Abstract
Background
Vitamin D has been shown to have an anticoagulant effect. A decrease in 25-hydroxyvitamin D [25(OH)D] concentration has also been associated with an increased risk of venous thromboembolism. Hence, we sought to determine the relationship between 25(OH) D levels and idiopathic lower-extremity deep vein thrombosis (DVT).
Methods
In a case control study, a total of 82 participants with idiopathic lower-extremity DVT were enrolled along with 85 sex- and age-matched healthy participants as controls. The plasma 25(OH)D levels were measured in all the studied samples.
Results
The participants’ mean age was 47.1±12.3 years. Baseline characteristics were not significantly different between the groups. The concentration of 25(OH)D was significantly lower in the DVT group compared to that of the control group (17.9±10.3 versus 23.1±12.5 ng/mL, P=0.004). The prevalence of participants with deficient 25(OH)D levels was significantly higher in the both DVT and control groups than those with sufficient 25(OH)D levels (68.3% versus 13.4%, and 49.4% versus 28.2%, respectively, P=0.027). In a multivariate analysis, 25(OH)D levels and sex were found to be the only independent predictors of DVT (odds ratio [OR] 1.05, 95% confidence interval [CI] 1.02–1.08, P=0.001 and OR 0.51, 95% CI 0.26–1.00, P=0.049, respectively).
Conclusion
Low levels of 25(OH)D are associated with idiopathic lower-extremity DVT. Further investigation is needed to establish determinants and probable causative role of 25(OH)D.
Introduction
Traditionally, vitamin D has been considered to be the main factor in skeletal health and in the prevention of rickets in children and osteomalacia and osteoporosis in adults. In addition, vitamin D has been recognized as a factor in reducing the risk of chronic diseases, particularly cancer, infectious diseases, and cardiovascular diseases.Citation1 The deficiency of 25-hydroxyvitamin D [25(OH)D], as a marker of vitamin D status,Citation1 contributes to an increase in inflammation, augmentation of insulin resistance and pancreas beta-cell dysfunction, and the dysfunction of renin-angiotensin-aldosterone system, which can lead to the development of atherosclerotic disease and consequent cardiovascularCitation2,Citation3 and cerebrovascular adverse events.Citation4,Citation5 Moreover, it has been shown that 1,25-dihydroxyvitamin D, as the active form of vitamin D, exerts anticoagulant effects in leukemia through upregulating thrombomodulin and downregulating tissue factor in monocytes and myelogenous cells.Citation6 It has also been demonstrated that vitamin D receptor may play an important role in thrombosis.Citation7,Citation8
Venous thromboembolism (VTE) is the most common cardiovascular disease affecting about 2 per 1,000 of the population and can lead to major morbidity and mortality.Citation9 Deep vein thrombosis (DVT) and pulmonary embolism (PE) form part of the spectrum of VTE,Citation10 and because they are associated with conventional cardiovascular risk factors, they may be considered as cardiovascular diseases. Therefore, the same risk factor modification and preventative strategies should be implemented for both conditions.Citation11 In addition, it has been demonstrated that an increase in 25(OH)D levels reduces the risk of VTE.Citation12 The use of 1,25-dihydroxyvitamin D led to a decrease in DVT incidence among patients with prostate cancer.Citation13 Investigating this correlation may be of great benefit in highlighting the potential role of vitamin D deficiency in developing DVT, particularly when excluding other causes of DVT and only considering idiopathic ones. Given these notions, we sought to examine the relationship between 25(OH)D levels and DVT in patients who had been hospitalized with idiopathic lower-extremity DVT in an Iranian population, West-Azerbaijan province, from March 2013 to October 2013.
Methods
Study design
Based on a case-control study, we prospectively evaluated the plasma levels of 25(OH)D of participants who had lower-extremity DVT and those of the age- and sex-matched healthy controls. Enrolled participants were hospitalized patients with suspected DVT and patients who visited our clinic for a routine checkup in Seyyed-al-Shohada Heart Center, Urmia, West-Azerbaijan province, Iran, from March 2013 to October 2013. The study population included 82 participants in the DVT group (with suspected DVT) and 85 healthy participants as control group. The participants in the control group did not have clinical probability of DVT or common risk factors of VTE. Participants gave their written informed consent for blood samples to be obtained for measuring laboratory markers and the study was also approved by our local ethics committee of Urmia University of Medical Sciences, Iran.
Study cohort
All adult patients (>18 years) who had been admitted to hospital due to suspected lower-extremity DVT were assessed for eligibility. They were evaluated for DVT based on the latest guidelines.Citation14 In order to diagnose lower-extremity DVT, all patients were assessed using clinical probability, D-dimer test, and compression ultrasound examination.Citation14 The patients in whom clinical evaluations suggested a low probability of DVT, the negative D-dimer test ruled out DVT, and if the D-dimer test was positive, an ultrasound examination confirmed the presence of DVT. In patients with a high probability of DVT, an ultrasound examination was used to confirm the diagnosis. All patients underwent conventional therapy for DVT consisting of low molecular weight heparin and/or the oral anti-coagulant regimen of warfarin.
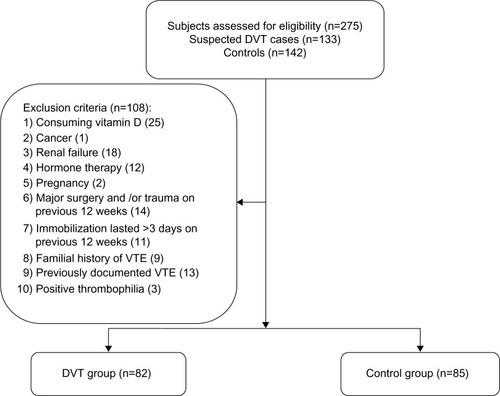
Exclusion criteria were as follows: 1) vitamin D supplementation (either orally or intravenously) in the last 2 years; 2) cancer (being under treatment and/or diagnosed with malignancies); 3) renal failure (chronic kidney disease stage >2); 4) hormone replacement therapy; 5) pregnancy; 6) major surgery or trauma in the last 12 weeks; 7) immobilization lasting >3 days during the last 12 weeks for any reason; 8) a family history of VTE; 9) previously documented VTE, and 10) positive markers of thrombophilia. Accordingly, a total of 82 patients with idiopathic lower-extremity DVT were enrolled in the DVT group, and 85 sex-and age-matched healthy participants served as the control group. The participants in the control group had their medical histories taken and underwent clinical and paramedical examinations. None of them showed signs and/or symptoms of VTE, had a history of VTE, had positive test results for thrombophilia, consumed vitamin D supplements, or met any of the other exclusion criteria mentioned above. From 133 subjects with suspected DVT, and 142 healthy controls who were assessed for eligibility, 82 participants and 85 healthy controls were selected to participate in the study and given consent forms ().
Study variables and laboratory markers
Demographics, including a history of diabetes mellitus, hypertension, dyslipidemia, smoking, and obesity were collected upon admission. A fasting blood glucose level of ≥126 mg/dL, a classic symptom of diabetes along with a plasma glucose level of >200 mg/dL, post-load glucose of >200 mg/dL following a tolerance test and/or consuming antidiabetic agents were defined as diabetes mellitus.Citation15 A blood pressure measurement of ≥140/90 mmHg and/or taking antihypertensive drugs was defined as hypertension.Citation16 Smoking was defined as patients who smoked ten or more cigarettes per day. Obesity was defined as subjects whose body mass index was ≥30 kg/m2.
Thrombophilia was determined using laboratory markers consisting of proteins C and S, factor V Leiden, homocysteine, anti-thrombin III, anti-phospholipid antibody, and anti-cardiolipin antibody. Plasma level of 25(OH)D, the commonly used marker of vitamin D status, was measured by chemiluminescence immunoassay without knowledge of the participant group. The controls’ blood samples were collected in the clinic and those of the DVT group were collected during hospitalization. All samples were analyzed within 1 hour of collection. The 25(OH)D levels were categorized into three groups: 1) sufficient group, 25(OH) D ≥30 ng/mL (n=35); 2) insufficient group, 25(OH)D 21–29 ng/mL (n=34); and 3) deficient group, 25(OH)D ≤20 ng/mL (n=98).Citation1,Citation3
Statistical analysis
Continuous and categorical variables were presented as mean (± standard deviation) and number (percentage), respectively. The Shapiro-Wilk test detected the normal distribution of continuous variables except the 25(OH)D levels. Therefore, categorical variables were analyzed using chi-square test, and continuous ones were analyzed using Student’s t-test, Mann–Whitney U test, or Kruskal–Wallis test, as appropriate. Moreover, a binary logistic regression model was used for detecting the independent predictors of DVT in the present studied population. The dependent variable was the status of having DVT, and covariates were the characteristics mentioned above. A two-tailed P-value <0.05 was considered to be statistically significant. All statistical analyses were performed by SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Univariate analyses
The mean age of participants was 47.1±12.3 years, and there was no significant difference between case and control groups (P=0.491). No significant differences were found between the risk factors in the two groups (). A 25(OH)D deficiency was the most frequent 25(OH)D level in both the DVT and control groups (68.3% versus 49.4%, respectively), while the sufficient 25(OH)D level was the least frequent in the DVT group compared to that in the control group (13.4% versus 28.2%, respectively). Accordingly, the number of participants in the 25(OH)D subgroups were found to be significantly different between the groups (P=0.027) (). As illustrated in , the 25(OH)D concentration was significantly lower in the DVT group compared to that of the control group (17.9±10.3 versus 23.1±12.5 ng/mL, P=0.004).
Figure 2 Comparison of 25-hydroxyvitamin D [25(OH)D] levels between the deep vein thrombosis (DVT) and control groups.
![Figure 2 Comparison of 25-hydroxyvitamin D [25(OH)D] levels between the deep vein thrombosis (DVT) and control groups.](/cms/asset/643636df-a0c9-4931-ab55-cc47be827e71/dijg_a_64812_f0002_b.jpg)
Table 1 Characteristics and vitamin D status of participants with idiopathic DVT compared to healthy controls
We analyzed the different characteristics among the subgroups, including the 25(OH)D sufficient, insufficient, and deficient groups, in patients who had been diagnosed with idiopathic DVT. There were no significant differences between subgroups, except the level of 25(OH)D, P<0.001 (). Moreover, there was a trend toward female sex in DVT patients with 25(OH)D deficiency compared with both insufficient and sufficient groups (27.3%, 33.3%, and 51.8% in sufficient, insufficient, and deficient groups respectively, P=0.196). No significant differences with respect to the level of 25(OH)D existed between subgroups defined by sex, obesity, diabetes mellitus, hypertension, dyslipidemia, and smoking ().
Figure 3 Comparison of 25-hydroxyvitamin D [25(OH)D] levels between the subgroups by characteristics among deep vein thrombosis (DVT) participants.
![Figure 3 Comparison of 25-hydroxyvitamin D [25(OH)D] levels between the subgroups by characteristics among deep vein thrombosis (DVT) participants.](/cms/asset/6169492d-578e-408d-a3d2-9a1b9c6c656e/dijg_a_64812_f0003_b.jpg)
Table 2 Characteristics and vitamin D status of participants in the DVT subgroups based on 25(OH)D status
Multivariate analysis
In the logistic regression analysis for detecting the predictors of DVT in our study, dependent variables were DVT or control group, while age, sex, obesity, diabetes mellitus, hypertension, dyslipidemia, smoking, and 25(OH)D concentration were considered as covariates. As a result of that model, the independent predictors of DVT were 25(OH)D level and female sex (odds ratio [OR] 1.05, 95% confidence interval [CI] 1.02–1.08, P=0.001 and OR 0.51, 95% CI 0.26–1.00, P=0.049, respectively) ().
Table 3 Logistic regression analysis for detecting the independent predictors of DVT
Discussion
Vitamin D has two forms, namely vitamin D2 (ergocalciferol) and D3 (cholecalciferol), which are naturally produced by ultraviolet irradiation of ergosterol and 7-dehydrocholesterol, respectively.Citation1 Vitamin D is produced through diet and ultraviolet irradiation and is converted to 25(OH)D in the liver, which is clinically used for evaluating vitamin D status.Citation1 In the last decades, vitamin D has emerged as a main factor in the regulation of bone metabolism. It has also been found that its deficiency can contribute to rickets in children and osteomalacia and osteoporosis in adults.Citation1,Citation3 In addition to controlling the above mentioned health problems, it has been demonstrated that vitamin D is not only a nutrient needed for bone metabolism, but is also an agent for decreasing the risk of some chronic diseases, including malignancies and cardiovascular disorders.Citation1 Vitamin D deficiency is a worldwide problem affecting many people. Although there is no consensus on defining 25(OH)D deficiency, most experts have agreed that 25(OH)D levels of <20 ng/mL may be considered as deficient.Citation1 Vitamin D deficiency has affected about 60% of the US and more than 60% of European populations.Citation1,Citation3 In addition, in a nationwide study performed among adults in Iran, it has been found that the rate of vitamin D deficiency [25(OH)D <25 ng/mL] is about 50%,Citation17 and also the rate of vitamin D deficiency [25(OH)D <20 ng/mL] was about 85% in a single city study in Iran.Citation18
The relationship between ischemic heart disease, especially myocardial infarction, and vitamin D deficiency has been demonstrated in a cohort study, in which decreased vitamin D was associated with an increase in ischemic heart disease, myocardial infarction, and early death at 29 year follow-up.Citation19 Moreover, similar findings have been reported that decreased 25(OH)D levels increased the incidence of subsequent cardiovascular and cerebrovascular events.Citation2,Citation20 Interestingly, the effects of vitamin D supplements on reducing cardiovascular events are another reason for considering vitamin D as a probable pathophysiological agent for developing cardiovascular diseases.Citation21
About one third of VTE cases are idiopathic.Citation9 Despite previous beliefs, it has been proposed that VTE and cardiovascular diseases are not definitely two separate problems, and are in fact related.Citation11 It has been suggested that the risk factors of atherosclerosis and thromboembolism are linked to each other, however there are inconsistent findings among studies.Citation22 Based on one of them, some conventional risk factors of atherosclerotic disease, including obesity, smoking, female sex, and increased diastolic blood pressure were associated with VTE, while diabetes mellitus, hypertension, and dyslipidemia were not.Citation22 In addition, in another investigation, diabetes mellitus and obesity were associated with high risk of thromboembolism.Citation23 Regarding the notion that atherosclerotic plaques were more prevalent in patients with idiopathic DVT than in those with secondary DVT, it may be suggested that atherosclerosis induces the development of VTE.Citation9 Furthermore, the higher risk of VTE in individuals who smoke, are physically inactive or less active, or drink excessive amounts of alcohol compared with non-smoking, physically active, moderate drinkers,Citation24 can be considered as evidence of the impact of lifestyle on the risk of VTE. In our study we could not show any significant differences between the control and DVT groups regarding cardiovascular risk factors, but 25(OH)D levels were significantly higher in DVT cases compared with controls. Although growing evidence exists about the relationship between VTE and cardiovascular diseases, it seems that further, large scale cohort studies are needed to clarifying this issue.
Vitamin D deficiency increases cardiovascular risk through mechanisms involved in increasing inflammation, raising insulin resistance and pancreas beta-cell dysfunction resulting in diabetes mellitus, and through increasing the activation of renin-angiotensin-aldosterone system contributing to hypertension and cardiac hypertrophy.Citation3,Citation25 In addition, atherosclerosis is associated with developing thrombosis as a result of platelet activation and consequent thrombotic adverse events,Citation9 and it has been demonstrated that vitamin D’s anticoagulant properties play a role in atherothrombosis.Citation6–Citation8,Citation26
In a large study comprised of 18,791 participants with a 30 year follow-up, decreased 25(OH)D levels during the seasonal change from summer to winter led to an increase in the risk of VTE.Citation27 In contrast, another study involving 6,021 participants during a 10 year follow-up demonstrated that there was no correlation between VTE and levels of 25(OH) D (≥28 ng/mL versus <18 ng/mL) and they also concluded that vitamin D deficiency did not have a pathological role in developing VTE.Citation28
In our study, we showed that the level of 25(OH)D was significantly higher in patients who had idiopathic DVT compared to that of the controls (P= 0.004), we also showed that 25(OH)D and sex were the only independent predictors of idiopathic DVT. Although we did not investigate the development of DVT, our cohort study consisted of patients with idiopathic DVT, in which the effects of other factors on DVT incidence were excluded. Therefore, higher 25(OH)D levels might demonstrate the probable pathological role of vitamin D in DVT pathogenesis. However, we cannot conclude that this relationship is a causative one; randomized clinical trials will need to be conducted to prove this.
Study limitations
Based on its nature, this study had some shortcomings. Firstly, there is increasing evidence in terms of seasonal effects on 25(OH)D levels and it has been demonstrated that vitamin D concentration is at its lowest in the winter months compared to summer.Citation29 On the other hand, recent study has found that an increase in sunbathing and consequent elevated vitamin D levels contributes to a decrease in the risk of VTE.Citation12 Moreover, a seasonal variation in the incidence of thrombotic events has been reported, in which the maximum risk of VTE had been observed during winter months.Citation12 However, in our study we could not investigate the seasonal impact on DVT incidence because our study was conducted during warm months. Secondly, prothrombin gene mutation, one of the causes of thrombophilia,Citation30 was not assessed in this study, and we could not exclude these patients; hence our cohort study might include some patients with thrombophilia, which cannot be considered as idiopathic DVT. Thirdly, the small sample size of our study was another restriction – large scale studies are needed to clarify the relationship between vitamin D and DVT.
In conclusion, it has been found that the level of 25(OH)D was significantly higher in patients who had been diagnosed with idiopathic lower-extremity DVT compared to those of healthy controls. Moreover, excluding other causes of DVT indicates that we need further studies to establish determinants and probable causative role of 25(OH)D in DVT.
Author contributions
KK, YR prepared the manuscript. KK, MHS-M, MA, YR, RE and AR participated in conceptualization and design of the study. KK, MHS-M, MA and AR collected data. YR contributed to the analyzing and interpretation of data. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest in terms of the research, authorship, and publication of this study.
References
- HolickMFVitamin D deficiencyN Engl J Med2007357326628117634462
- WangTJPencinaMJBoothSLVitamin D deficiency and risk of cardiovascular diseaseCirculation2008117450351118180395
- LeeJHO’KeefeJHBellDHensrudDDHolickMFVitamin D deficiency an important, common, and easily treatable cardiovascular risk factor?J Am Coll Cardiol200852241949195619055985
- PilzSDobnigHFischerJELow vitamin D levels predict stroke in patients referred to coronary angiographyStroke20083992611261318635847
- KojimaGBellCAbbottRDLow dietary vitamin D predicts 34-year incident stroke: the Honolulu Heart ProgramStroke20124382163216722627988
- KoyamaTShibakuraMOhsawaMKamiyamaRHirosawaSAnticoagulant effects of 1alpha, 25-dihydroxyvitamin D3 on human myelogenous leukemia cells and monocytesBlood19989211601679639512
- Wu-WongJRNakaneMMaJVitamin D analogs modulate the expression of plasminogen activator inhibitor-1, thrombospondin-1 and thrombomodulin in human aortic smooth muscle cellsJ Vasc Res2007441111817159355
- AiharaKAzumaHAkaikeMDisruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in miceJ Biol Chem200427934357983580215205460
- PrandoniPBiloraFMarchioriAAn association between atherosclerosis and venous thrombosisN Engl J Med2003348151435144112686699
- MokhtariMSalamehPKouchekMThe AVAIL ME Extension: a multinational Middle Eastern survey of venous thromboembolism risk and prophylaxisJ Thromb Haemost2011971340134921605327
- AgenoWBecattiniCBrightonTSelbyRKamphuisenPWCardiovascular risk factors and venous thromboembolism: a meta-analysisCirculation200811719310218086925
- LindqvistPGEpsteinEOlssonHDoes an active sun exposure habit lower the risk of venous thrombotic events? A D-lightful hypothesisJ Thromb Haemost20097460561019335448
- BeerTMVennerPMRyanCWHigh dose calcitriol may reduce thrombosis in cancer patientsBr J Haematol2006135339239416984385
- WellsPAndersonDThe diagnosis and treatment of venous thromboembolismHematology Am Soc Hematol Educ Program2013201345746324319219
- American Diabetes AssociationDiagnosis and Classification of Diabetes MellitusDiabetes Care200629suppl 1s43s4816373932
- ChobanianAVBakrisGLBlackHRThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA2003289192560257212748199
- HeshmatRMohammadKMajdzadehSVitamin D Deficiency in Iran: A Multi-center Study among Different Urban AreasIranian J Publ Health200837sup7278
- KaykhaeiMAHashemiMNarouieBHigh prevalence of vitamin D deficiency in Zahedan, southeast IranAnn Nutr Metab2011581374121304235
- Brondum-JacobsenPBennMJensenGBNordestgaardBG25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studiesArterioscler Thromb Vasc Biol201232112794280222936341
- KilkkinenAKnektPAroAVitamin D status and the risk of cardiovascular disease deathAm J Epidemiol200917081032103919762371
- WangLMansonJESongYSessoHDSystematic review: Vitamin D and calcium supplementation in prevention of cardiovascular eventsAnn Intern Med2010152531532320194238
- HolstAGJensenGPrescottERisk factors for venous thromboembolism: results from the Copenhagen City Heart StudyCirculation2010121171896190320404252
- TsaiAWCushmanMRosamondWDCardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiologyArch Intern Med2002162101182118912020191
- LindqvistPGEpsteinEOlssonHThe relationship between lifestyle factors and venous thromboembolism among women: a report from the MISS studyBr J Haematol2009144223424019036105
- TargherGPichiriILippiGVitamin D, thrombosis, and hemostasis: more than skin deepSemin Thromb Hemost201238111412422314609
- OhsawaMKoyamaTYamamotoK1α, 25-Dihydroxyvitamin D3 and Its Potent Synthetic Analogs Downregulate Tissue Factor and Upregulate Thrombomodulin Expression in Monocytic Cells, Counteracting the Effects of Tumor Necrosis Factor and Oxidized LDLCirculation2000102232867287211104746
- Brondum-JacobsenPBennMTybjaerg-HansenANordestgaardBG25-Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18, 791 participantsJ Thromb Haemost201311342343123279309
- BrodinELerstadGGrimnesGSerum levels of vitamin D are not associated with future risk of venous thromboembolism. The Tromso StudyThromb Haemost2013109588589023446951
- HovsepianSAminiMAminorroayaAAminiPIrajBPrevalence of vitamin D deficiency among adult population of Isfahan City, IranJ Health Popul Nutr201129214915521608424
- De StefanoVRossiETesting for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working GroupsThromb Haemost2013110469770523846575