Abstract
Background
Celecoxib is an effective treatment for osteoarthritis (OA). However, information on its efficacy and safety profile in different racial/ethnic groups is limited. Noticeable differences among racial groups are found in other disease states, but a thorough investigation of OA is lacking. The objective of this study was to determine if celecoxib 200 mg once daily is as effective as naproxen 500 mg twice daily in the treatment of OA of the knee in Hispanic patients.
Methods
Hispanic patients aged ≥45 years with knee OA were randomized to receive celecoxib 200 mg once daily, naproxen 500 mg twice daily, or placebo for 6 weeks. The primary efficacy variable was the change in Patient’s Assessment of Arthritis Pain at 6 weeks compared with baseline. Secondary variables were change in Patient’s and Physician’s Global Assessments of Arthritis from baseline to week 6/early termination, change in Western Ontario and McMaster Universities OA Index (WOMAC) from baseline to week 6/early termination, change in American Pain Society pain score, Pain Satisfaction Scale, Patient Health Questionnaire (PHQ-9), and measurements of upper gastrointestinal tolerability.
Results
In total, 239 patients completed the trial (96 celecoxib, 96 naproxen, 47 placebo). Celecoxib was as effective as naproxen in reducing OA pain (least squares mean change from baseline [standard error] −39.7 [2.7] for celecoxib and −36.9 [2.6] for naproxen). Patient’s and Physician’s Global Assessments of Arthritis, WOMAC scores, upper gastrointestinal tolerability, Pain Satisfaction Scale, and PHQ-9 showed no statistically significant differences between the celecoxib and naproxen groups. The incidence of adverse events and treatment-related adverse events were similar among the treatment groups.
Conclusion
Celecoxib 200 mg once daily was as effective as naproxen 500 mg twice daily in the treatment of signs and symptoms of knee OA in Hispanic patients. Celecoxib was shown to be safe and well tolerated in this patient population.
Introduction
It is predicted that nearly one in three US residents will be Hispanic by 2060.Citation1 The exact prevalence of osteoarthritis (OA) in the Hispanic population is unknown, but research suggests that between 12% and 22% of Hispanics have arthritis, of which OA is the most common form.Citation2,Citation3 The prevalence is lowest (12%) in Cubans/Cuban Americans and highest (22%) in Puerto Ricans.Citation3 Research also suggests that severe pain, work impairment, and poor outcomes are higher in this population compared with Caucasians.Citation4 Older Hispanics with OA of the knee and obesity have an unduly elevated loss of quality-adjusted life-years.Citation5 In addition, a negative impact on the perception of quality of life has been reported in association with pain severity, age, and poor socioeconomic status in Venezuelan patients with OA.Citation6 This suggests that Hispanic patients with OA could benefit from early and effective medical intervention.
Limited information is available on how Hispanic patients with OA compare with other ethnic groups in their OA treatment approaches and responsiveness. The literature indicates that, compared with Caucasians, Hispanic patients are less likely to undergo total knee replacement and are more likely to use oral herbs and magnets/copper jewelry therapy.Citation7–Citation12 Another study has shown that these patients are less likely to receive treatment with a cyclo-oxygenase-2 selective non-steroidal anti-inflammatory drug (NSAID; odds ratio 0.47, P<0.01) and more likely to discontinue treatment early.Citation13
Celecoxib is a selective NSAID indicated for the treatment of signs and symptoms associated with OA.Citation14 Its efficacy has been established, and it has a favorable gastrointestinal tolerability profile relative to nonselective NSAIDs, ie, fewer patients report gastrointestinal adverse events, such as dyspepsia, with celecoxib.Citation15 The purpose of this study was to confirm the noninferiority of celecoxib to naproxen, a nonselective NSAID, with regard to analgesic effects and gastrointestinal tolerability in patients with OA who were of Hispanic descent.
Materials and methods
Objectives
The primary objective of this study was to determine whether celecoxib 200 mg once daily was as effective as naproxen 500 mg twice daily for the treatment of symptoms associated with OA of the knee in a Hispanic population. The secondary objective was to confirm the tolerability of celecoxib 200 mg once daily versus placebo in these patients. The use of complementary and alternative medicines in this population was also evaluated at baseline.
Study population
Patients aged ≥45 years and of self-reported Hispanic descent with OA of the knee (diagnosed according to American College of Rheumatology criteriaCitation16) who were determined to be in a fare state and had a functional capacity classification of I to III met the study eligibility criteria. Other inclusion criteria that applied have been described in a previously published report that assessed the response to NSAIDS in an African American population with OA.Citation17 Briefly, patients actively being treated with an NSAID or other analgesic therapy discontinued treatment at least 48 hours prior to the baseline assessments. Eligible patients indicated a Patient’s Assessment of Arthritis Pain visual analog scale (VAS) score between 40 mm and 90 mm (range 0–100 mm) and had a minimum rating of 3 on the Physician’s and Patient’s Global Assessment of Arthritis at baseline. Exclusion criteria were the same as those described in the previous report.Citation17
Study design
This was a 6-week, randomized, double-blind, placebo-controlled, active-comparator, parallel-group trial carried out in 31 US centers in compliance with the principles of Good Clinical Practice and the Declaration of Helsinki. Each study site received protocol approval from an institutional review board, and all patients gave written informed consent.
Four clinic visits were required, ie, at screening, baseline, week 2, and week 6. During the screening visit, patients underwent a physical examination and laboratory tests. Both the patient and physician provided an assessment of arthritis. Patients were randomized in a 2:2:1 ratio to one of three treatments, ie, celecoxib 200 mg once daily, naproxen 500 mg twice daily, or placebo, according to a predetermined computer randomization schedule. Patients were assigned their randomization number based on the order in which they enrolled in the study. Both the investigator and patient were blinded to the study medications and to placebo, and all assessments were made by individuals who had been blinded. Each study medication had a matching placebo that was of similar appearance (capsule size, color, smell, and taste).
Efficacy evaluation
The primary efficacy outcome was defined as the change from baseline to week 6 in the Patient’s Assessment of Arthritis Pain, which was measured on a VAS of 0 mm (no pain) to 100 mm (worst pain). All pain assessments were based on the one knee selected by the patient to be the “index joint”. Secondary outcomes included change in Patient’s and Physician’s Global Assessments of Arthritis and Western Ontario and McMaster Universities OA Index (WOMAC) from baseline to week 6, change in American Pain Society (APS) pain scores from baseline to day 7 (week 1), change in Pain Satisfaction Scale and Patient Health Questionnaire (PHQ-9) scores from screening to week 6, and measurement of upper gastrointestinal tolerability.
The population evaluable for efficacy was used for the primary efficacy analysis and the modified intent-to-treat population was used for secondary efficacy analyses. The modified intent-to-treat population included all patients who were randomized, received at least one dose of study medication, and had at least one post-baseline follow-up efficacy measure. The efficacy evaluable population included modified intent-to-treat patients who had no major protocol violations, were assessed at both baseline and week 6 for the primary efficacy variable, had adequate treatment, and belonged to the protocol-specified ethnic group.
Safety evaluation
General clinical safety was assessed by monitoring treatment-emergent adverse events and serious adverse events and by physical examination. Upper gastrointestinal tolerability was assessed as described in a previously published study.Citation17 Safety analyses were carried out in the safety population, which included all randomized patients who received at least one dose of study medication.
Statistical analysis
Sample size calculation was based on the maximum clinically acceptable difference for declaring noninferiority, which was compared with the lower bound of the two-sided 95% confidence interval (CI) for the difference between the two treatment groups.Citation17 A total of 120 patients per active treatment group were randomized to adjust for the differences between the intent-to-treat and efficacy evaluable populations to allow for any nonevaluable patients (eg, those who are lost to follow-up). Sixty patients were randomized to placebo in order to have 80% power to detect a difference of 15 mm between the active treatment group and placebo in the VAS score.
Change in VAS score from baseline to week 6 was analyzed using a general linear model with treatment and center effects in the model and baseline score as a covariate. Pairwise comparisons were conducted. Celecoxib was declared to be as effective as naproxen if the lower bound of the two-sided 95% CI of the treatment difference (naproxen – celecoxib) lay above −10 mm.Citation18 As a test of internal control, differences in the mean change in VAS score were also analyzed for celecoxib versus placebo and for naproxen versus placebo.
The 2 4-item WOMAC scale and subscales were analyzed using a general linear model with treatment and center effects in the model, and baseline WOMAC score as a covariate. The WOMAC total domain score (range 0–96) was the sum of the pain, stiffness, and physical function domain scores. Responses to the Patient’s and Physician’s Global Assessments of Arthritis were analyzed and the patients’ conditions were classified as “improved”, “no change”, or “worsened” using the Cochran–Mantel–Haenszel test, stratified by center. APS questions were analyzed using the Cochran–Mantel–Haenszel test stratified by center (question 1, “Have you experienced any pain in the past 24 hours?”; yes or no). Change from baseline for the remaining questions was analyzed using a general linear model with treatment, center, and baseline APS value (questions 2–5) as a covariate. Change in PHQ-9 score was analyzed using a general linear model with treatment, center, and screening PHQ-9 score as a covariate. Patient pain satisfaction was analyzed at screening and week 6 using the Cochran–Mantel–Haenszel test, stratified by center.
Safety and upper gastrointestinal events were analyzed in the safety population, defined as randomized patients receiving at least one dose of study medication. The incidence of upper gastrointestinal events was analyzed using two-tailed Fisher’s exact tests.
Results
Patient disposition and baseline demographics
A total of 318 patients were randomized to treatment (127, 129, and 62 patients in the celecoxib, naproxen, and placebo groups, respectively), and 315 patients received treatment (). Baseline characteristics of the patients were similar among the treatment groups, and are summarized in . Patients were mostly female (60%–72%), and with an age range of 40–88 years. Mean duration of OA ranged from 5.3 to 6.6 years. The majority of patients were assessed as “poor” or “very poor” at baseline on both the Patient’s and Physician’s Global Assessments of Arthritis, and were in the functional capacity classification of II and III. Mean WOMAC total domain scores ranged from 55.7 to 58.6.
Figure 1 Patient disposition.
Abbreviations: AE, adverse events; mITT, modified intent-to-treat; qd, once daily; bid, twice daily.
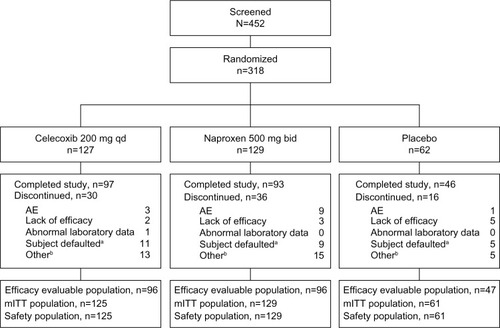
Table 1 Baseline demographic and clinical characteristics
There were no significant differences noted between groups with regard to responses to the Pain Satisfaction Scale at the screening visit except for question 8 (“have better relationships with others”), for which a statistically significant difference was seen in favor of the celecoxib group over the placebo group (P=0.015).
Responses to the Complementary and Alternative Medicines Questionnaire indicated that prescription medicines, self-determined over-the-counter medicines, and physician-recommended over-the-counter medicines were used by 6 9%, 45%, and 4 4% of the screened population, respectively (data not shown). “Store bought lotions, oils, and creams” (56%) were the most frequently used alternatives to conventional medical OA treatments, while other herbal, homemade, or household lotions or oils were used by fewer than 15% of individuals screened. Dietary modifications were also a common alternative to medical OA treatments; ≥50% of patients avoided alcohol and saturated fats or fried foods, 44% ate a high-fiber diet or whole grain foods, and 35%–38% avoided white flour, sugar, and/or red meats, and/or increased the amount of cabbage, broccoli, kale, and Brussels sprouts in their diets. There were also reports indicating the use of special vitamins, vitamin combinations, or minerals (27%) and glucosamine and/or chondroitin sulfate (23%). The use of other dietary supplements occurred in <12% of the screened individuals.
Further commonly-used alternative treatments included nutritional therapy (25%), massage (30%), and prayer (34%). Less than 18% of the population used herbal medicine, reflexology, acupuncture, chiropractic, and spiritual healing. Miscellaneous treatments such as venom, magnets, and biofeedback were used by <10% of the population.
Efficacy outcomes
Improvement in all three groups was seen on the primary efficacy outcome, ie, Patient’s Assessment of Arthritis Pain (VAS, ). Least squares mean changes from baseline to week 6 were −39.7 mm, −36.9 mm, and −28.6 mm in the celecoxib, naproxen, and placebo groups, respectively. The lower bound of the two-sided 9 5% CI of the treatment difference (naproxen – celecoxib) was above −10 mm (−3.8 mm). Hence, celecoxib was as effective as naproxen at reducing OA pain. Also, the P-values for celecoxib – placebo (P=0.0077) suggest celecoxib is significantly more effective than placebo at relieving pain, as recorded on the Patient’s Assessment of Arthritis Pain VAS.
Table 2 Patient’s assessment of arthritis pain (VAS) at week 6 (efficacy evaluable population)
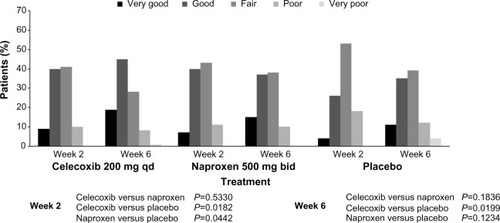
The results for the secondary efficacy end points were similar between active treatments, supporting the noninferiority of celecoxib to naproxen. The outcomes of Patient’s and Physician’s Global Assessments of Arthritis are presented in and . Physicians described the arthritis condition of 60% of patients in the celecoxib group and 52% of patients in the naproxen group as “improved” by the week 6/early termination visit, compared with 46% of patients in the placebo group. Between-treatment differences were statistically significant in favor of celecoxib over placebo (P=0.0369). The OA condition of 5 7% of the patients in each active treatment group had “improved” by the week 6/early termination visit compared with 43% of patients in the placebo group. Between-treatment differences were not statistically significant (data not shown).
Figure 2 Patient’s Global Assessment of Arthritis: modified intent-to-treat population.
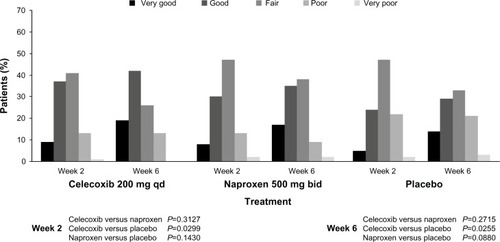
Figure 3 Physician’s Global Assessment of Arthritis: overall ratings (modified intent-to-treat population).
The mean change from baseline in the total and individual domain scores of the WOMAC indicated improvement from baseline in each treatment group (). Although differences between celecoxib and naproxen were not statistically significant, between-treatment differences for both celecoxib and naproxen compared with placebo were statistically significant for all but the stiffness domain.
Table 3 WOMAC, upper gastrointestinal tolerability, and APS pain scores
For APS pain measurements, the number of patients who experienced pain within 24 hours prior to completion of the questionnaire decreased from 100% at baseline to 91%, 84%, and 95% on day 7 for the celecoxib, naproxen, and placebo groups, respectively. In general, APS pain scores improved from baseline to day 7 (). There were statistically significant differences in favor of active treatments compared with placebo in total pain interference from baseline to day 7 (P<0.001).
Overall, a greater percentage of patients in the celecoxib and naproxen groups responded positively to the questions on the Pain Satisfaction Scale at the week 6/early termination visit compared with placebo. More of the patients using active treatment than those using placebo were happy with the duration and speed of pain relief and agreed or somewhat agreed that their study pain medication positively affected their physical health, outlook, ability to perform daily and leisure activities, independence, relationships, mood, concentration, and ease of movement. Differences between celecoxib and naproxen were not statistically significant. Results on the PHQ-9 were similar between the celecoxib and naproxen groups (data not shown).
Safety outcomes
All 315 patients who received treatment were evaluated for adverse events. A total of 110 patients (28%–37% per treatment group) reported 157 adverse events. The incidence of adverse events was similar among the treatment groups, with most being mild to moderate in severity. Of the 157 adverse events, 70 were considered to be treatment-related (25, 36, and nine in the celecoxib, naproxen, and placebo groups, respectively). No deaths were reported. Treatment-related adverse events occurring in ≥2% of patients are summarized in .
Table 4 Treatment-related adverse events occurring in ≥2% of patients (in decreasing order of occurrence)
The majority of patients who reported adverse events complained of gastrointestinal system and psychiatric disorders. The most commonly occurring adverse events were depression, headache, abdominal pain, constipation, and dyspepsia. Of these, only depression occurred in >5% of the subject population: 11% of subjects overall reported depression (10%, 9%, and 20% in the celecoxib, naproxen, and placebo groups, respectively). There were few reports of upper gastrointestinal intolerability in this study. A total of eight patients (three in the celecoxib group, four in the naproxen group, and one in the placebo group) experienced an upper gastrointestinal event, defined as moderate or severe nausea, abdominal pain, and/or dyspepsia (). Between-treatment differences were not statistically significant (P=1.0000).
Thirteen patients in total discontinued the study as a result of adverse events, while ten were withdrawn because of adverse events that were deemed to be treatment-related (two, seven, and one patients in the celecoxib, naproxen, and placebo groups, respectively). One patient in the naproxen group experienced a severe adverse event (gastrointestinal hemorrhage) that was considered related to the study treatment, resulting in discontinuation of the study medication.
Discussion
Despite the growing population of minority ethnic groups in the USA and a greater interest in their clinical experience of pain, non-white groups remain substantially underrepresented in clinical trials for a multitude of reasons, including mistrust of the health care system, sociocultural barriers, and a shortage of investigators of diverse ethnic backgrounds.Citation19–Citation23 This lack of representation poses a challenge with regard to the generalizability and external validity of clinical trial results and leaves a need for safety and efficacy data in minority groups.
Cultural and ethnic differences exist in the perception of pain and how it is treated from the perspectives of both patients and health care providers. In addition, the response to medication may differ in various ethnic populations because of genetic and metabolic factors.Citation24,Citation25 For these reasons, individual prescribers and payers are increasingly requesting efficacy and safety data for medications that have been studied in a greater variety of ethnic populations, so as to reflect the diversity of their beneficiary groups. The present study was conducted to further our understanding of the efficacy, safety, and tolerability of celecoxib in individuals of Hispanic descent.
In this population, mean changes in the Patient’s Assessment of Arthritis Pain VAS improved in all three treatment groups. Given that the lower boundary of the two-sided 95% CI of the treatment difference (naproxen – celecoxib) was above −10 mm (−3.8 mm), celecoxib was as effective as naproxen at reducing OA pain. Compared with placebo, celecoxib was significantly more effective at relieving pain (P=0.0077). Secondary efficacy findings were indicative of the noninferiority of celecoxib to naproxen, because similar results were seen on the Patient’s and Physician’s Global Assessment of Arthritis, WOMAC scores, Pain Satisfaction Scale, PHQ-9, and upper gastrointestinal tolerability. These findings are consistent with other studies showing comparable efficacy of celecoxib with naproxen and other NSAIDs.Citation26–Citation28 However, in these studies, the racial composition of the study subjects was primarily white or undefined.
Several recent publications have highlighted disparities in treatment approaches and outcomes in Hispanic patients with cardiovascular disease,Citation29,Citation30 asthma, and depression. Hispanics are less likely to receive or use medications for asthma,Citation31 cardiovascular disease,Citation32 human immunodeficiency virus infection/acquired immunodeficiency syndrome,Citation33 mental illness,Citation34 or pain,Citation35 as well as prescription medications in general.Citation36,Citation37 These disparities in pharmaceutical treatment are substantial and often persist, even after adjustment for differences in income, age, insurance coverage, and coexisting medical conditions.Citation38 There is a paucity of data evaluating differences in response to medications between Hispanic and non-Latino populations.Citation38 Emerging research demonstrates that genetic variations affect Hispanic Americans and may require dosage adjustments to achieve an optimal therapeutic effect.Citation39,Citation40 The published literature highlights that Hispanics are cautious about American medicines, in part because of concerns about addiction, and often initiate downward dosage adjustments to avoid even minor side effects.Citation41 Given the increasing percentage of Hispanic Americans in the US population, studying the efficacy and safety of various medications in Hispanic populations will become increasingly important to health care practitioners and payers as they make treatment and formulary decisions for their populations.Citation39 To meet the data needs of payers and health care practitioners, more studies such as this one, which prospectively evaluated a specific medication in a Hispanic population, or the implementation of measures to increase the participation of Hispanic patients in broader clinical trials, will be required.Citation39
Safety and tolerability are important considerations when prescribing analgesic therapies. As noted above, they may be even more important in the Hispanic community. The composite measures of upper gastrointestinal tolerability presented in this paper, in addition to individually recorded adverse events, may provide a more clinically relevant assessment of treatment for the practicing physician. With only three upper gastrointestinal tolerability events (defined as moderate or severe nausea, abdominal pain, and/or dyspepsia) reported in the celecoxib treatment group, coupled with the low incidence of patient discontinuation due to treatment-related adverse events, the tolerability profile of celecoxib was demonstrated to be favorable.
Conclusion
This prospective, well controlled study of Hispanic patients provides insight into the efficacy and tolerability of celecoxib in the effective management of OA symptoms in a minority population. Celecoxib 200 mg once daily was noninferior to naproxen 500 mg twice daily for treating the signs and symptoms associated with OA of the knee in this patient group. In addition, both celecoxib and naproxen were shown to be safe and well tolerated. Research into the role of race or ethnicity in the response to treatment is still needed.
Disclosure
This study was sponsored by Pfizer Inc. The authors are fulltime employees of Pfizer Inc. Editorial support was provided by K Bradford of PAREXEL and was funded by Pfizer Inc.
References
- United States Census Bureau US census bureau projections show a slower growing, older, more diverse nation a half century from nowUS Department of Commerce Available from: https://www.census.gov/newsroom/releases/archives/population/cb12-243.htmlAccessed October 22, 2013
- LawrenceRCFelsonDTHelmickCGEstimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part IIArthritis Rheum2008581263518163497
- Centers for Disease Control and PreventionPrevalence of doctor-diagnosed arthritis-attributable effects among Hispanic adults, by Hispanic subgroup – United States, 2002, 2003, 2006, and 2009MMWR Morb Mortal Wkly Rep201160616717121330965
- Centers for Disease Control and PreventionRacial/ethnic differences in the prevalence and impact of doctor-diagnosed arthritis – United States, 2002MMWR Morb Mortal Wkly Rep200554511912315703693
- LosinaEWalenskyRPReichmannWMImpact of obesity and knee osteoarthritis on morbidity and mortality in older AmericansAnn Intern Med2011154421722621320937
- ChaconJGGonzalezNEVelizAEffect of knee osteoarthritis on the perception of quality of life in Venezuelan patientsArthritis Rheum200451337738215188322
- 2008 National Population Projections1970, 1980, 1990 and 2000 Decennial CensusesUS Census Bureau Available from: http://www.census.gov/prod/2011pubs/12statab/pop.pdfAccessed March 7, 2013
- DunlopDDSongJManheimLMChangRWRacial disparities in joint replacement use among older adultsMed Care200341228829812555056
- HermanCJDenteJMAllenPHuntWCEthnic differences in the use of complementary and alternative therapies among adults with osteoarthritisPrev Chronic Dis200633A8016776881
- OlsonJCFolandJTracking racial and ethnic disparities of knee replacement rates in ConnecticutConn Med200569421121515926636
- SkinnerJWeinsteinJNSporerSMWennbergJERacial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patientsN Engl J Med2003349141350135914523144
- Suarez-AlmazorMESouchekJKellyPAEthnic variation in knee replacement: patient preferences or uninformed disparityArch Intern Med2005165101117112415911724
- DominickKLBosworthHBJeffreysASGrambowSCOddoneEZHornerRDRacial/ethnic variations in non-steroidal anti-inflammatory drug (NSAID) use among patients with osteoarthritisPharmacoepidemiol Drug Saf2004131068369415386734
- Celebrex [US prescribing information]New York, NY, USAPfizer Inc2013
- NiculescuLLiCHuangJMallenSPooled analysis of GI tolerability of 21 randomized controlled trials of celecoxib and nonselective NSAIDsCurr Med Res Opin200925372974019210159
- AltmanRAschEBlochDDevelopment of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism AssociationArthritis Rheum1986298103910493741515
- EssexMNO’ConnellMBhadraBPResponse to nonsteroidal anti-inflammatory drugs in African Americans with osteoarthritis of the kneeJ Int Med Res20124062251226623321182
- EhrichEWDaviesGMWatsonDJBologneseJASeidenbergBCBellamyNMinimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritisJ Rheumatol200027112635264111093446
- WendlerDKingtonRMadansJAre racial and ethnic minorities less willing to participate in health research? PLoS Med200632e1916318411
- ShaversVLLynchCFBurmeisterLFFactors that influence African-Americans’ willingness to participate in medical research studiesCancer200191Suppl 123323611148585
- RochonPAMashariACohenAThe inclusion of minority groups in clinical trials: problems of under representation and under reporting of dataAccount Res2004113–421522315812967
- Hussain-GamblesMAtkinKLeeseBWhy ethnic minority groups are under-represented in clinical trials: a review of the literatureHealth Soc Care Community200412538238815373816
- BartlettCDoyalLEbrahimSThe causes and effects of socio-demographic exclusions from clinical trialsHealth Technol Assess2005938 iii–iv, ix–x1152
- CronsteinBNPharmacogenetics in the rheumatic diseasesBull NYU Hosp Jt Dis2006641–2161917121484
- AsanumaYXieHGSteinCMPharmacogenetics and rheumatology: molecular mechanisms contributing to variability in drug responseArthritis Rheum20055251349135915880820
- BensenWGFiechtnerJJMcMillenJITreatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trialMayo Clin Proc199974111095110510560596
- KivitzAJMoskowitzRWWoodsEComparative efficacy and safety of celecoxib and naproxen in the treatment of osteoarthritis of the hipJ Int Med Res200129646747911803730
- McKennaFBorensteinDWendtHWallemarkCLefkowithJBGeisGSCelecoxib versus diclofenac in the management of osteoarthritis of the kneeScand J Rheumatol2001301111811252686
- Cooper-DeHoffRMZhouQGaxiolaEInfluence of Hispanic ethnicity on blood pressure control and cardiovascular outcomes in women with CAD and hypertension: findings from INVESTJ Womens Health (Larchmt)200716563264017627399
- Cooper-DeHoffRMArandaJMJrGaxiolaEBlood pressure control and cardiovascular outcomes in high-risk Hispanic patients – findings from the International Verapamil SR/Trandolapril Study (INVEST)Am Heart J200615151072107916644338
- LieuTALozanoPFinkelsteinJARacial/ethnic variation in asthma status and management practices among children in managed medicaidPediatrics2002109585786511986447
- HerholzHGoffDCRamseyDJWomen and Mexican Americans receive fewer cardiovascular drugs following myocardial infarction than men and non-Hispanic whites: the Corpus Christi Heart Project, 1988–1990J Clin Epidemiol19964932792878676174
- MooreRDStantonDGopalanRChaissonRERacial differences in the use of drug therapy for HIV disease in an urban communityN Engl J Med1994330117637688107743
- HarrisKMEdlundMJLarsonSRacial and ethnic differences in the mental health problems and use of mental health careMed Care200543877578416034291
- PletcherMJKerteszSGKohnMAGonzalesRTrends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departmentsJAMA20082991707818167408
- HahnBAChildren’s health: racial and ethnic differences in the use of prescription medicationsPediatrics19959557277327724312
- XuKTRojas-FernandezCHAncillary community pharmacy services provided to older people in a largely rural and ethnically diverse region: a survey of consumers in West TexasJ Rural Health2003191798612585778
- ReyesCVan de PutteLFalcónAPLevyRAGenes, culture, and medicines: bridging gaps in treatment for Hispanic AmericansNatioanal Alliance for Hispanic Health and the National Pharmaceutical Council Available from: http://www.hispanichealth.org/assets/resource_library/hispanic_report04.pdfAccessed October 28, 2013
- BurroughsVJMaxeyRWLevyRARacial and ethnic differences in response to medicines: towards individualized pharmaceutical treatmentJ Natl Med Assoc200294Suppl 1012612401060
- HuangSMTempleRIs this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practiceClin Pharmacol Ther200884328729418714314
- GrissingerMCultural diversity and medication safetyP&T Community Available from: http://www.ptcommunity.com/ptJournal/fulltext/32/9/PTJ3209471.pdfAccessed April 22, 2014
