Abstract
Nine pretreated patients aged >19 years with relapsed/refractory acute lymphoblastic leukemia (ALL) were treated with a combination of bortezomib plus chemotherapy before allogeneic hematopoietic stem cell transplantation (allo-HSCT). Eight (88.9%) patients, including two Philadelphia chromosome-positive ALL patients, achieved a complete remission. Furthermore, the evaluable patients have benefited from allo-HSCT after response to this reinduction treatment. We conclude that bortezomib-based chemotherapy was highly effective for adults with refractory/relapsed ALL before allo-HSCT. Therefore, this regimen deserves a larger series within prospective trials to confirm these results.
Introduction
The prognosis in patients with refractory/relapsed adult acute lymphoblastic leukemia (ALL) is dismal. Currently, there is no standard salvage therapy for such patients and the only realistic strategy resides in getting another complete remission (CR) followed by a successful allogeneic hematopoietic stem cell transplantation (allo-HSCT),Citation1–Citation3 provided that the toxicity of the salvage regimen is acceptable. In the reinduction setting, the use of chemotherapeutic agents similar to those administered during initial induction chemotherapy may get CR, but the possibility of achieving a second CR is less than 50%.Citation4 In addition, this regimen is limited, particularly in adults with refractory ALL. Treatment strategies to further improve the efficacy of antileukemic therapy are often based on the introduction of novel agents. Recently, Dewar et alCitation5 reported that a patient diagnosed with refractory adult Philadelphia chromosome-positive (Ph+) ALL was treated successfully with a bortezomib-containing chemotherapy regimen. In addition, Hu et alCitation6 obtained encouraging results in a relapsed adult T-cell acute lymphoblastic leukemia (T-ALL) by administration of bortezomib combined with chemotherapy. Thus far, no series has reported the use of bortezomib for the treatment of relapsed/refractory adult ALL. We report here the outcomes of nine such patients treated by a combination of bortezomib plus chemotherapy as part of a pilot study. Favorable responses were observed.
Case report
Between March 2008 and November 2010, a total of nine consecutive patients with refractory/relapsed adult ALL, including Ph+ ALL, were enrolled in this study at the Henan Institute of Haematology (). Informed consent was obtained from all patients and the study was approved by the Institutional Review Board at Henan Tumor Hospital. Other eligibility criteria included a performance status of 2 or lower according to the Eastern Cooperative Oncology Group (ECOG), adequate hepatic and renal function, and adequate cardiac status.
Table 1 Characteristics of patients and outcomes after bortezomib-based therapy
As a salvage reinduction regimen, all patients received a bortezomib-based therapy at a median time of 6 months (range 1.9–13.5 months) after ALL diagnosis. Five patients received the bortezomib (1.3 mg/m2/day on days 1, 4, 8, and 11) + fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD) regimen and two patients received the bortezomib (1.3 mg/m2/day on days 1, 4, 8, and 11) + (high-dose methotrexate and ara-C) regimen. The other two patients with refractory Ph+ ALL were treated with bortezomib (1.3 mg/m2/day on days 1, 4, 8, and 11) + imatinib (400 mg/day) + Hyper-CVAD regimen. CR was defined as ≤5% lymphoblasts in a normocellular or hypercellular marrow with no evidence of circulating blasts or extramedullary disease, and with absolute neutrophil count 1×109/L and platelet count ≥100×109/L. Treatment-related toxicities were assessed and graded according to the National Cancer Institute Expanded Common Toxicity Criteria (version 3.0). After a salvage reinduction regimen, patients getting CR could receive one or more first consolidation courses, followed by allo-HSCT as soon as possible. After allo-HSCT, patients with refractory/relapsed Ph+ ALL were maintained on dasatinib (70 mg twice a day). Patients who failed to obtain CR after two courses were taken off the study. A third-line regimen called FLAG-IDA was suggested for patients who failed to obtain CR after the salvage treatment and for those who relapsed after the salvage treatment and before allo-HSCT.Citation7 Overall survival (OS) was calculated from the beginning of salvage therapy to the date of the last follow-up or death for survivors, whatever the cause, while disease-free survival (DFS) was calculated from CR until relapse, death from any cause, or the last contact for survivors. Probability of OS rate was estimated according to the Kaplan–Meier method.
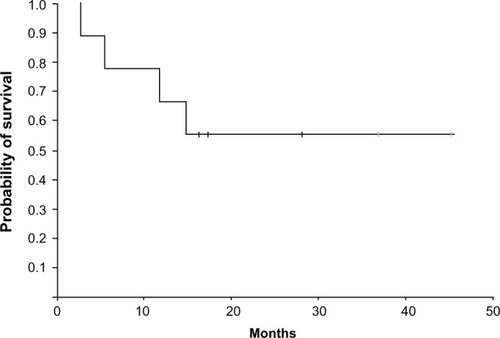
shows the responses and outcome details of therapy. Patient 3, who failed to respond to the first course of salvage treatment, gave up therapy and died 1.7 months after stopping treatment. The other eight patients achieved CR after 1–2 courses of bortezomib-containing chemotherapy. Then, after receiving one or more first consolidation courses, all underwent an allo-HSCT. At the time of the last follow-up, three of eight eligible patients who had achieved CR died of leukemia relapse (at 4.2, 10.6, and 13.8 months, respectively), while the other five patients were still alive and in CR at 15.1, 16.3, 35.8, and 44.3 months, respectively. The median DFS was 15.7 months (range 8.1–44.3 months) and the median OS was 16.4 months (range 2.7–45.4 months). Two-year OS rate was 56.4 months (95% CI 8.9–30.7 months) (). The combination of bortezomib plus chemotherapy was well tolerated. No patient discontinued therapy because of toxicity and there was no treatment-related mortality. All patients experienced grade 3–4 anemia, neutropenia, and thrombocytopenia. The median time to reach absolute neutrophil count >0.5×109/L was 19 days (range 15–24 days) from the start of chemotherapy. Platelet count >20×109/L was achieved in a median time of 21 days (range 18–26 days). The most common grade 1–2 nonhematologic toxicities in the study included constipation (50%), vomiting (100%), fatigue (100%), diarrhea (37.5%), and skin rashes (12.5%). In addition, Grade 1–2 peripheral neuropathy and grade 3 pulmonary infections occurred in one (12.5%) and four (50%) patients, respectively.
Discussion
Treatment of refractory/relapsed adult ALL, including Ph+ ALL, represents a considerable clinical challenge. These patients are often refractory to currently available treatment options, and the alternative salvage therapeutic approaches remain elusive.
Single-agent bortezomib was studied in adults with refractory/relapsed acute leukemia and found to be ineffective in controlling disease progression,Citation8 but the results from a combination trial have suggested bortezomib is a rational strategy to overcome chemoresistance and induce chemosensitization. Attar et alCitation9 reported that the treatment of bortezomib plus cytarabine and idarubicin in patients with relapsed acute myeloid leukemia showed encouraging activity, and that the regimen had also been well tolerated. In addition, in patients with fludarabine-refractory chronic lymphocytic leukemia, single-agent bortezomib demonstrated biologic activity.Citation10 Recently, Hu et alCitation6 reported that administration of bortezomib plus dexamethasone and liposomal doxorubicin to a heavily pretreated adult with relapsed ALL achieved another CR.
Our results suggest that the addition of bortezomib to conventional chemotherapy programs in adults with refractory/relapsed ALL is feasible and that the salvage treatment was generally well tolerated. Indeed, we can confidently conclude that CR achievement was due to the addition of bortezomib. All eight evaluable patients had refractory disease after receiving Hyper-CVAD-based or imatinib-based induction regimens, but they were all in CR after receiving the bortezomib + Hyper-CVAD, or bortezomib + Hyper-CVAD + imatinib, respectively, indicating the efficacy of bortezomib and a synergistic effect of the combination. Thus, the combined bortezomib-based reinduction therapy may be a promising salvage alternative for this population with a very poor prognosis as a springboard to allo-HSCT, the only potential curative intervention.
Furthermore, all eight eligible patients have benefited from allo-HSCT as consolidation after CR was assessed. However, the idea that bortezomib could overcome or reverse chemoresistance and increase sensitivity to conventional or targeted agents was not evaluated in this study. This could be a pivotal factor in selecting optimal patients who might respond to the combination of bortezomib plus conventional chemotherapy, and this should be addressed in future studies.
Conclusion
Reinduction with the bortezomib-based regimen followed by allo-HSCT may be a feasible approach for adults with refractory/relapsed ALL. Larger series within prospective trials are needed to evaluate this concept.
Author contributions
All authors designed the research, enrolled the study patients, supervised the study, collected clinical data, analyzed the data, and drafted and revised the manuscript.
Acknowledgments
The authors would like to thank all patients for their cooperation. This study was supported by grants from the National Natural Science Foundation of China (Number 30900637) and the National Natural Science Foundation of China (Number 81370661).
Disclosure
The authors declare no potential conflicts of interest in this work.
References
- AtraAGerrardMHobsonRImesonJDHannIMPinkertonCROutcome of relapsed or refractory childhood B-cell acute lymphoblastic leukaemia and B-cell non-Hodgkin’s lymphoma treated with the UKCCSG 9003/9002 protocolsBr J Haematol2001112496596811298592
- MartinoRBellidoMBrunetSAllogeneic or autologous stem cell transplantation following salvage chemotherapy for adults with refractory or relapsed acute lymphoblastic leukemiaBone Marrow Transplant19982110102310279632276
- MartinoRBellidoMBrunetSIntensive salvage chemotherapy for primary refractory or first relapsed adult acute lymphoblastic leukemia: results of a prospective trialHaematologica199984650551010366793
- WeissMATreatment of adult patients with relapsed or refractory acute lymphoblastic leukemia (ALL)Leukemia199711Suppl 4S28S309179279
- DewarRChenSTYeckes-RodinHMillerKKhosravi-FarRBortezomib treatment causes remission in a Ph+ ALL patient and reveals FoxO as a theranostic markerCancer Biol Ther201111655255821282974
- HuXXuJSunAShenYHeGGuoFSuccessful T-cell acute lymphoblastic leukemia treatment with proteasome inhibitor bortezomib based on evaluation of nuclear factor-κB activityLeuk Lymphoma201152122393239521745166
- SpecchiaGPastoreDCarluccioPFLAG-IDA in the treatment of refractory/relapsed adult acute lymphoblastic leukemiaAnn Hematol2005841279279516047203
- CortesJThomasDKollerCPhase I study of bortezomib in refractory or relapsed acute leukemiasClin Cancer Res200410103371337615161691
- AttarECDe AngeloDJSupkoJGPhase I and pharmacokinetic study of bortezomib in combination with idarubicin and cytarabine in patients with acute myelogenous leukemiaClin Cancer Res20081451446145418316568
- FaderlSRaiKGribbenJPhase II study of single-agent bortezomib for the treatment of patients with fludarabine-refractory B-cell chronic lymphocytic leukemiaCancer2006107591692416832816
- HuguetFLeguayTRaffouxEPediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 studyJ Clin Oncol200927691191819124805
- ChalandonYThomasXHayetteSFirst Results of the GRAAPH-2005 Study in younger Adult Patients with De Novo Philadelphia Positive Acute Lymphoblastic Leukemia: 50th ASH Annual Meeting and Exposition, San Francisco, CA, USA, 6–9 December 2008Blood20081122
- KantarjianHMO’BrienSSmithTLResults of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemiaJ Clin Oncol200018354756110653870