHrP levels were within normal limits (7.8 pg/mL, 1–15pg/mL) (). The patient was treated with IV fluids and multiple doses of bisphosphonates (zoledronic acid 4 mg, pamidronate disodium 60 mg) and given a trial of cinacalcet hydrochloride 30 mg twice daily. The patient was not able tolerate the cinacalcet for more than 2 days before it was discontinued because of worsening nausea.
Chemotherapy was restarted with doxorubicin 74 mg IV, vincristine 1.8 mg IV, and cyclophosphamide 1480 mg IV. After the first cycle, her calcium levels normalized (2.34 mmol/L) and following the second cycle, her PTH level decreased to 16.6 pmol/L (). A repeat CT scan of her abdomen also showed a reduction in the size of the pancreatic mass (49 mm × 42 mm) (). Immunohistochemical staining of tissue from liver biopsy was done but failed to show evidence of PTH immunoreactivity within the tumor cells.
Despite chemotherapy, the patient died 15 months after diagnosis from recurrent hypercalcemia. An autopsy was declined by her family.
Discussion
Pancreatic neuroendocrine tumors are uncommon, with an incidence of 1 per 100,000 patients per year.Citation32 Clinical presentation and symptoms correlate with the functionality of the tumor and the type of hormones that are overexpressed. Among this type of malignancy, insulin-secreting pancreatic neuroendocrine tumors are the most common, followed by gastrinomas, glucagonoma, vasoactive intestinal peptide secreting tumors (VIPomas), and somatostatinomas.Citation33 PTH-secreting pancreatic neuroendocrine tumors are rare, with the present case being only the third reported in the literature to date.
Hypercalcemia associated with a high PTH level commonly results from a primary parathyroid disorder. This was initially suspected in our patient. However, the clinical and biochemical findings in our case provide evidence to suggest that this pancreatic neuroendocrine tumor was secreting ectopic PTH. In our patient, serum calcium and PTH levels were found to be elevated, and PTHrP remained in the normal range. Despite a number of imaging modalities, there was no evidence of a parathyroid adenoma or hyperplasia to explain the high PTH levels. Chemotherapy treatment led to a reduction of the pancreatic tumor, normalization of calcium levels, and a reduction in PTH levels.
Although paraneoplastic secretion of PTH is not commonly known to cause hypercalcemia of malignancy it has been described in association with a number of different malignancies (). In the few cases reported to date, it appears to be more common in males (11 of 17 cases reported)Citation10–Citation12,Citation16–Citation18,Citation22–Citation26 and predominantly affects those over the age of 60 (14 of 17 cases).Citation10–Citation14,Citation16,Citation17,Citation19,Citation21–Citation26 This is only the second case report of ectopic PTH production in a patient under the age of 30.Citation18
Table 3 A summary and description of the cases of ectopic production of PTH reported in the literature since the discovery of PTHrP
As summarized in , a number of techniques have been reported in the literature as being used to confirm the diagnosis of hypercalcemia secondary to ectopic PTH production. Immunohistochemical staining for PTH in tissue samples from the liver metastases was attempted in our patient; however, these were negative. The negative results may have been due to the quality of biopsy, the immunohistochemical staining antibody that was used, differences between metastatic tissue and primary malignancy, ectopic hormone production being restricted to a subpopulation of tumor cells, or structural differences between the ectopic and the natural form of the hormone not detected with immunohistochemical staining. A biopsy of the primary pancreatic mass was not performed as the procedure was not in the best interest of the patient. The cancer diagnosis was obtained from the biopsy of the liver metastases and therefore tissue from the primary tumor was not available for PTH staining.
Although PTH mRNA was not available at our center, other authors have successfully demonstrated ectopic PTH production by comparing PTH mRNA sequencing from tumor extracts to PTH mRNA from parathyroid tissue using northern blot analysis.Citation10,Citation14,Citation18,Citation21 Despite the lack of immunohistochemical staining or PTH mRNA to confirm ectopic production of PTH, the reduction in tumor burden and improvements in calcium and PTH levels with chemotherapy provides good evidence of PTH secretion from this neuroendocrine tumor.
Outcomes with ectopic PTH secretion are variable, as described in . In almost half of the studies reported, patients succumbed to their disease shortly after diagnosis. Resistant hypercalcemia resulted in only transient improvements with standard therapy for hypercalcemia such as fluids and bisphosphonates reported in some cases of ectopic PTH secretion.Citation10,Citation20,Citation21 Our patient had a similar course prior to the second course of chemotherapy, wherein the patient required weekly dosing of intravenous bisphosphonates and large amounts of IV fluids to maintain calcium levels at a reasonable albeit elevated level. A positive prognosis and long-term management of hypercalcemia in ectopic PTH secretion appears to be limited to resection of the malignancy or treatment with chemotherapy. There have been cases of hepatocellular carcinoma with a tumor producing intact PTH described where hypercalcemia was successfully controlled through transcatheter arterial embolization.Citation16,Citation17
Another option for management of severe or resistant hypercalcemia caused by hyperparathyroidism may be cinacalcet. Cinacalcet is a calcimimetic that increases the sensitivity of the calcium-sensing receptor (CaSR) and has been shown to reduce both calcium and PTH levels in other forms of hyperparathyroidism.Citation34 Cinacalcet was tried in our patient, but unfortunately it was not tolerated because of inducing severe nausea and vomiting. We are not aware of any other case reports where this therapy has been used to treat ectopic PTH secretion.
The benefit of cinacalcet may extend beyond the calcium lowering effects. The CaSR is known to be expressed in a number of tissues not classically considered to play a role in calcium regulation.Citation35 Recent studies have found that CaSR expression and activity in various tissues correlates with both malignancy proliferation and suppression. In breast and prostate cancer, increased expression and activity of CaSR may facilitate bone metastases.Citation36,Citation37 In contrast, parathyroid carcinomas have been associated with decreased or absent expression of the CaSR.Citation38 Animal studies have demonstrated that cinacalcet leads to both activation and increased expression of the CaSR, suppression of parathyroid hyperplasia and reduced PTH secretion in rodents with secondary hyperparathyroidism.Citation39 It is possible that the CaSR may also play a role in ectopic-PTH producing malignancies, and that cinacalcet may have the potential benefit of suppressing the proliferation of PTH-secreting tumor cells. Although further research is required, the potential role of the CaSR to influence tumor growth and suppression presents a novel and important target for investigation of new malignancy therapies.
Conclusion
Hypercalcemia of malignancy resulting from ectopic production of PTH, although not common, should be considered when PTH levels are significantly elevated and there is no evidence of a parathyroid-related cause. Recognizing the association between elevated PTH levels and hypercalcemia of malignancy may prevent unnecessary parathyroid or exploratory neck surgeries and also could possibly lead to the early detection of an undiagnosed malignancy. More research is needed to determine whether there is a role for calcimimetics in treating resistant hypercalcemia secondary to ectopic PTH production and on the potential for suppressing tumor growth in these circumstances.
Acknowledgments
We would like to thank Dr Andrew Karaplis and Dr Dibyendu Panda (Lady Davis Institute for Medical Research, Jewish General Hospital, McGill University) for their assistance with the immunohistochemistry staining.
Disclosure
The authors report no conflict of interest in this work.
References
- Al-AzemHKhanAPrimary hyperparathyroidismCMAJ201118310E685E68921540163
- Consensus Development Task Force on Diagnosis and Management of Asymptomatic Primary HyperparathyroidismAsymptomatic primary hyperparathyroidism: standards and guidelines for diagnosis and management in Canada: Position paperEndocr Pract200395400405 Available at http://www.stjoes.ca/pdfs/PHPT%20position2003.pdfAccessed September 18, 201314583424
- MarcocciCCetaniFClinical practice. Primary hyperparathyroidismN Engl J Med2011365252389239722187986
- AlbrightFCase 27461N Engl J Med194122520789791
- MoseleyJMKubotaMDiefenbach-JaggerHParathyroid hormone-related protein purified from a human lung cancer cell lineProc Natl Acad Sci U S A19878414504850522885845
- BurtisWWuTBunchCIdentification of a novel 17, 000-dalton parathyroid hormone-like adenylate cyclise-stimulating protein from a tumour associated with humoral hypercalcemia of malignancyJ Biol Chem198726215715171563584110
- StrewlerGJSternPHJacobsJwParathyroid hormonelike protein from human renal carcinoma cells. Structural and functional homology with parathyroid hormoneJ Clin Invest1987806180318073680530
- SuvaLJWinslowGAWettenhallREA parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expressionScience198723748178938963616618
- MundyGREdwardsJRPTH-related peptide (PTHrP) in hypercalcemiaJ Am Soc Nephrol200819467267518256357
- YoshimotoKYamasakiRSakaiHEctopic production of parathyroid hormone by small cell lung cancer in a patient with hypercalcemiaJ Clin Endocrinol Metab19896859769812541161
- NeilsenPKRasmussenAKFeldt-RasmussenUBrandtMChristensenLOlgaardKEctopic production of intact parathyroid hormone by a squamous cell lung carcinoma in vivo and in vitroJ Clin Endocrinol Metab199681379337968855839
- UchimuraKMokunoTNagasakaALung cancer associated with hypercalcemia induced by concurrently elevated parathyroid hormone and parathyroid hormone-related protein levelsMetabolism200251787187512077733
- WeissESDotyJBrockMVHalvorsonLYangSCA case of ectopic parathyroid hormone production by a pulmonary neoplasmJ Thorac Cardiovascular Surg20061314923924
- NussbaumSRGazRDArnoldAHypercalcemia and ectopic secretion of parathyroid hormone by an ovarian carcinoma with rearrangement of the gene for parathyroid hormoneN Engl J Med199032319132413282215618
- ChenLDihnTAHaqueASmall cell carcinoma of the ovary with hypercalcemia and ectopic parathyroid hormone productionArch Pathol Lab Med2005129453153315794681
- KoyomaYIshijimaHIshibashiAIntact PTH-producing hepatocellular carcinoma treated by transcatheter arterial embolizationAbdom Imaging199924214414610024399
- MahoneyEJMonchikJMDonatiniGDe LellisRLife-threatening hypercalcemia from a hepatocellular carcinoma secreting intact parathyroid hormone: localization by sestamibi single-photon emission computed tomographic imagingEndocr Pract200612330230616772205
- RizzoliRPacheJ-CDiderjeanLBürgerABonjourJPA thymoma as a cause of true ectopic hyperparathyroidismJ Clin Endocrinol Metab19947939129158077382
- IguchiHMiyagiCTomitaKHypercalcemia caused by ectopic production of parathyroid hormone in a patient with papillary adenocarcinoma of the thyroid glandJ Clin Endocrinol Metab1998838265326579709927
- Vacher-CoponatHOprisADenizotADussolPBerlandYHypercalcemia induced by excessive parathyroid hormone secretion in a patient with a neuroendocrine tumourNephrol Dial Transplant200520122832283516188904
- VanHoutenJNYuNRimmDHypercalcemia of malignancy due to ectopic transactivation of the parathyroid hormone geneJ Clin Endocrinol Metab200691258058316263810
- EidWWheelerTMSharmaMDCurrent hypercalcemia due to ectopic production of parathyroid hormone-related protein and intact parathyroid hormone in a single patient with multiple malignanciesEndocr Pract200410212513415256329
- StrewlerGJBudayrAAClarckOHNissensonRAProduction of parathyroid hormone by a malignant nonparathyroid tumour in a hypercalcemic patientJ Clin Endocrinol Metab1993765137313757684395
- WongKTsudaSMukaiRSumidaKArakakiRParathyroid hormone expression in a patient with metastatic nasopharyngeal rhabdomyosarcoma and hypercalcemiaEndocrine2005251838616077176
- KandilENoureldineSKhalekMDarocaPFriedlanderPEctopic secretion of parathyroid hormone in a neuroendocrine tumour: a case report and review of the literatureInt J Clin Exp Med20114323424021977238
- NakajimaKTamaiMOkaniwaSHumoral hypercalcemia associated with gastric carcinoma secreting parathyroid hormone: a case report and review of the literatureEndocr J201360555756223303131
- StewartAFHypercalcemia associated with cancerN Engl J Med2005352437337915673803
- ShuSTMartinCKThudiNKDirksenWPRosolTJOsteolytic bone resorption in adult T-cell leukemia/lymphomaLeuk Lymphoma201051470271420214446
- SyedMHorwitzMTedescoMGarcia-OcañaAWisniewskiSRStewartAFParathyroid hormone-related protein (1–36) stimulates renal tubular calcium reabsorption in normal human volunteers: implications for the pathogenesis of humoral hypercalcemia of malignancyJ Clin Endocrinol Metab2001861525153111297578
- SchillingTPecherstorferMBlindELeidigGZieglerRRaueFParathyroid hormone-related protein (PTHrP) does not regulate 1,25-dihydroxyvitamin D serum levels in hypercalcemia of malignancyJ Clin Endocrinol Metab19937638018038445039
- MoeSMDisorders involving calcium, phosphorus and magnesiumPrimary Care200835221523718486714
- KlimstraDSNonductal neoplasms of the pancreasMod Pathol200720Suppl 1S94S11217486055
- MunirajTVigneshSShettySThiruvengadamSAslanianJRPancreatic neuroendocrine tumoursDis Mon201359151923312533
- WüthrichRMartinDBilezikianJThe role of calcimimetics in the treatment of hyperparathyroidismEur J Clin Invest2007371291592218036025
- BrennanSCThiemURothSCalcium sensing receptor signalling in physiology and cancerBiochim Biophys Acta2013183371732174423267858
- MihaiRStevensJMcKinneyCIbrahimNBExpression of the calcium receptor in human breast cancer-a potential new marker predicting the risk of bone metastasesEur J Surg Oncol200632551151516564154
- LiaoJSchneiderADattaNSMcCauleyLKExtracellular calcium as a candidate mediator of prostate cancer skeletal metastasisCancer Res200666189065907316982748
- HavenCJvan PuijenbroekMKarperienMFLeurenGJMorreauHDifferential expression of the calcium receptor and combined loss of chromosome 1q and 11q in parathyroid carcinomaJ Pathol20042021869414694525
- MillerGDavisJShatzenECollotonMMartinDHenleyCMCinacalcet HCL prevents development of parathyroid gland hyperplasia and reverses established parathyroid gland hyperplasia in a rodent model of CKDNephrol Dial Transplant20122762198220522036941
