Abstract
Objective
Anticonvulsants are increasingly being used in the symptomatic management of several neuropathic pain disorders. The present observational study was designed to evaluate the efficacy, tolerability, and quality of life (QoL) of carbamazepine use for 12 weeks in patients with painful diabetic neuropathy, in Pakistan.
Methods
This was a 12-week, multicenter, open-label, uncontrolled trial in adult type 2 diabetic patients (aged 18–65 years) suffering from clinically confirmed neuropathic pain (Douleur Neuropathique en 4 [DN4] score ≥4). Change in neuropathic pain at week 12 compared with baseline was assessed using the Brief Pain Inventory Scale–Short Form (pain severity score and pain interference score). QoL was determined by the American Chronic Pain Association QoL scale. Safety was assessed based on patient reported adverse events (AEs) and serious AEs.
Results
Of the total 500 screened patients, 452 enrolled and completed the study. The mean (± standard deviation [SD]) pain interference score decreased from 4.5±2.0 at baseline to 3.1±1.9 at week 12 (P<0.001). The mean (± SD) pain severity score decreased from 5.8±2.0 at baseline to 3.6±2.2 at week 12 (P<0.001). There was a decrease of ≥30% in the pain severity score between visits. The mean (± SD) QoL scale score improved from 5.9±1.6 at baseline to 8.0±1.7 at week 12. A total of ten (2.2%) patients reported AEs during the study period. No patient discontinued the study due to AEs.
Conclusion
In this real-life experience study, carbamazepine, when prescribed for 12 weeks to adult diabetic patients suffering from neuropathic pain, showed pain-relief effect, with reduced mean pain severity and mean pain interference scores and with improved QoL and good tolerability profile.
Introduction
Neuropathic pain is a chronic condition caused by damage to or pathological change in the peripheral or central nervous system. The prevalence of chronic pain of neuropathic origin is common in the general population (6%–8%), as reported in epidemiological surveys.Citation1 The International Association for the Study of Pain defines neuropathic pain as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system”.Citation2
Painful diabetic neuropathy (PDN) is the most common microvascular complication of diabetes mellitus with peripheral nerve dysfunction. Diabetes affects 8.3% of the US population,Citation3 and 26% to 47% of adult patients with diabetes mellitus have been reported to experience neuropathic pain.Citation4 Hyperglycemia can be highly correlated to diabetic neuropathy, where the magnitude and duration are proportional to the gradual development and progression of neuropathy.Citation5–Citation7
Diabetic neuropathy involves small and large sensory fibers and is associated with the symptoms of neuropathic pain that begins in the lower limb, first affecting the toes and then gradually progressing upward. Patients experience numbness, a tingling or burning sensation in the extremities, and further lose the ability to sense pain and temperature. PDN has a major impact on quality of life (QoL) affecting mood, sleep, and daily living activities.Citation7,Citation8
In a small population-based study from Wales (UK) of 269 patients who were recruited for assessment, 26.4% had PDN and a significantly lower QoL (difference in mean scores of 1.7) compared with those with nonneuropathic pain.Citation9 In another study, conducted in Turkey, in diabetic patients, 62% had diabetic neuropathy based on abnormal nerve conduction and clinical examination, and 16% had neuropathic pain according to the Leeds Assessment of Neuropathic Symptoms and Signs score.Citation10 A population-based study in the UK that looked at painful neuropathic characteristics revealed that diabetic nonneuropathic patients of South Asian origin had a 50% increased risk of developing painful neuropathic symptoms compared with European and African Caribbeans.Citation11 Although there is a lack of data from Pakistan, the burden of neuropathic pain in Pakistan is likely to be high, considering the large population and high prevalence of diabetes mellitus and stroke.Citation12
Anticonvulsants and antidepressants are among the most commonly used therapeutic options for diabetic neuropathy. A systematic review by Wong et alCitation7 reported that oral tricyclic antidepressants and traditional anticonvulsants were better than the newer generation anticonvulsants for short-term pain relief. Another work reported that carbamazepine (CBZ), a traditional anticonvulsant, provided pain relief and improved symptoms of patients with PDN.Citation13 However, the studies reported were conducted in a small patient population or for a short duration.Citation14–Citation16 Thus, the present study was designed to evaluate the efficacy, tolerability, and QoL of CBZ use for a long duration, 12 weeks, in patients with PDN.
Study objectives
The primary objective of the study was to evaluate the change in neuropathic pain at week 12 compared with baseline, using the Brief Pain Inventory–Short Form (BPI-SF) scale.Citation17 The secondary objectives included assessment of the tolerability of CBZ and the QoL of diabetic neuropathic patients after treatment with CBZ. Tolerability was assessed by the incidences of adverse events (AEs) and serious adverse events (SAEs), and the QoL was assessed using the American Chronic Pain Association QoL scale.Citation18
Materials and methods
Study design and intervention
This trial was a 1-year observational, noninterventional, prospective clinical trial with open-label, uncontrolled study design, conducted at multiple sites (Karachi, Lahore, Islamabad, Peshawar, and Rawalpindi) in Pakistan. Type 2 diabetic patients being treated with an oral anti diabetic medication who were aged between 18 and 65 years and who had neuropathic pain (Douleur Neuropathique en 4 [DN4] score ≥4)Citation19 and creatinine clearance >60 mL/minute were enrolled in the study (). The first patient was enrolled on October 7, 2011, and the last patient completed the study on September 29, 2012. The protocol was approved by the ethics committee of Hamdard College of Medicine and Dentistry (HCMD). This study was performed in accordance with the principles of the Declaration of Helsinki and local applicable laws and regulations.
Table 1 Baseline characteristics of patients
CBZ tablets were administered twice daily orally at the recommended dose of 400–800 mg daily. The daily dose was titrated gradually from 100 mg (day 1) to 400 mg by the end of the first week (days 6 and 7). The dose was later increased up to 600–800 mg daily, according to the clinical response and the tolerability of the drug. Each patient was followed up for a period of 12 weeks.
Outcome measures
Change in neuropathic pain at week 12 compared with baseline was assessed by the pain severity score and pain interference scores of the BPI-SF, a self-rated questionnaire. The pain severity score was calculated by taking the mean of scores for pain at its worst, pain at its least, pain on average, and pain experienced at a particular time point. The pain interference score was calculated by taking the mean of scores for the interference of pain with general activity, mood, walking, normal work, relations, sleep, and enjoyment of life. The QoL was evaluated, at baseline and week 12, using the American Chronic Pain Association QoL scale.Citation20
Statistical analysis
Data collected were analyzed using SPSS Statistics for Windows, Version 17 (SPSS Inc., Chicago, IL, USA). It was estimated that considering an error rate of 4.5%, a sample size of 474 would be required to yield 95% confidence. Allowing for some patient dropout, a total of 500 patients were considered sufficient to produce statistical significance. Descriptive analysis of all baseline variables was performed. The results were presented as mean ± standard deviation (SD) and range, for continuous variables, and as frequency with 95% confidence interval, for categorical variables. Student’s t-test for paired samples was used to compare changes in the means of BPI-SF scores at baseline and week 12.
Results
Out of the 500 patients screened, 452 (90.4%) patients were enrolled and completed the study. The majority of the patients were male (63.7%), with a mean age of 48.1 years (). At baseline, 15.9% of patients experienced retinopathy, and 11.6% were suffering from kidney disease. The mean duration of diabetes was 10.5 years and neuropathic mean (± SD) pain severity score at baseline was 5.8±2.0.
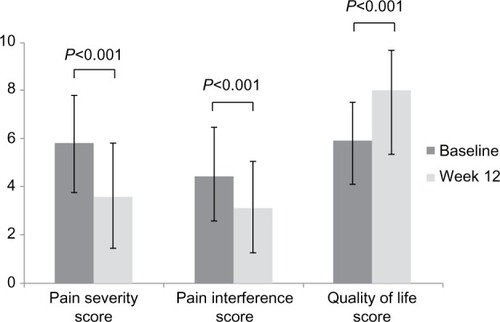
After 12 weeks of CBZ treatment, about 330 (73%) of the patients reported at least 50% relief in pain, whereas three (0.7%) patients did not report any pain relief. For 32 (7.0%) patients, 100% pain relief was observed. Moreover, at week 12, there was a significant (P<0.001) decrease in the mean (± SD) pain severity score, from 5.8±2.0 at baseline to 3.6±2.2 at week 12, and mean (± SD) pain interference score, from 4.5±2.0 at baseline to 3.1±1.9 at week 12 (). A decrease of >30% in the pain severity score and pain interference score was observed between the visits. The mean (± SD) QoL scale score was significantly (P<0.001) improved, from 5.9±1.6 at baseline to 8.0±1.7, at week 12 ().
Figure 1 Change in neuropathic pain and quality of life (QoL) from baseline to week 12.
Ten (2.2%) patients experienced at least one AE during the study period (). The most common AEs were drowsiness, headache, mild dizziness, and nausea. Except for drowsiness, which was considered as drug-related, none of the other AEs were considered to be related to the study. Most of the AEs were mild in severity. No deaths or other SAEs were reported.
Table 2 Adverse events (AEs) during the study period
Discussion
This study assessed the efficacy, tolerability, and impact on QoL of treatment with CBZ, in adult patients with diabetic neuropathy. Results of the present study showed that CBZ provided at least 50% pain relief in more than 70% of the patients by the end of 12 weeks of treatment. A significant reduction in mean pain severity and mean pain interference scores was observed from baseline to week 12. The decrease in pain severity and pain interference scores was $30% between the visits. Previously, traditional anticonvulsants, such as phenytoin (100 mg), have been studied in the treatment of peripheral neuropathies in small populations and demonstrated improved pain relief compared with placebo.Citation21,Citation22 In one double-blind, placebo-controlled trial in 40 patients over a period of 4 weeks, CBZ significantly improved pain from baseline (P<0.01) compared with placebo (both patient and observer assessment).Citation15 In another active controlled trial, CBZ improved paresthesia and pain in diabetic neuropathic patients compared with the combination of nortriptyline and fluphenazine, although the difference between the treatment groups was not statistically significant.Citation21 The findings of our study are consistent with the results of previous studies in which CBZ improved pain relief.Citation15,Citation21
QoL is substantially impaired in diabetic patients with PDN versus diabetic patients without PDN.Citation9 PDN interferes with basic daily life activities and decreases QoL.Citation23 Davies et al revealed that clinical manifestations of diabetic neuropathy have a significant negative impact on QoL.Citation9 Previous studies using CBZ and gabapentin reported that improvement in pain results in substantial improvement in sleep quality and increased mobility, general activity, and mood.Citation15,Citation24 In our study, CBZ significantly improved the QoL score in diabetic patients with PDN. Moreover, the patients were able to work/volunteer for at least 6 hours daily and also had energy to make plans for one evening of social activity during the week and were active over the weekends. The safety profile of CBZ in this study was consistent with earlier studies.Citation14,Citation25 Only ten patients (2.2%) experienced one or more AE during the study period. The most frequent AEs reported during the study period were drowsiness, headache, mild dizziness, and nausea.
A recent systematic review has suggested that the use of traditional anticonvulsants, such as CBZ, should be preferred over the newer generation anticonvulsants.Citation3 Cepeda and Farrar further revealed that traditional anticonvulsants and antidepressants are reported to be cost effective compared with the newer anticonvulsants, such as gabapentin, in the treatment of neuropathic pain of diabetic or postherpetic origin.Citation26
To our knowledge, this is the first real-life experience study of 12 weeks duration to prospectively evaluate the efficacy, tolerability, and QoL of CBZ use in a large population with PDN, in Pakistan. This study demonstrated that CBZ was effective in reducing pain and improving QoL in patients with PDN. A limitation of this study was the open-label and noninterventional study design. A strong placebo effect was previously observed in a randomized, placebo-controlled study of oxcarbazepine in PDN;Citation27 however, the contribution of placebo effect in the efficacy of CBZ could not be denied in the present study. Nevertheless, given the prospective real-life setting and the high number of patients enrolled, this data provides compelling evidence for the use of CBZ in patients with PDN. To establish the efficacy of CBZ in PDN, further large scale, double-blind, randomized, placebo-controlled studies are required.
Conclusion
The results of this real-life experience study in adult patients with PDN, in Pakistan, showed that CBZ provided pain relief, with significant reduction in mean pain scores and with improved QoL and a good tolerability profile.
Author contributions
Tariq Saeed participated in the study design and in data collection and critical review. Muhammad Nasrullah and Nadeem Islam performed data collection and analysis and wrote the description of the results. Riaz Shahid, Adnan Ghafoor, and Mohammad Usman Khattak participated in data collection and wrote the Discussion. Ahson Siddiqi participated in design of the study and critical review. Neeta Maheshwary performed data management and data review. Muhammad Athar Khan performed data analysis. All authors participated in the drafting of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Authors thank Srujana Takkallapally and Venugopal Peta, professional medical writers (Novartis Healthcare Private Limited), for writing and editorial assistance. The study was funded by Novartis Pharma Pakistan, Karachi, Pakistan.
Disclosure
The sponsor of the study, Novartis Pharma Pakistan, was involved in study design, data management, data review, drafting and critical review of the manuscript.
NM and AS are full-time employees of Novartis Pharma Pakistan. The authors report no other conflicts of interest.
References
- FreynhagenRBennettMIDiagnosis and management of neuropathic painBMJ2009339b300219675082
- TreedeRDJensenTSCampbellJNNeuropathic pain: redefinition and a grading system for clinical and research purposesNeurology200870181630163518003941
- National Diabetes Information Clearinghouse (NDIC)National diabetes statistics. Fast facts on diabetesNational Diabetes Information Clearinghouse2011 [updated September 9, 2011; cited Feb 2011]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/statistics/#NervousAccessed October 30, 2013
- BarrettAMLuceroMALeTRobinsonRLDworkinRHChappellASEpidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a reviewPain Med20078Suppl 2S50S6217714116
- FowlerMJMicrovascular and macrovascular complications of diabetesClinical Diabetes20082627782
- HuizingaMMPeltierAPainful diabetic neuropathy: a management-centered reviewClinical Diabetes2007251615
- WongMCChungJWWongTKEffects of treatments for symptoms of painful diabetic neuropathy: systematic reviewBMJ200733576108717562735
- LindsayTJRodgersBCSavathVHettingerKTreating diabetic peripheral neuropathic painAm Fam Physician201082215115820642268
- DaviesMBrophySWilliamsRTaylorAThe prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetesDiabetes Care20062971518152216801572
- ErbasTErtasMYucelAKeskinaslanASenocakMTURNEP Study GroupPrevalence of peripheral neuropathy and painful peripheral neuropathy in Turkish diabetic patientsJ Clin Neurophysiol2011281515521221008
- AbbottCAMalikRAvan RossERKulkarniJBoultonAJPrevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UKDiabetes Care201134102220222421852677
- KhealaniBANaumanAKhosoNAGuidelines for Evaluation and Management of Neuropathic PainKarachiPakistan Society of Neurology2010 Available from: http://www.pakneurology.net/images/Neuropathic_pain_guidelines_detailed_version.pdfAccessed October 30, 2013
- WiffenPJDerrySMooreRAKalsoEACarbamazepine for chronic neuropathic pain and fibromyalgia in adultsCochrane Database Syst Rev20144CD00545124719027
- RullJAQuibreraRGonzález-MillánHLozano CastañedaOSymptomatic treatment of peripheral diabetic neuropathy with carbamazepine (Tegretol): double blind crossover trialDiabetologia1969542152184902717
- WiltonTDTegretol in the treatment of diabetic neuropathyS Afr Med J197448208698724597907
- Gómez-PérezFJChozaRRíosJMNortriptyline-fluphenazine vs carbamazepine in the symptomatic treatment of diabetic neuropathyArch Med Res19962745255298987189
- Iowa Geriatric Education CenterBrief Pain Inventory (Short Form)Iowa CityIowa Geriatric Education Center1991 Available from: http://www.healthcare.uiowa.edu/igec/tools/pain/briefPain.pdfAccessed June 4, 2104
- American Chronic Pain AssociationQuality of Life Scale, A measure of function for people with painRocklinAmerican Chronic Pain Association2003 Available from: http://www.theacpa.org/uploads/documents/Quality_of_Life_Scale.pdfAccessed June 4, 2014
- BouhassiraDAttalNAlchaarHComparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4)Pain2005114293615733628
- American Chronic Pain AssociationQuality of Life Scale: A Measure of Function for People with PainRocklin, CAAmerican Chronic Pain Association2003 Available from: http://www.theacpa.org/uploads/Life_Scale_3.pdfAccessed March 19, 2014
- HussainAMAfshanGUse of anticonvulsant drugs for neuropathic painful conditionsJ Pak Med Assoc2008581269069619157324
- ChaddaVSMathurMSDouble blind study of the effects of diphenylhydantoin sodium on diabetic neuropathyJ Assoc Physicians India1978265403406365857
- BenbowSJWallymahmedMEMacFarlaneIADiabetic peripheral neuropathy and quality of lifeQJM1998911173373710024935
- GalerBSGianasAJensenMPPainful diabetic polyneuropathy: epidemiology, pain description, and quality of lifeDiabetes Res Clin Pract200047212312810670912
- AskmarkHWiholmBEEpidemiology of adverse reactions to carbamazepine as seen in a spontaneous reporting systemActa Neurol Scand19908121311402327233
- CepedaMSFarrarJTEconomic evaluation of oral treatments for neuropathic painJ Pain20067211912816459277
- GrosskopfJMazzolaJWanYHopwoodMA randomized, placebo-controlled study of oxcarbazepine in painful diabetic neuropathyActa Neurol Scand2006114317718016911345
