Abstract
Background and objectives
Diabetic retinopathy is the main microvascular complication in diabetes mellitus and needs to be diagnosed early to prevent severe sight-threatening retinopathy. The purpose of this study was to quantify the retinal microvasculature pattern and analyze the influence of blood glucose level and the duration of diabetes mellitus on the retinal microvasculature.
Methods
Two groups were analyzed: patients with diabetes (N=26) and patients without diabetes, ie, controls (N=26). A quantitative semiautomated method analyzed retinal microvasculature. The diameters of arterioles and venules were measured. The total numbers of arterioles and venules were counted. The ratio of arteriole diameter to venule diameter was calculated. The retinal microvasculature pattern was related to clinical and biochemical parameters.
Results
Patients with diabetes exhibited larger venule diameters in the upper temporal quadrant of the retina compared to the lower temporal quadrant (124.85±38.03 µm vs 102.92±15.69 µm; P<0.01). Patients with diabetes for 5 or more years had larger venule diameters in the upper temporal quadrant than patients without diabetes (141.62±44.44 vs 112.58±32.11 µm; P<0.05). The degree of venodilation in the upper temporal quadrant was positively correlated with blood glucose level and the estimated duration of diabetes mellitus.
Interpretation and conclusion
The employed quantitative method demonstrated that patients with diabetes exhibited venule dilation in the upper temporal quadrant, and the duration of diabetes mellitus was positively correlated with blood glucose level. Therefore, the early assessment of retinal microvascular changes is possible prior to the onset of diabetic retinopathy.
Introduction
Diabetic retinopathy (DR) is the main microvascular complication in diabetes mellitus (DM), and it is a primary cause of low visual acuity.Citation1 Changes to the retinal micro-vasculature, especially dilation of venules and arterioles, result from structural and functional alterations, such as pericyte degenerationCitation2 and thickening of the basement membrane.Citation3 Endothelial dysfunction is a characteristic functional change that precedes structural alterations.Citation4 Retinal blood flow is autoregulated by the interaction between myogenic and metabolic mechanisms,Citation5 and an imbalance of endothelium-derived relaxing and contracting factors could be important for the development of vascular ophthalmic complications like diabetes and retinal ischemia.Citation6
The study suggests that retinal vessel caliber and geometry of the retinal vasculature may be important risk factors for the progression to proliferative DR.Citation7 In addition, the Asian population was shown to have wider retinal arterioles in diabetes and wider venules in those with DR, supporting the concept that a quantitative assessment of retinal vasculature may provide further insights into early diabetic microvascular damage.Citation8
The early diagnosis of DR using high precision instruments is extremely important to improve our understanding of diabetic microangiopathy and develop new treatment options. DR is a major cause of blindness.Citation9 The qualitative changes in DR initially appear in the upper temporal quadrant.Citation10 The quantification of retinal vessels can estimate the progression of DR, and this technique can predict treatment success.Citation11 The present study quantified the retinal microvasculature pattern and analyzed the influence of blood glucose level and the duration of DM on the retinal microvasculature.
Materials and methods
Individuals were invited to participate voluntarily in this study. After being informed about all relevant procedures and aspects of the study, participants signed an informed consent form in accordance with national and international standards for research conducted using human subjects. The study was approved by the Ethics in Human Research Committee of the Federal University of Espirito Santo. Initially, 180 patients were screened for this study. The patients were treated at the Ophthalmology Outpatient Clinics of the municipal public health care system and the Cassiano Antônio de Moraes University Hospital. Individuals of both sexes aged 25–55 years were included in the study with no restrictions on race. The following exclusion criteria were used: prior diagnosis of ametropy greater than or equal to 4 D, cataract, glaucoma, corneal damage, systemic arterial hypertension, and patients who did not complete all stages of the study. Patients without diabetes (N=26) and with (N=26) diabetes with fasting glucose ≥7.8 mmol/L or using oral hypoglycemics after the initial assessment remained in the study. Clinical assessment, retinography, and blood collection for biochemical assays were performed during fasting at the Clinic for Cardiovascular Research of the Graduate Program in Physiological Sciences at the Federal University of Espírito Santo, Vitoria, Espirito Santo, Brazil.
Blood pressure (BP) was measured casually, and the mean of three BP measurements was considered. BP was measured with the patient seated, after resting for 5 minutes and at least 30 minutes since last consuming coffee, alcohol, cigarettes, or food.Citation12 A mercury sphygmomanometer and stethoscope were used. A single observer performed the assessments based on Phases 1 and 5 of the Korotkoff sounds. The pulse pressure (PP) was calculated as the difference between systolic arterial pressure (SAP) and diastolic arterial pressure (DAP). The heart rate (HR) was assessed using the arterial pulse (bpm). The mean arterial pressure (MAP) was calculated using the following formula: MAP = DAP + (SAP – DAP)/3.Citation12 Hypertension was defined as a mean systolic blood pressure ≥140 mmHg and/or mean diastolic blood pressure ≥90 mmHg and/or a history of taking antihypertensive medication at the time of examination.
The ophthalmological examination was performed with the participants under pharmacological mydriasis with 1% tropicamide (Mydriacyl®). Retinography (photograph of the fundus – noninvasive method) was performed using a Nikon® NF 505 retinograph attached to a 35-mm camera with a 50° aperture, and images of the fundus were recorded on Fuji® ASA 400 film. The film was developed, and the highest quality picture was selected for one eye and digitized on a model 3670 HP scanner (200 dpi resolution). Quantitative semiautomated analysis of the retinal vessels was performed using a computer program that automatically defined the radius of the optic disk as ~0.9 mm.Citation13
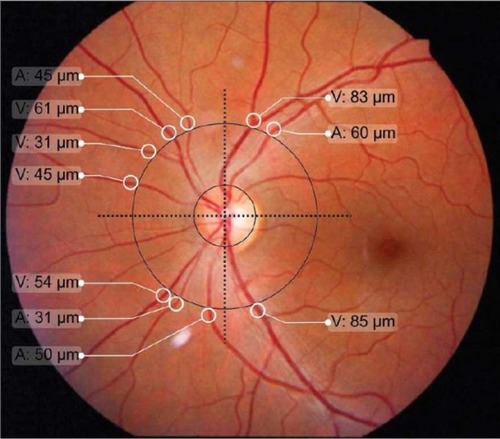
The program drew a circle with a radius three times that of the optic disk, which is similar to the procedure of Stanton et al.Citation14 The observer marked the edges of retinal vessels around the greater circle using a caliper and measured in micron (µm). Two perpendicular lines were drawn through the center of the optical disk to divide the retina into quadrants (). Vessels with diameters smaller than 30 µm were excluded due to the lack of precision in edge definition. The following variables were recorded: total number of arterioles (TNA); total number of venules (TNV); arteriole diameter (AD), defined as the sum of diameters for all arterioles; sum of arteriole diameters in the upper temporal quadrant (ADUT); sum of arteriole diameters in the lower temporal quadrant (ADLT); sum of arteriole diameters in the upper nasal quadrant (ADUN); sum of arteriole diameters in the lower nasal quadrant (ADLN); venule diameter (VD), defined as the sum of diameters for all venules; sum of venule diameters in the upper temporal quadrant (VDUT); sum of venule diameters in the lower temporal quadrant (VDLT); sum of venule diameters in the upper nasal quadrant (VDUN); sum of venule diameters in the lower nasal quadrant (VDLN); arteriole diameter/venule diameter ratio, obtained by dividing these two variables (AD/VD ratio); the AD/VD ratio in the upper temporal quadrant (AD/VD ratio UT); AD/VD ratio in the lower temporal quadrant (AD/VD ratio LT); AD/VD ratio in the upper nasal quadrant (AD/VD ratio UN); and AD/VD ratio in the lower nasal quadrant (AD/VD ratio LN).
Figure 1 Definition of the optical disk, the greater circle and quadrants of the retina for quantification of the arteriole (A) and venule (V) microvasculature.
Statistical analysis
The data are presented as the means ± standard deviation. Student’s t-test was used to compare the clinical, biochemical, and ophthalmological data between groups with and without diabetes, when required. Correlations between the studied variables in the total sample were tested using Pearson’s correlation coefficient, and the results are shown in linear regression plots. One-way analysis of variance (ANOVA) with Tukey’s post hoc test were used to compare the control group with the group with diabetes for less than 5 years and the group with diabetes for 5 or more years. Multiple linear regression was used to test the effects of DM duration and blood glucose level on retinal microvasculature. The level of significance was set at 0.05. The SPSS 11.0 software for Windows was used to perform the statistical analyses.
Results
The clinical and biochemical characteristics of the sample are shown in . The groups did not differ in age, sex, SAP, DAP, PP, MAP, or HR. The biochemical data in indicate that fasting glucose, VLDL (very-low-density lipoprotein) cholesterol, and triglycerides were significantly higher in patients with diabetes than patients without diabetes (controls). The groups did not differ in mean values for total cholesterol, HDL (high-density lipoprotein) cholesterol, LDL (low-density lipoprotein) cholesterol, hematocrit, erythrocyte number, or hemoglobin. The microvascular features of the retina were distinct in the various quadrants. shows significant differences among patients with diabetes in the mean ADUN vs ADLN, VDUT vs VDLT, and VDUN vs VDLN. The remaining microvascular features of the retina were not significantly different. The mean VDUT was ~22% greater in patients with diabetes for 5 or more years than in patients with diabetes for less than 5 years and in patients without diabetes (controls) (). The differences for the group with diabetes described in remained when the distribution of diabetes duration was considered, and the means for VDUT vs VDLT and VDUN vs VDLN differed significantly among patients with diabetes for 5 or more years. Quantitative changes in the retinal microvasculature in the upper temporal quadrant of patients with diabetes were more evident when associated with blood plasma glucose levels (). Significant positive correlations were observed between blood glucose level and VDUT (r=0.37, P<0.01) and blood glucose level and ADUT (r=0.31, P<0.05). The TAD, ADLT, ADUN, ADLN, TVD, VDLT, VDUN, VDLN, AD/VD ratio, TNA, and TNV were not correlated with blood glucose level. A positive correlation was observed between VDUT and duration of diabetes (). The duration of diabetes exhibited an r2 of 0.23 (P<0.05) and explained 23% of VDUT dilation. The partial correlation coefficient was adjusted for SAP, and a positive correlation was observed between blood glucose level and VDUT (r=0.37 vs r=0.37 after adjusting for SAP, P<0.01) and blood glucose level and ADUT (r=0.31 vs r=0.30 after adjusting for SAP, P<0.05), independent of SAP. The remaining retinal microvascular parameters (TAD, ADLT, ADUN, ADLN, TVD, VDLT, VDUN, VDLN, AD/VD Ratio, TNA, and TNV) were not correlated with blood glucose level (). The partial correlation with adjustment for SAP was calculated between DM duration and VDUT. SAP did not affect this correlation (r=0.33 vs r=0.33 after adjusting for SAP, P<0.01). The remaining retinal microvascular parameters were not correlated with DM duration after adjusting for SAP ().
Figure 2 Linear regression between blood glucose level and VDUT (A) and ADUT (B).
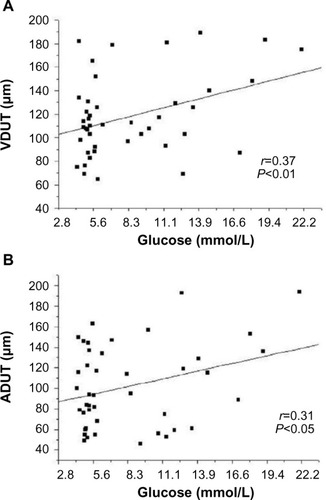
Figure 3 Linear regression between duration of DM and VDUT.
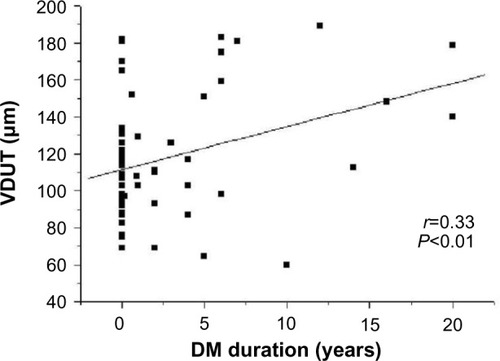
Table 1 Clinical and biochemical characteristics of patients with and without diabetes
Table 2 Characteristics of arteriole diameter and venule diameter of the retinal micro vasculature by quadrant, in patients with and without diabetes (controls)
Table 3 Characteristics of the AD, VD, and the AD/VD ratio of the retinal microvasculature by quadrant, in patients with and without diabetes (controls) distributed according to disease duration
Table 4 Correlation between blood glucose level and retinal microvascular parameters before and after adjusting for SAP
Table 5 Correlation between DM duration and the retinal microvascular parameters before and after adjusting for SAP
Discussion
Aiming at minimizing the limitations in the qualitative forms of diagnosing DR, it was observed in the literature that researchers have looked for different methods to quantify retina microvascularity. The quantitative methods intend to measure the diameter in the retina vessels,Citation8 estimate the equivalent diameter in the central retina artery and central retina vein,Citation15 and measure blood flow.Citation16
The quantitative analysis of retinal microvasculature in the present study revealed that patients with diabetes exhibited larger retinal vessel diameters, specifically in the upper temporal quadrant relative to the lower temporal quadrant. Previous population studies have shown a significant increase in arteriole and venule diameters in individuals with diabetes.Citation8,Citation17
Changes in retinal microvasculature were found in the upper temporal quadrant in the early stage of the disease, but the lower temporal quadrant, which is responsible for supplying the macula, was spared. This difference was most likely due to a differential regulation of blood flow. The inferior temporal quadrant of the retina is, in comparison with the superior temporal region, less responsive to vasodilation.Citation18 One study in children with diabetes showed that the superior temporal vein was dilated more than the inferior temporal vein, which suggests that vasodilation could precede other signs of DR.Citation19 Blood flow increases during the initial stages of DR, and retinal blood flow decreases during more severe stages (proliferative DR) due to a reduction in arterial blood flow. Grunwald et alCitation16 analyzed retinal blood flow in patients with diabetes and found that venous blood flow was greater in the upper retina than in lower retina. Changes in upper temporal venule diameter can occur at early stages of diabetes before the onset of retinopathy. Therefore, the early identification of these changes would allow control measures and treatments to be adopted sooner. The quantitative method identified changes in VDUT in patients with diabetes and showed the absence of these changes in patients without diabetes. This analysis provided an early diagnosis of DR in a simple and reliable manner. Other methods can perform this assessment,Citation20,Citation21 but these methods are often costly and complex complicating the assessment of patients in locations that lack resources. Therefore, the method in the present study can be a useful tool for early diagnosis.
The most important risk factors for the development of DR are DM duration, poor metabolic control, and hypertension.Citation22 Our study showed that patients with diabetes for 5 years or more exhibited significant changes in VDUT, and VDUT was greater in these individuals than in individuals with diabetes for less than 5 years and in individuals without diabetes (controls). DM duration appeared to directly affect the development of DR, which is consistent with Moloney and Drury’s findingsCitation23 that a positive correlation between DM duration and DR exists. Population-based studies have shown a positive association between DM duration and the prevalence and severity of DR.Citation24,Citation25 The increase in retinal vein diameter is greater in patients who have had DM for a longer duration, and retinal microvasculature changes can be useful for an early DM diagnosis.Citation26,Citation27 The prevalence of DR is strongly associated with DM duration.Citation28,Citation29
Retinal microvasculature parameters are related to age, arterial pressure, blood glucose level, and other factors. Only blood glucose level was positively correlated with VDUT after adjusting for SAP, and AP did not affect this correlation. Therefore, blood glucose control is the primary modifiable risk factor for the prevention of DR onset and progression.Citation30,Citation31 The present results showed that DM duration remained positively correlated with VDUT, even after adjusting for SAP.
Blood glucose level and the estimated DM duration correlated with VDUT, and the estimated DM duration was an independent predictor that explained ~23% of the venodilation in the upper temporal quadrant. Multiple regression and multivariate analyses have shown that DM duration is the most important risk factor for DR, and it is associated with changes in venule diameter.Citation15
The quantification of retinal microvasculature can be a useful tool for the detection of early microvascular changes in the retinas of patients with diabetes. The quantitative method in the present study showed vasodilation, especially in the venular bed, of the upper temporal quadrant in patients with diabetes. The main risk factors of the described venule changes were blood glucose level and the duration of DM. The early diagnosis of DR and the detection of risk factors are indispensable for the prevention of this incapacitating complication of DM. More longitudinal prospective studies will be needed to further explore the findings of this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- BuchHVindingTNielsenNVPrevalence and causes of visual impairment according to World Health Organization and United States Criteria in an aged, urban Scandinavian populationOphthalmology2001108122347235711733284
- CurtisTMScholfieldCNThe role of lipids and protein kinase Cs in the pathogenesis of diabetic retinopathyDiabetes Metab Res Ver20042012843
- CockburnDMDiabetic retinopathy: classification, description and optometric managementClin Exp Optom1999822–3597312482294
- CooperMEBonnetFOldfieldMJandeleit-DahmKMechanisms of diabetic vasculopathy: an overviewAm J Hypertens2001145 Pt 147548611368471
- PournarasCJRungger-BrändleERivaCEHardarsonSHStefanssonERegulation of retinal blood flow in health and diseaseProg Retin Eye Res200827328433018448380
- HaefligerIOMeyerPFlammerJLüscherTFThe vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology?Surv Ophthalmol19943921231327801220
- Crosby-NwaobiRHengLZSivaprasadSRetinal vascular calibre, geometry and progression of diabetic retinopathy in type 2 diabetes mellitusOphthalmologica20122282849222517193
- IslamFMNguyenTTWangJJQuantitative retinal vascular calibre changes in diabetes and retinopthy: the Singapore Malay eye studyEye2009231719172419079148
- AlderVASuENYuDYCringleSJYuPKDiabetic retinopathy: early functional changesClin Exp Pharmacol Physiol1997249–107857889315390
- TaylorEDobreeJHProliferative diabetic retinopathyBr J Ophthalmol197054811185417648
- KleinRKleinBEMossSEThe relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy. XIX: The Wisconsin Epidemiologic Study of Diabetic RetinopathyArch Ophthalmol20041221768314718299
- Mion JrDMachadoCAGomesMAIV Diretrizes Brasileiras de Hipertensão Arterial [Brazilian guidelines in arterial hypertension]Arq Bras Cardiol199156Suppl AA1A161859291
- JonasJBPapastathopoulosOphthalmoscopic measurement of the optic discOphthalmology19951027110211069121759
- StantonAVMullaneyPMeeFO’BrienETO’MalleyKA method of quantifying retinal microvascular alterations associated with blood pressure and ageJ Hypertens199513141487759850
- KleinRMeyersCELeeKEGanongRKleinBEChanges in retinal vessel diameter and incidence and progression of diabetic retinopathyArch Ophthalmol2012130674975522332203
- GrunwaldJERivaCEBaineJBruckerAJTotal retinal volumetric blood flow rate in diabetic patients with poor glycemic controlInvest Ophthalmol Vis Sci19923323563631740366
- TikellisGWangJJTappRThe relationship of retinal vascular caliber to diabetes and retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) studyDiabetologia200750112263227117891374
- ChungHSHarrisAHalterPJRegional differences in retinal vascular reactivityInvest Ophthalmol Vis Sci199940102448245310476818
- FalckALaatikainenLRetinal vasodilation and hyperglycaemia in diabetic children and adolescentsActa Ophthalmol Scand19957321191247656137
- EuvrardGGenevoisORivalsIA semiautomated computer tool for the analysis of retinal vessel diameter dynamicsComput Biol Med201343551352323566397
- MirsharifQTajeripourFPourrrezaHAutomated characterization of blood vessels as arteries and veins in retinal imagesComput Med Imaging Graph20133760761723849699
- YauJWRogersSLKawasakiRMeta-Analysis for Eye Disease (META-EYE) Study GroupGlobal prevalence and major risk factors of diabetic retinopathyDiabetes Care201235355656422301125
- MoloneyJDruryMIRetinopathy and retinal function in insulin-dependent diabetes mellitusBr J Ophthalmol198266127597616756466
- WestSKKleinRRodriguezJDiabetes and diabetic retinopathy in a Mexican-American populationDiabetes Care20012471204120911423503
- HaddadOASaadMKPrevalence and risk factors for diabetic retinopathy among Omani diabeticsBr J Ophthalmol19988289019069828774
- IrvingRJWalkerBRNoonJPWattGCWebbDJShoreACMicro-vascular correlates of blood pressure, plasma glucose, and insulin resistance in healthCardiovasc Res200253127127611744037
- WongTYRetinal vessel diameter as a clinical predictor of diabetic retinopathy progression: time to take out the measuring tapeArch Ophthalmol20111291959621220635
- StrattonIMKohnerEMAldingtonSJfor the UKPDS GroupUKPDS 50: risk factors for incidence and progression of retinopathy in the type II diabetes over 6 years from diagnosisDiabetologia200144215616311270671
- American Diabetes AssociationDiabetic retinopathyDiabetes Care200225S90S93
- LookerHCKrakoffJKnowlerWBennettPHKleiRHansonRLLongitudinal studies of incidence and progression of diabetic retinopathy assessed by retinal photography in Pima IndiansDiabetes Care200326232032612547856
- ShichiriMKishikawaHOhkuboYWakeNLong-term results of the Kumamoto study on optimal diabetes control in type 2 diabetic patientsDiabetes Care200023Suppl 2B21B2910860187
