Abstract
Type 2 diabetes mellitus (T2DM) is characterized by hyperglycemia, insulin resistance, and/or progressive loss of β-cell function. T2DM patients are at increased risk of micro- and macrovascular disease, and are often considered as representing an atherosclerotic coronary heart disease (CHD) risk equivalent. Interventions directed at glucose and lipid level control in T2DM patients may reduce micro- and macrovascular disease. The optimal T2DM agent is one that lowers glucose levels with limited risk for hypoglycemia, and with no clinical trial evidence of worsening CHD risk. Lipid-altering drugs should preferably reduce low-density lipoprotein cholesterol and apolipoprotein B (apo B) and have evidence that the mechanism of action reduces CHD risk. Statins reduce low-density lipoprotein cholesterol and apo B and have evidence of improving CHD outcomes, and are thus first-line therapy for the treatment of hypercholesterolemia. In patients who do not achieve optimal lipid levels with statin therapy, or who are intolerant to statin therapy, add-on therapy or alternative therapies may be indicated. Additional available agents to treat hypercholesterolemic patients with T2DM include bile acid sequestrants, fibrates, niacin, and ezetimibe. This review discusses the use of these alternative agents to treat hypercholesterolemia in patients with T2DM, either as monotherapy or in combination with statin therapy.
Keywords:
Introduction
Type 2 diabetes mellitus (T2DM) is a disease characterized by hyperglycemia, insulin resistance, and/or progressive loss of β-cell function. T2DM is associated with high cardiovascular disease (CVD) risk,Citation1 and hyperglycemia induces vascular changes that contribute to atherosclerosis and vasculopathy ().Citation2–Citation4
Table 1 Effects of hyperglycemia on atherosclerotic processes
Intensive glucose control in patients with T2DM may reduce CVD, depending upon how early and the speed at which such intervention is implemented, the types of agents used for glucose control, and the medical status of the patient.Citation4,Citation5 Overall, the best approach for reducing CVD risk is a comprehensive one that not only includes glucose and lipid control, but also the introduction of therapeutic lifestyle changes such as smoking cessation, optimal nutrition, increased physical activity, appropriate body weight management, blood pressure management, and possible aspirin therapy for patients with high CVD risk.Citation6
In some patients, T2DM may be considered a coronary heart disease (CHD) risk equivalent,Citation7 which may necessitate more stringent lipid control for primary prevention than in individuals without diabetes mellitus. While the recent American College of Cardiology/American Heart Association guidelines emphasize reducing risk in patient groups at high risk for CVD rather than focusing on specific low-density lipoprotein (LDL) cholesterol (LDL-C) lipid treatment goals, other guidelines recommend a LDL-C treatment goal of <100 mg/dL for high risk patients with diabetes mellitus, and <70 mg/dL for those at very high CVD risk (eg, diabetes mellitus patients with existing CVD or multiple other risk factors).Citation6,Citation8,Citation9 Unfortunately, a substantial proportion of T2DM patients do not achieve those goals. In a study of 17,306 patients with diabetes that aimed to determine levels of therapeutic goal achievement, only 42% of patients achieved an LDL-C goal of <100 mg/dL over the 7-year period from 1999 to 2006.Citation10
Hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) are first-line lipid-lowering therapy for patients with T2DM. In patients with T2DM, statins generally reduce LDL-C levels by about 24%–52%, depending upon the statin and dose (eg, atorvastatin, fluvastatin, lovastatin, and rosuvastatin).Citation11–Citation13 The reduction in LDL-C levels achieved by statins is associated with reductions in CVD events. A meta-analysis by the Cholesterol Treatment Trialists’ Collaborators showed that, among 18,686 patients with diabetes mellitus (92% type 2/8% type 1) receiving statin therapy, for each mmol/L (39 mg/dL) reduction in LDL-C, there was a 21% proportional reduction in major vascular events (P<0.0001).Citation14
Although the efficacy of statins is well established, a considerable proportion of patients do not achieve lipid goals with statin monotherapy and may require add-on or alternative therapies to statins to better achieve LDL-C treatment goals. In the National Cholesterol Education Program Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II study, the percentage of patients who achieved LDL-C treatment goals decreased as the number of risk factors increased; 89% and 76% of patients with zero to one and two or more risk factors, respectively, achieved LDL-C goal, while only 57% of patients with CHD or CHD risk equivalents achieved goal.Citation15 Moreover, in the subset of patients with CHD or CHD risk equivalents, 55% of patients with diabetes mellitus but without CHD achieved LDL-C goal compared with 62% of patients with CHD and only 40% of patients with other CHD risk equivalents (without CHD).Citation15 Of the patients in the CHD or CHD risk equivalents subgroup who had triglycerides ≥200 mg/dL (≥2.25 mmol/L), 50% of patients with diabetes mellitus (without CHD) and 57% of patients with CHD achieved LDL-C goal, whereas 44% of patients with other CHD risk equivalents (without CHD) achieved LDL-C goal.Citation15
One strategy for improving LDL-C goal attainment is to increase statin therapy, often to the maximal approved dose; however, doubling the statin dose does not double the LDL-C lowering efficacy. In a pooled analysis of 37 studies of 32,258 patients receiving rosuvastatin, atorvastatin, or simvastatin, doubling the statin dose reduced LDL-C levels by only an additional 5%–7%.Citation16 High-dose statin therapy is generally well tolerated in many patients, at least as determined by clinical trial data.Citation11 However, increasing statin dose to the highest doses may not be the best strategy for all patients. A meta-analysis of studies investigating intensive-and moderate-dose statin regimens showed that patients receiving higher-dose statin were more likely to experience an adverse event, discontinue therapy because of an adverse event, and demonstrate liver abnormalities and increased creatine kinase levels compared with patients receiving moderate-dose statins.Citation17 Also, while the clinical significance is unclear, statins (particularly at intensive doses) may be associated with increased risk of developing new-onset diabetes and/or unfavorable glycemic effects.Citation18–Citation21
In patients who are unable to achieve desired LDL-C treatment goals with statin therapy, a number of other agents, including bile acid (BA) sequestrants (BASs), fibrates, niacin, and cholesterol absorption inhibitors (eg, ezetimibe), may be combined with statins to facilitate goal achievement or be used in place of statins for patients who cannot tolerate statins.Citation6,Citation22 This review describes these add-on or alternative therapy options for the lowering of LDL-C levels in patients with T2DM. Because of their dual effects on lowering glucose and LDL-C, the role of BASs will be discussed in greater detail.
BASs
Before statins were approved as agents to reduce elevated cholesterol levels, BASs were recommended as first-line therapy for reducing LDL-C levels.Citation23 In the Lipid Research Clinics Coronary Primary Prevention Trial, the BAS cholestyramine was shown to improve cardiovascular (CV) outcomes in a population of asymptomatic middle-aged men with primary hypercholesterolemia (diabetes mellitus was an exclusion criteria). This was the first study to demonstrate that a reduction in LDL-C levels (mean reduction of 12.6% compared with placebo) significantly reduced CV risk (primary endpoint of CHD death and/or nonfatal myocardial infarction reduced by 19%, P<0.05), which was associated with a 24% reduction in CHD death and a 19% reduction in nonfatal myocardial infarction.Citation24 BASs are nonsystemic agents; however, BASs may bind to certain drugs in the gastrointestinal tract and it is therefore recommended that agents such as warfarin, digoxin, thyroid hormones, and fat-soluble vitamins be taken either 1 hour before or 4–6 hours after BAS administration. The primary adverse events reported for the older BASs cholestyramine and colestipol are constipation and flatulence, and these agents are associated with high discontinuation rates within clinical trials of 40%–60%.Citation25,Citation26 In comparison, the primary adverse events reported for the specifically engineered BAS colesevelam are constipation and dyspepsia, with an observed compliance rate within clinical trials of 88%–93%.Citation27,Citation28
The synthesis of BAs occurs exclusively in the liver, and the BA pool is tightly regulated within the liver and intestine. BAs are known ligands for the nuclear receptor farnesoid X receptor (FXR) and self-regulate their own synthesis. Published literature suggests the following proposed model for the regulation of BAs. In the intestine, BAs secreted in response to an ingested meal activate FXR, which induces expression of fibroblast growth factor (FGF)-19.Citation29,Citation30 FGF-19 binds to surface hepatocyte FGF receptor 4 (FGFR4), which subsequently results in a c-Jun N-terminal kinase-mediated repression of cytochrome P450 enzyme cholesterol 7 α-hydroxylase (CYP7A1), thus inhibiting the rate-limiting step in the conversion of cholesterol to BAs, and subsequently resulting in the downregulation of HMG-CoA reductase (the rate-limiting step of cholesterol synthesis) (). Metabolic pathways in the liver also play a major role in the regulation of BAs. More specifically, increasing BA levels in the liver upregulate the small heterodimer partner (SHP) via increased FXR activation, which results in both inhibition of the liver X receptor (LXR) and liver receptor homolog-1 (LRH-1), and ultimately further repression of CYP7A1 to reduce BA synthesis.Citation30,Citation31
Figure 1 Proposed mechanism of action for the lipid-lowering and glycemic effects of a BAS.
Abbreviations: A1C, hemoglobin A1C; BA, bile acid; BAS, bile acid sequestrant; CYP7A1, cholesterol-7-alpha-hydroxylase; FGF15/19, fibroblast growth factor 15/19; FGFR4, fibroblast growth factor receptor 4; FPG, fasting plasma glucose; FXR, farnesoid X receptor; GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide-1; hLDLR, hepatic low-density lipoprotein receptors; HNF-4α, hepatocyte nuclear factor 4 alpha; JNK, c-Jun N-terminal kinase; LDL-C, low-density lipoprotein cholesterol; LRH-1, liver receptor homolog-1; LXR, liver X receptor; MOA, mechanism of action; PEPCK, phosphoenolpyruvate carboxykinase; SHP, small heterodimer partner; T2DM, type 2 diabetes mellitus.
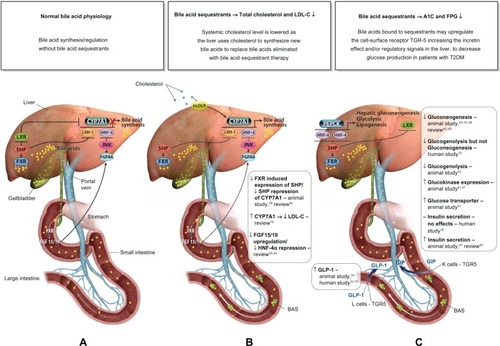
BASs bind BAs in the intestine, thus increasing BA excretion in the feces. Consequently, fewer BAs are returned to the liver. Binding BAs also “deactivate” FXR activity. Thus, the alteration of the BA pool reduces nuclear receptor FXR-mediated repression of key regulatory elements (eg, FGF15/19, FGFR4, SHP) responsible for BA synthesis, in particular, CYP7A, which ultimately results in increased BA synthesis.Citation29,Citation30 The upregulation of CYP7A in the BA synthesis pathway increases HMG-CoA transcriptional activity. Subsequent increased conversion of cholesterol to synthesize BAs results in a compensatory upregulation in hepatic LDL receptors (hLDLR), increased hepatic LDL-C uptake, and decreased circulating LDL-C.Citation32,Citation33 The proposed mechanism by which BA sequestration leads to LDL-C lowering is shown in .
In studies in patients with T2DM, the BASs colesevelam and cholestyramine reduced LDL-C levels,Citation34–Citation37 which may be accompanied by a modest increase in high-density lipoprotein (HDL) cholesterol (HDL-C) and triglyceride levels. In a double-blind, randomized, crossover study of 21 patients with well-controlled T2DM but fasting LDL-C levels of >130 mg/dL receiving cholestyramine or placebo for 6 weeks, cholestyramine produced a 28% reduction in LDL-C (P<0.001 versus placebo), a 13.5% increase in triglycerides (P=0.02 versus placebo), and a non-statistically significant increase in HDL-C (1 mg/dL; P>0.2 versus placebo).Citation34 In three pivotal randomized, double-blind, placebo-controlled studies in patients with T2DM (n>280), colesevelam reduced LDL-C by 13%–17% compared with placebo (P<0.001 for all); the placebo-adjusted mean change from baseline in triglyceride levels in colesevelam recipients ranged from +5% to +22% () and HDL-C changed by −0.9% to +0.9% (P=not significant for all).Citation35–Citation37 Among patients from these studies who were taking concomitant statins, the addition of colesevelam reduced LDL-C by 16% compared with an increase of 1% with placebo, and had no significant effect on HDL-C levels (+0.02% versus placebo; P=not significant).Citation38
Table 2 Least squares mean percent treatment difference in glycemic and lipid parameters in patients with type 2 diabetes mellitus receiving COL or PL
BASs may also lower glucose levels in patients with T2DM.Citation34–Citation37,Citation39 The BAS colesevelam was approved in 2008 by the US Food and Drug Administration (FDA) to improve glycemic control in adults with T2DM. The precise glucose-lowering mechanisms of BASs are unknown. Possible mechanisms involved with the glucose-lowering effects of BASs are summarized in . In brief, decreased activity of both FXR and SHP resulting from the reduction of the enterohepatic BA pool after BAS administration promotes phosphoenolpyruvate carboxykinase (PEPCK) production, which increases hepatic gluconeogenesis and glycolysis.Citation30,Citation31 However, increased LXR activity suppresses expression of PEPCK and results in a reduction in gluconeogenesis,Citation40–Citation46 as well as increased insulin secretionCitation45,Citation47 and increased expression of glucokinaseCitation32,Citation41,Citation47 and glucose transporter.Citation41 Furthermore, BA bound to a BAS may activate the G-protein-coupled receptor TGR5 in the intestine leading to the increased secretion of glucagon-like peptide-1 ([GLP-1] [L cells]) resulting in reduced hepatic glucose production via the suppression of hepatic glycogenolysis.Citation33,Citation48
In three randomized, double-blind, placebo-controlled studies, colesevelam significantly lowered hemoglobin A1C by 0.5% or more compared with placebo (P<0.001 for all) in adults with T2DM when added to stable metformin-, insulin-, or sulfonylurea-based therapy ().Citation35–Citation37 Subgroup analysis of the metformin-based therapy study by pre-study use or nonuse of statins (which continued during the study) indicated that, regardless of any potential effect of statins on glycemia, concomitant statins did not attenuate the effects of colesevelam.Citation49 In both statin users and nonusers, colesevelam produced significantly greater reductions than placebo in hemoglobin A1C (mean treatment differences −0.63% [P=0.0003] and −0.49% [P=0.001], respectively) and LDL-C (−16.4% [P=0.0024] and −15.8% [P<0.0001], respectively).
Fibrates
Fibrates are synthetic ligands for peroxisome proliferator-activated α-receptors. It is through binding to these nuclear receptors that they act to alter lipid levels.Citation50 Fibrates primarily reduce triglycerides (which are often elevated in patients with T2DM),Citation51 have a modest effect on HDL-C levels, and, depending upon the baseline triglyceride levels, may decrease LDL-C levels (in patients without baseline elevation in triglyceride levels) or may substantially increase LDL-C levels (in patients with very high baseline triglyceride levels). In the Diabetes Atherosclerosis Intervention Study,Citation52 the improvements in lipid levels with fenofibrate were associated with reductions in the angiographic progression of coronary artery disease. However, while fenofibrate significantly improved LDL-C, HDL-C, triglyceride, and total cholesterol levels compared with placebo in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study (relative treatment difference: −5.8%, 1.2%, −21.9%, and −6.9%, respectively; all P<0.05), it did not significantly reduce the risk of CHD death or nonfatal myocardial infarction in patients with T2DM, although there was a significant reduction in the rate of total CVD events, a composite of CVD death, myocardial infarction, stroke, and coronary and carotid revascularization (hazard ratio, 0.89; 95% confidence interval: 0.80–0.99; P=0.035).Citation53 It is noteworthy that, in the FIELD study, fenofibrate did significantly reduce the need for retinal laser treatment in patients with retinopathy (5.2% versus 3.6%; P=0.0003), and resulted in significantly less albuminuria progression (P=0.002) in patients with T2DM.Citation53 Thus, fenofibrate may have a beneficial effect in reducing microvascular complications in this population.
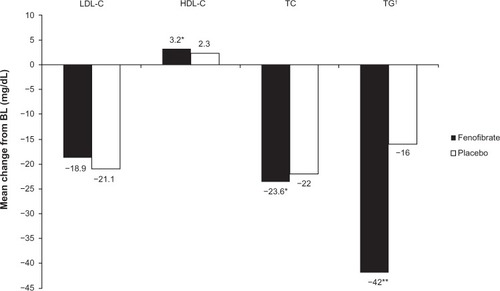
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) lipid trial investigated the efficacy of fenofibrate versus placebo in 5,518 patients with T2DM at high risk of CVD who were receiving simvastatin therapy; the changes from baseline to the end of the study in lipid parameters are presented in . While fenofibrate treatment resulted in significant improvements in total cholesterol, triglycerides and HDL-C compared with placebo, it did not significantly reduce the rate of fatal CV events, nonfatal myocardial infarction, or nonfatal stroke.Citation54 However, an analysis by lipid subgroup suggested a possible benefit among patients with both a high baseline triglyceride level and a low baseline HDL-C level (P=0.057).Citation54
Figure 2 Mean change from BL in lipid parameters in fenofibrate and placebo recipients from the ACCORD study.
Abbreviations: ACCORD, Action to Control Cardiovascular Risk in Diabetes; BL, baseline; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Some reports suggest fibrates may mildly reduce glucose levels, which, in addition to the triglyceride lowering, likely helps to account for the reduction in metabolic syndrome in patients treated with fibrates. When combined with statins, fibrates may mitigate the increase in glucose levels sometimes found associated with statins.Citation55 The risk of rhabdomyolysis associated with combination therapy with statins and fibrates appears to differ among the fibrates, with a higher incidence observed with gemfibrozil, at least partially due to a higher risk of drug–drug interactions with statins.Citation56
Niacin
Niacin is believed to exert its effects via a number of potential mechanisms including: 1) directly and noncompetitively inhibiting hepatocyte diacylglycerol acyltransferase 2, thereby reducing hepatic triglyceride synthesis and subsequent very low-density lipoprotein/LDL secretion; 2) inhibiting the surface expression of β-chain adenosine triphosphate synthase by hepatocytes, which inhibits HDL-apolipoprotein (apo) A-I removal, thus increasing apo A-I containing HDL particles; and 3) potentially stabilizing the circulation of secreted apo A-I via increased HDL biogenesis resulting from increased hepatic adenosine triphosphate-binding cassette transporter A-I-mediated apo A-I lipidation.Citation57 At therapeutic doses, niacin significantly reduces LDL-C, non-HDL-C, apo B, and triglyceride levels and increases HDL-C levels. Niacin may also influence lipoprotein particle size and the distribution of lipid subparticles and improve lipid ratios.Citation58 The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) trial investigated the addition of extended-release niacin to simvastatin in 3,414 patients with established CVD (~34% of whom had diabetes), aiming to determine its effect on lipid levels and the composite endpoint of death from CHD, nonfatal myocardial infarction, ischemic stroke, hospitalization for an acute coronary syndrome, or symptom-driven coronary or cerebral revascularization.Citation59 After 2 years of treatment, HDL-C levels had increased 25% with niacin treatment (versus 10% with placebo; P<0.001), while triglyceride and LDL-C levels had decreased by 29% and 12% (versus decreases of 8% and 6% with placebo), respectively; however, improvement in the lipid profile did not translate into a reduction of adverse CV events, with the primary endpoint occurring in 16.4% of patients receiving add-on niacin and 16.2% receiving placebo.Citation59
Niacin causes flushing, which can be intolerable to some patients. Niacin-induced flushing is caused primarily by the promotion of prostaglandin D2 release from skin cells, which stimulates the action of prostaglandin D2 receptors in smooth muscle cells in the dermal arteriole vasculature. Stimulated dermal arterioles then dilate, increasing blood flow and causing flushing.Citation58 Flushing is reduced with extended-release formulations and the fixed combination of extended-release niacin and laropiprant, a selective inhibitor of the prostaglandin D2 receptor subtype.Citation58 As was observed in previous studies, preliminary results from the large Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) trial demonstrated no reduction in risk of CV events with extended-release niacin/laropiprant combination therapy. As a result of these disappointing findings, the extended-release niacin/laropiprant development program was discontinued.Citation60
Examination of the literature regarding the safety profile of niacin shows that, in patients without diabetes, niacin therapy may result in insulin resistance and hyperglycemia; in patients with diabetes, niacin treatment may worsen glucose and hemoglobin A1C control. Although the changes are generally small, in clinical trials this translates into a need for intensification of antidiabetes medications.Citation58,Citation61–Citation64 During a 9-month study in 796 patients with T2DM, a significantly greater proportion of those receiving niacin/laropiprant, compared with placebo, required intensification of their antihyperglycemic regimen (17.6% versus 8.2%; P<0.001).Citation64
Ezetimibe
Ezetimibe acts to block intestinal cholesterol absorption, which leads to a reduction in cholesterol delivery to the liver and an enhanced clearance of LDL-C, which reduces plasma LDL-C levels.Citation65 In patients with T2DM, the addition of ezetimibe to statin therapy provides a significantly greater reduction in LDL-C,Citation66–Citation68 even more so than doubling the statin dose.Citation69–Citation71
While ezetimibe has no known effect on glycemic parameters in patients with T2DM, ezetimibe was studied in diabetes mellitus patients. In two studies (>500 patients) investigating the addition of ezetimibe to existing statin therapy in patients with and without T2DM, patients receiving the combination therapy had significantly greater reductions in LDL-C levels compared with statin therapy alone, irrespective of diabetes status, and similarly, improvements were observed in total cholesterol, triglycerides, and HDL-C levels ().Citation66,Citation68 A study comparing the efficacy of simvastatin/ezetimibe combination therapy (10/20 or 40 mg/day) with atorvastatin monotherapy (10, 20, or 40 mg/day) in 1,229 patients with T2DM showed that simvastatin + ezetimibe recipients had significantly (P≤0.001) greater improvements in LDL-C, total cholesterol, and HDL-C levels than patients receiving any dose of atorvastatin alone.Citation67 Generally, patients receiving simvastatin/ezetimibe combination therapy in this trial achieved LDL-C goals (<100 mg/dL or <70 mg/dL) more frequently than patients receiving atorvastatin.Citation67
Table 3 Least squares mean percent change from baseline in lipid parameters in patients with or without T2DM receiving EZE or PL on a background of statin therapy
Simvastatin/ezetimibe (Vytorin®; Merck & Co., Inc., Whitehouse Station, NJ, USA) combination therapy is generally well tolerated. But, as with simvastatin monotherapy, the simvastatin/ezetimibe combination agent may increase the risk for myopathy and rhabdomyolysis, which increases among patients taking higher simvastatin doses, as is often true with other statins at higher doses.Citation72 In addition, the prescribing information lists other very rare adverse effects, including anaphylaxis, angioedema, rash, and urticaria.Citation72
The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) is an ongoing trial that aims to determine if simvastatin/ezetimibe combination therapy improves CV outcomes in patients with acute coronary syndromes to a greater degree than simvastatin alone.Citation73 The study has enrolled >18,000 patients, and follow-up will continue until >5,250 patients experience the primary endpoint (a composite of CV-related death, nonfatal coronary events, and nonfatal stroke) and each patient is followed for >2.5 years; at present, the trial is expected to report results in September 2014.Citation74
Conclusion
For patients with T2DM, the therapeutic goal is to lower LDL-C and favorably affect other CV risk factors. Statins are LDL-C-lowering agents with the best clinical trial evidence of CVD outcome benefits, and are first-line therapy for hypercholesterolemia. However, statins may not be tolerated by all patients in doses large enough to attain LDL-C goal. Moreover, evidence suggests that statins may be associated with risk (particularly at high doses) for increasing new-onset diabetes and unfavorable glycemic effects. Other lipid-lowering agents, eg, fibrates and ezetimibe, have little to no impact on glucose parameters. BASs are the only class of agents with dual benefits in the management of glucose and lipids in patients with T2DM. Colesevelam is currently the only BAS with an approved indication for use in combination with other classes of lipid- (and glucose-) lowering drugs in patients with T2DM to both lower LDL-C and improve glycemic control.
Acknowledgments
Sheridan Henness, PhD, Alan J Klopp, PhD, CMPP, and Sushma Soni of inScience Communications, Springer Healthcare, provided medical writing support funded by Daiichi Sankyo, Inc.
Disclosure
In the past year, Dr Harold Bays has served as a clinical investigator for (and has received research grants from) pharmaceutical companies such as Abbott, Amarin, Arena, Cargill, California Raisin Board, Daiichi Sankyo, Inc., Esperion, Essentialis, Forest, Gilead, GlaxoSmithKline, Johnson & Johnson, Merck, Novo Nordisk, Omthera, Orexigen, Pfizer, Pozen, Schering Plough, Shionogi, Stratum Nutrition, Takeda, Trygg, and TWI Bio. Dr Bays has received consultant, advisory, or speaking fees from Amarin, AstraZeneca, Boston Scientific, Essentialis, Daiichi Sankyo, Inc., Merck, Novartis, Regeneron, Sanofi, Valeant, Vivus, and Zeomedex. The development of this manuscript was supported by Daiichi Sankyo, Inc. Medical writing support was funded by Daiichi Sankyo, Inc.
References
- KannelWBMcGeeDLDiabetes and cardiovascular risk factors: the Framingham studyCirculation1979591813758126
- AronsonDRayfieldEJHow hyperglycemia promotes atherosclerosis: molecular mechanismsCardiovasc Diabetol20021112119059
- BaysHE“Sick fat,” metabolic disease, and atherosclerosisAm J Med2009122Suppl 1S26S3719110085
- BaysHEAdiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating “sick fat” through improving fat function with antidiabetes therapiesAm J Cardiol2012110Suppl 94B12B
- RayKKSeshasaiSRWijesuriyaSEffect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trialsLancet200937396771765177219465231
- American Diabetes AssociationStandards of medical care in diabetes – 2013Diabetes Care201336 Suppl 1S11S6623264422
- GrundySMCleemanJIMerzCNNational Heart, Lung, and Blood InstituteAmerican College of Cardiology FoundationAmerican Heart AssociationImplications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelinesCirculation2004110222723915249516
- JellingerPSSmithDAMehtaAEAACE Task Force for Management of Dyslipidemia and Prevention of AtherosclerosisAmerican Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of AtherosclerosisEndocr Pract201218Suppl 117822522068
- StoneNJRobinsonJLichtensteinAH2013ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesCirculation Epub11122013
- CheungBMOngKLChernySSShamPCTsoAWLamKSDiabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006Am J Med2009122544345319375554
- BerneCSiewert-DelleAURANUS study investigatorsComparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: results from the URANUS studyCardiovasc Diabetol20054715935095
- GargAGrundySMLovastatin for lowering cholesterol levels in non-insulin-dependent diabetes mellitusN Engl J Med1988318281863422105
- KnoppRHFrohlichJJokubaitisLADawsonKBroylesFEGomez-CoronadoDEfficacy and safety of fluvastatin in patients with non-insulin-dependent diabetes mellitus and hyperlipidemiaAm J Med1994966A69S78S8017470
- Cholesterol Treatment Trialists’ (CTT) CollaboratorsKearneyPMBlackwellLEfficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysisLancet2008371960711712518191683
- DavidsonMHMakiKCPearsonTAResults of the National Cholesterol Education (NCEP) Program Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendationsAm J Cardiol200596455656316098311
- NichollsSJBrandrup-WognsenGPalmerMBarterPJMeta-analysis of comparative efficacy of increasing dose of atorvastatin versus rosuvastatin versus simvastatin on lowering levels of atherogenic lipids (from VOYAGER)Am J Cardiol20101051697620102893
- SilvaMMatthewsMLJarvisCMeta-analysis of drug-induced adverse events associated with intensive-dose statin therapyClin Ther200729225326017472818
- PreissDSeshasaiSRWelshPRisk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysisJAMA2011305242556256421693744
- RautioNJokelainenJOksaHDo statins interfere with lifestyle intervention in the prevention of diabetes in primary healthcare? One-year follow-up of the FIN-D2D projectBMJ Open201225
- SattarNPreissDMurrayHMStatins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trialsLancet2010375971673574220167359
- ZaharanNLWilliamsDBennettKStatins and risk of treated incident diabetes in a primary care populationBr J Clin Pharmacol20137541118112422845189
- HandelsmanYMechanickJIBlondeLAACE Task Force for Developing Diabetes Comprehensive Care PlanAmerican Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care planEndocr Pract201117Suppl 215321474420
- [No authors listed]Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. The Expert PanelArch Intern Med1988148136693422148
- [No authors listed]The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol loweringJAMA198425133653746361300
- AndradeSEWalkerAMGottliebLKDiscontinuation of antihyperlipidemic drugs – do rates reported in clinical trials reflect rates in primary care settings?N Engl J Med199533217112511317700285
- AvornJMonetteJLacourAPersistence of use of lipid-lowering medications: a cross-national studyJAMA199827918145814629600480
- FlorentinMLiberopoulosENMikhailidisDPElisafMSColesevelam hydrochloride in clinical practice: a new approach in the treatment of hypercholesterolaemiaCurr Med Res Opin2008244995100918291066
- WELCHOL® (colesevelam hydrochloride) [prescribing information]Daiichi Sankyo IncParsippany, NJ2014 [revised Jan 2014]
- GoodwinBJonesSAPriceRRA regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesisMol Cell20006351752611030332
- LefebvrePCariouBLienFKuipersFStaelsBRole of bile acids and bile acid receptors in metabolic regulationPhysiol Rev200989114719119126757
- StaelsBA review of bile acid sequestrants: potential mechanism(s) for glucose-lowering effects in type 2 diabetes mellitusPostgrad Med20091213 Suppl 1253019494475
- BaysHEGoldbergRBThe ‘forgotten’ bile acid sequestrants: is now a good time to remember?Am J Ther200714656758018090882
- PotthoffMJPottsAHeTColesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO miceAm J Physiol Gastrointest Liver Physiol20133044G371G38023257920
- GargAGrundySMCholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. A short-term, double-blind, crossover trialAnn Intern Med199412164164228053615
- BaysHEGoldbergRBTruittKEJonesMRColesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effectsArch Intern Med2008168181975198318852398
- FonsecaVARosenstockJWangACTruittKEJonesMRColesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapyDiabetes Care20083181479148418458145
- GoldbergRBFonsecaVATruittKEJonesMREfficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapyArch Intern Med2008168141531154018663165
- JialalIAbbySLMisirSNagendranSConcomitant reduction in low-density lipoprotein cholesterol and glycated hemoglobin with colesevelam hydrochloride in patients with type 2 diabetes: a pooled analysisMetab Syndr Relat Disord20097325525819344229
- YamakawaTTakanoTUtsunomiyaHKadonosonoKOkamuraAEffect of colestimide therapy for glycemic control in type 2 diabetes mellitus with hypercholesterolemiaEndocr J2007541535817102570
- CaoGLiangYBroderickCLAntidiabetic action of a liver X receptor agonist mediated by inhibition of hepatic gluconeogenesisJ Biol Chem200327821131113612414791
- LaffitteBAChaoLCLiJActivation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissueProc Natl Acad Sci U S A200310095419542412697904
- BaranowskiMBiological role of liver X receptorsJ Physiol Pharmacol200859 Suppl 7315519258656
- GoldfineABModulating LDL cholesterol and glucose in patients with type 2 diabetes mellitus: targeting the bile acid pathwayCurr Opin Cardiol200823550251118670263
- ReasnerCAReducing cardiovascular complications of type 2 diabetes by targeting multiple risk factorsJ Cardiovasc Pharmacol200852213614418670366
- StaelsBHandelsmanYFonsecaVBile acid sequestrants for lipid and glucose controlCurr Diab Rep2010101707720425070
- PotthoffMJBoney-MontoyaJChoiMFGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathwayCell Metab201113672973821641554
- EfanovAMSewingSBokvistKGromadaJLiver X receptor activation stimulates insulin secretion via modulation of glucose and lipid metabolism in pancreatic beta-cellsDiabetes200453 Suppl 3S75S7815561926
- HarachTPolsTWNomuraMTGR5 potentiates GLP-1 secretion in response to anionic exchange resinsSci Rep2012243022666533
- JonesMBaysHColesevelam HCl: glycemic and lipid parameter effects in patients with type 2 diabetes mellitus treated with metformin-based therapy and a statinPaper presented at: AACE 21st Annual Scientific and Clinical CongressMay 23–27, 2012Philadelphia, PA
- GoldenbergIBenderlyMGoldbourtUUpdate on the use of fibrates: focus on bezafibrateVasc Health Risk Manag20084113114118629356
- MooradianADDyslipidemia in type 2 diabetes mellitusNat Clin Pract Endocrinol Metab20095315015919229235
- [No authors listed]Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised studyLancet2001357926090591011289345
- KeechASimesRJBarterPFIELD study investigatorsEffects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trialLancet200536695001849186116310551
- ACCORD Study GroupGinsbergHNElamMBEffects of combination lipid therapy in type 2 diabetes mellitusN Engl J Med2010362171563157420228404
- BaysHERothEMMcKenneyJMThe effects of fenofibric acid alone and with statins on the prevalence of metabolic syndrome and its diagnostic components in patients with mixed dyslipidemiaDiabetes Care20103392113211620573750
- JonesPHDavidsonMHReporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statinAm J Cardiol200595112012215619408
- KamannaVSGanjiSHKashyapMLRecent advances in niacin and lipid metabolismCurr Opin Lipidol201324323924523619367
- BaysHEBallantyneCWhat’s the deal with niacin development: is laropiprant add-on therapy a winning strategy to beat a straight flush?Curr Opin Lipidol200920646747619779335
- AIM-HIGH InvestigatorsBodenWEProbstfieldJLNiacin in patients with low HDL cholesterol levels receiving intensive statin therapyN Engl J Med2011365242255226722085343
- Merck Sharp & Dohme CorpMerck Announces HPS2-THRIVE Study of TREDAPTIVE™ (Extended-Release Niacin/Laropiprant) Did Not Achieve Primary Endpoint [press release]Whitehouse Station, NJMerck Sharp & Dohme Corp20121220 Available from: http://www.mercknewsroom.com/press-release/prescription-medicine-news/merck-announces-hps2-thrive-study-tredaptive-extended-relea#sthash.8dgN5z3w.dpufAccessed June 6, 2014
- ElamMBHunninghakeDBDavisKBEffect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial Disease Multiple Intervention TrialJAMA2000284101263127010979113
- GrundySMVegaGLMcGovernMEDiabetes Multicenter Research GroupEfficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trialArch Intern Med2002162141568157612123399
- GuytonJRBaysHESafety considerations with niacin therapyAm J Cardiol2007996A22C31C
- MacLeanAMcKenneyJScottREfficacy and safety of extended-release niacin/laropiprant in patients with type 2 diabetes mellitusBr J Cardiol2011183745
- BaysHENeffDTomassiniJETershakovecAMEzetimibe: cholesterol lowering and beyondExpert Rev Cardiovasc Ther20086444747018402536
- DenkeMPearsonTMcBridePGazzaraRABradyWETershakovecAMEzetimibe added to ongoing statin therapy improves LDL-C goal attainment and lipid profile in patients with diabetes or metabolic syndromeDiabetes Vasc Dis Res20063293102
- GoldbergRBGuytonJRMazzoneTEzetimibe/simvastatin vs atorvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia: the VYTAL studyMayo Clin Proc200681121579158817165637
- SimonsLTonkonMMasanaLEffects of ezetimibe added to on-going statin therapy on the lipid profile of hypercholesterolemic patients with diabetes mellitus or metabolic syndromeCurr Med Res Opin20042091437144515383192
- BardiniGGiordaCBPontiroliAELe GrazieCRotellaCMEzetimibe + simvastatin versus doubling the dose of simvastatin in high cardiovascular risk diabetics: a multicenter, randomized trial (the LEAD study)Cardiovasc Diabetol201092020492655
- GaudianiLMLewinAMeneghiniLEfficacy and safety of ezetimibe co-administered with simvastatin in thiazolidinedione-treated type 2 diabetic patientsDiabetes Obes Metab200571889715642080
- ConardSBaysHLeiterLAEzetimibe added to atorvastatin compared with doubling the atorvastatin dose in patients at high risk for coronary heart disease with diabetes mellitus, metabolic syndrome or neitherDiabetes Obes Metab201012321021820151997
- Vytorin® [prescribing information]Whitehouse Station, NJMerck Sharp and Dohme Corp, a subsidiary of Merck & Co Inc2014
- CannonCPGiuglianoRPBlazingMAIMPROVE-IT InvestigatorsRationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromesAm Heart J2008156582683219061694
- Merck Sharp & Dohme CorpIMPROVE-IT: Examining Outcomes in Subjects With Acute Coronary Syndrome: Vytorin (Ezetimibe/Simvastatin) vs Simvastatin (P04103 AM5) Available from: http://clinicaltrials.gov/show/NCT00202878Accessed January 12, 2014 NLM identifier: NCT00202878
- BeysenCMurphyEJDeinesKEffect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled studyDiabetologia201255243244222134839
- SmushkinGSathananthanMPiccininiFThe effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetesDiabetes20136241094110123250357
- SuzukiTObaKIgariYColestimide lowers plasma glucose levels and increases plasma glucagon-like PEPTIDE-1 (7–36) levels in patients with type 2 diabetes mellitus complicated by hypercholesterolemiaJ Nippon Med Sch200774533834317965527
- BaysHDujovneCColesevelam HCl: a non-systemic lipid-altering drugExpert Opin Pharmacother20034577979012740000
