Abstract
Hepatic encephalopathy is a common neuropsychiatric abnormality, which complicates the course of patients with liver disease. It was probably first described by Hippocrates over 2000 years ago, who said that “those whose madness arises from phlegm are quiet and neither shout nor make a disturbance, while those whose madness arises from bile shout, play tricks and will not keep still, but are always up to some mischief ”. He was presumably describing the differences between patients with pneumonia and acute liver failure. Despite the fact that the syndrome was probably first recognized thousands of years ago, the exact pathogenesis still remains unclear. Furthermore, a precise definition of the syndrome is lacking, as are definitive methods of diagnosing this condition. It is important as both patients with cirrhosis and the general population with whom they interact may be affected as a consequence. At a minimum, the individual may be affected by impaired quality of life, impaired ability to work, and slowed reaction times, which are relevant to the population at large if affected individuals operate heavy machinery or drive a car. Pathogenic mechanisms, diagnostic tools, and treatment options are discussed.
Background
Hepatic encephalopathy (HE) is a complex, reversible neuropsychiatric syndrome, complicating the course of liver disease. In recent guidelines published jointly by the European and American Associations for the Study of the Liver, HE was defined as “brain dysfunction caused by liver insufficiency or portal systemic shunting”.Citation1,Citation2 Despite the fact that the syndrome was probably recognized thousands of years ago, the exact pathogenesis remains unclear.Citation3
The pathogenesis is thought to be attributable to both neurochemical and neurophysiological disorders of the brain.Citation4 The essentially reversible nature suggests a metabolic cause. The broad spectrum of cerebral disturbance is likely to be a reflection of the range of metabolic disturbances responsible for the syndrome, rather than one causal abnormality.
One of the central factors contributing to HE in cirrhosis is unfiltered blood from the portal system reaching the brain. Neuropathologically, the most frequently described change is to astrocytes, which undergo cell swelling.Citation5 The morphological change seen is known as Alzheimer type II astrocytosis. However, neurons remain structurally normal. Theories on pathogenesis of HE are outlined in .
Table 1 Theories for pathogenesis of HE
Gut-derived neurotoxins
Ammonia has been the most studied gut-derived neurotoxin, produced from the breakdown of proteins and amino acids. Ammonia is produced by the gastrointestinal tract in two ways: by direct ammonia liberation from breakdown products of dietary protein and metabolism of circulating glutamine (GLN) and by the gut microbiome acting upon urea and ingested food. Concentrations of ammonia are kept relatively constant in the blood by efficient detoxification processes, involving hepatic production of urea and synthesis of GLN from glutamate (GLU) by the action of glutamine synthetase (GS), which is located in the liver, muscle, and brain.Citation6 Hyperammonemia is commonly seen in chronic liver disease, as are high levels of circulating endotoxins, as the liver fails to detoxify the portal circulation draining the intestines, which are heavily colonized by metabolically active bacteria, or else because the portal blood supply bypasses the liver through the development of a collateral circulation in the presence of portal hypertension.
The first suggestion that ammonia may be involved in HE was in 1893 by investigators from Pavlov’s group.Citation7 A rise in arterial blood ammonia in dogs was associated with behavioral disturbances after high protein meals.Citation8 Fifty years later, Gabuzda et al inadvertently produced HE in chronic liver disease patients, treated with ion-exchange resins to diminish ascites.Citation9 The resins absorbed sodium, but released ammonium ions, bringing about HE.
More recent animal studies using portacaval shunts showed blood and brain ammonia concentrations to be increased two- and three folds, respectively.Citation10 Primary cultured astrocytes exposed to ammonia have been shown to develop features of Alzheimer type II astrocytosis seen neuropathologically in human beings.Citation11
Short- and medium-chain fatty acids are increased in the blood of patients with HE.Citation12 These fatty acids produce coma in animal models and possibly interfere with ureagenesis.Citation13 Nevertheless, these agents have been given to patients without any adverse consequences.Citation14
Amino acid disturbances have been observed in HE patients, typically increased aromatic amines and decreased or normal levels of branched-chain amino acids (BCAAs).Citation15,Citation16 Low plasma concentrations of BCAAs are a result of increased use by muscle, heart, kidney, and adipose tissue as an energy source,Citation17 since alternatives such as glucose and ketone bodies are reduced in liver disease. Hyperammonemia results in increased brain uptake of aromatic acids, including tryptophan, the precursor of the neurotransmitter, serotonin. Tryptophan’s increased availability leads to altered serotonin synthesis, increasing neuroinhibition.Citation18
Cerebral neurotransmission
GLU is the predominant excitatory neurotransmitter in the brain. In HE, astrocytes play an important role in detoxification of ammonia through conversion of GLU to GLN. Astrocytic GLU reuptake mechanisms may become impaired; thereby increasing extracellular concentrations of GLU.Citation19 Furthermore, GLU binding sites appear to be downregulated on postsynaptic neurons. This, therefore, contributes to decreased neuroexcitatory activityCitation20 and increased cerebral GLN.Citation21
γ-Aminobutyric acid (GABA) is the predominant inhibitory neurotransmitter in the brain,Citation22 binding to specific GABA receptors, which are part of larger receptor complexes, activated by benzodiazepines and barbiturates. For the most part, GABA is synthesized by gut bacteria and would normally enter the portal vein to be metabolized by the liver. In cirrhosis, blood bypasses the liver by collaterals and enters the systemic circulation. Increased plasma levels of GABA have been observed in patients with HE.Citation23 However, autopsied brain tissue from HE patients did not support GABA involvement.Citation24
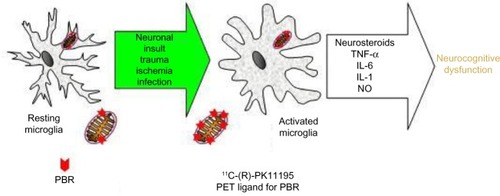
Translocator proteins (TSPOs), formerly known as peripheral benzodiazepine binding sites, may play an important role in neuroinhibition. TSPOs are 18 kDa proteins, located on the outer mitochondrial membrane of astrocytes, and facilitate cholesterol ingress into mitochondria, among other roles.Citation25 TSPOs are widely distributed throughout the body and are also present in microglia.Citation26 TSPOs are upregulated by ammoniaCitation27 and have been shown to be present in increased density in autopsied brains of HE patients.Citation28 TSPO upregulation promotes synthesis of neurosteroids, such as tetrahydroprogesterone and tetrahydrodeoxycorticosterone, which are potent agonists of GABAA receptors (). In vivo positron emission tomography (PET) studies support the hypothesis that TSPOs could play an active role in impaired brain functioning in HE.Citation29
Figure 1 Schematic representation of microglial activation.
Abbreviations: PBR, peripheral benzodiazepine receptor; TSPO, translocator protein; PET, positron emission tomography; TNF-α, tumor necrosis factor-alpha; IL, interleukin; NO, nitric oxide.
Inflammation
It is frequently observed that cirrhosis patients with active infections may exhibit HE, leading several investigators to consider a direct link between HE and inflammation. One group medically induced hyperammonemia, using amino acid solution, in a group of stable cirrhosis patients.Citation30 Patients with abnormal psychometric performance had more markedly elevated inflammatory markers (white cell count, neutrophil count, C-reactive protein, nitrate/nitrite, and interleukin [IL]-6). Psychometric performance deteriorated in 44.8% of patients with the amino acid solution. In “deteriorators”, there was an increase in inflammatory markers, compared to “nondeteriorators”. Further support for this theory is provided by another group, which studied effects of alteration of gut flora with administration of fiber and probiotics upon HE patients.Citation31 They showed that improvement in HE was associated with reductions in venous endotoxin and ammonia levels. This is an area of increasing interest that requires further investigation.
Low-grade cerebral edema
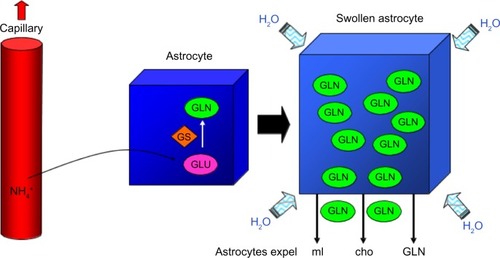
It has been proposed by Häussinger et al that a major contributory event to the development of HE in patients with chronic liver disease is an increase in astrocyte hydration (low-grade cerebral edema without a clinically overt increase in intracranial pressure).Citation32 This may occur as a consequence of astrocytic ammonia uptake and subsequent detoxification by GS, forming GLN from GLU.Citation10 This deamidation process is thought to result in accumulation of GLN within astrocytes, acting as a cerebral osmolyte. The cells respond by expelling osmolytes, myo-inositol (mI), choline (cho), and GLN, but the membrane transport systems are not able to keep up with greatly increased production of GLN. Equilibrium cannot be re-established and there is resultant cell swelling (). Cerebral magnetic resonance spectroscopy (MRS) studies in vivo demonstrated reduced levels of mI and increased GLN and GLU (Glx).Citation33 Additionally, benzodiazepines, hyponatremia, and inflammatory cytokines can induce astrocyte swelling in vitro, so these factors may act synergistically with ammonia to produce low-grade cerebral edema.Citation32
Clinical features
Recognized clinical features of HE are listed in . The first widely accepted clinical grading system to attempt to categorize HE was described by Parsons-Smith et al.Citation34 This classification system was later modified and is now known as either the “modified Parsons-Smith” or West Haven criteriaCitation35 ().
Table 2 Recognized features of HE
Table 3 Modified Parsons-Smith or West Haven criteria for HE
Minimal hepatic encephalopathy
The first published work to propose that subtle mental changes can occur before the onset of clinically detectable neurological changes was by Zeegen et al in 1970.Citation36 They performed paper-based psychometric tests, consisting of the construction of five-pointed stars and modified “Reitan” trail making tests on a population of patients after portal decompression surgery. They found that 13 out of 34 patients (38%), without clinical signs of HE on standard clinical examination, had impaired performance on Reitan trail making tests.
Nomenclature
Subsequently, the presence of these subtle abnormalities has been observed by numerous investigators and gaged by a variety of psychometric tests.Citation37,Citation38 The presence of this abnormality was first termed subclinical or latent HE owing to the lack of clinical signs on examination. Subsequently, nomenclature was replaced by the term “minimal” HE (mHE)Citation39 (). To confuse matters further, the 2014 AASLD/EASL guidelines also suggest an alternative classification considering mHE (West Haven grade 0) and West Haven grade 1 overt HE as “covert” HE, while West Haven grade 2 and above are considered as “overt” HE.Citation1 However, this classification is rarely used outside the United States.
Table 4 Proposed nomenclature of HE
Clinical significance
mHE has been shown to have effects upon quality of life, earning potential, driving performance, and possibly survival.
Quality of life
Groeneweg et al used the “Sickness Impact Profile” (SIP) to determine the effects of mHE on patients’ “activities of daily living”.Citation40 The SIP is a questionnaire assessing influence of the disease and treatment on daily functioning.Citation41 The questionnaire consists of 136 items, grouped into 12 scales encompassing different aspects of daily living, eg, sleep, eating, social interaction, and emotional behavior. They found that mHE patients performed significantly worse in every dimension of the SIP.Citation40
Earning potential
Schomerus and Hamster selected 110 ambulatory outpatients with cirrhosis, who were part of a larger study undergoing psychometric testing.Citation42 Of white-collar workers, 40% had abnormal psychometry, but only 20% were unable to continue working, whereas 55% of blue-collar workers had abnormal psychometry and 60% were unable to earn their living.Citation42
Driving ability
Wein et al measured “on-the-road” driving performance. Of 274 consecutive patients with cirrhosis, 48 fulfilled medical and driving inclusion criteria, 14 of them with mHE and 34 without mHE. The likelihood of an accident in mHE patients was nearly ten times higher than that of patients without mHE.Citation43 Bajaj et al studied self-reported driving behavior in postal questionnaires. Patients with cirrhosis had a higher percentage of driving violations over the previous year (13%), previous 5 years (25%), and a higher number of accidents at 1 year (9%) and 5 years (17%).Citation44
Diagnosis
Despite agreement between investigators that mHE warrants further investigation and probably screening and treatment, there is little consensus as to the optimal instruments with which to diagnose and monitor the condition.Citation39 This has a significant role in the discrepancy between studies, as to the prevalence of mHE, with quoted prevalence varying from 27% to 75% according to the battery of tests used, interpretation of these tests, and populations studied.Citation45,Citation46 Some studies have used just one psychometric test,Citation47 whereas other studies have used up to 26 tests.Citation38 In the latter case, tests needed to be spread over 2 days to avoid fatigue.
In response to the variability of diagnostic tests being used to define mHE, a consensus was reached by a working party,Citation39 which proposed use of a psychometric hepatic encephalopathy score, based on the results of five neuropsychometric tests.Citation48 They also suggested that when possible, quantitative neurophysiological tools, such as electroencephalography (EEG) with mean dominant frequency and P300 auditory evoked potentials, should be used.Citation1,Citation2
Assessment of HE
HE affects cognitive, affective/emotional, behavioral, and bioregulatory domains.Citation39 Each broad domain may be subdivided into various components. For example, cognition may include evaluation of psychomotor speed, visuopraxis, attention, concentration, and level of consciousness.Citation39 Overt or clinically apparent HE should be excluded by careful and detailed neuropsychiatric examination and anamnestic enquiry. Particular attention should be paid to cognitive and motor function, ability to perform activities of daily living, and sleep–wake cycle abnormalities. To diagnose mHE, at least two neuropsychological tests from the following psychometric hepatic encephalopathy score system should be used:
Number connection test-A (NCT-A);
NCT-B;Citation49
Block design test;
Digit symbol substitution test.Citation50
The recommendation was to use a standardized battery including NCT-A and NCT-B, the line tracing, serial dotting, and digit symbol substitution tests.Citation1,Citation2 This battery is easy to apply, but reference test scores are normalized for German populations, so it may be necessary to adjust these for other patient populations. Reference ranges are now available for Spanish,Citation51 Italian, and British populations.Citation52
Paper-based tests
The theoretical advantages of paper-based tests are that they are easy to administer, require no sophisticated technical equipment, and can be performed at the bedside. However, in practice, they may be subject to a greater degree of subjectivity. Ideally, they should be performed in a quiet, well-lit room, under standardized conditions, which apply to both examiner and examinee. In practice, these conditions do not exist in hospital wards. The paper-and-pencil tasks may be affected by subjective “one-off influences”, such as lack of sleep, recent arguments, or hunger. Theoretically, the tests should also be easily interpretable, but reference scores should take account of education, cultural background, and language difficulties.
Quality of life scores
Other paper-based assessments of mHE include nonpsychometric tests such as validated quality of life scores, such as the short form 36 health survey, SIP, and fatigue impact questionnaires.Citation53–Citation55
Computer-based psychometric tests
The use of computerized psychometric testing is widespread in both clinical research settings and for monitoring effects of pharmacological compounds upon cognitive function.Citation56 The choice of system varies from center to center, depending upon investigators’ personal experience, developmental input, and financial restraints. The advantages of computerized systems above conventional paper-and-pencil tests include reproducibility of stimulus presentation, accurate recording of responses/reaction times, and easier to facilitate data analysis. Furthermore, some tests are entirely visual and therefore do not depend on literacy and numeracy, which can hamper the interpretation of paper-based tests. For example, the use of the Stroop test on smartphones and computer tablets has proven to be an easily accessible, useful tool in many countries of the developed world.Citation57
Electroencephalography
The EEG detects and records patterns of electrical activity within the brain through electrodes placed in multiple areas on the scalp. EEG abnormalities in hepatic coma were described in 1950.Citation58 One of the more constant features in the studies of HE is the presence of a generalized slowing of background EEG activity, which appears to be a constant, progressive, and a quantifiable finding. It is important to note that EEG slowing is nonspecific as it is observed in other metabolic and drug-induced encephalopathies.Citation4
Evoked potentials
Evoked potentials are electrical potentials, elicited in conjunction with sensory, motor, and cognitive events. They are a measure of the conduction and function of afferent pathways between stimulated peripheral nerves and the cortex. Abnormalities of visual evoked potentials, brainstem auditory evoked potentials, and somatosensory evoked potentials have been demonstrated in HE, but the sensitivity of the techniques appear to vary significantly between studies.Citation4 P300 is a cognitive evoked potential elicited when a subject receives an infrequent stimulus embedded within a group of irrelevant stimuli (oddball paradigm). The subject is asked to identify the oddball stimulus and a potential is elicited independent of the sensory modality being stimulated.Citation4 The potential occurs ~300 ms after the stimulus, hence its name. The P300 appears to be useful as an investigative research tool in lower grades of HE.Citation4
Critical flicker frequency
The critical flicker frequency (CFF) of an individual is the highest frequency in Hertz that an individual perceives the “flicker” of a flickering light. All frequencies above that threshold will be observed to be continuous. CFF is thought to reflect both the efficiency of the visual pathways and the cerebral cortex. A single study has used CFF in a wellcharacterized cohort of patients with cirrhosis and healthy controls.Citation59 CFF thresholds were found to be significantly lower in patients with mHE and overt HE than in healthy controls or unimpaired patients. Furthermore, they were able to define a threshold value of 39 Hz, which divided impaired from unimpaired patients.
Brain imaging
Currently, there is no established imaging modality or technique for the assessment of HE in clinical practice. The most widely utilized modality is magnetic resonance imaging (MRI), with the most consistently reported sequence being MRS. MRS is a noninvasive technique used to determine the biochemical profile of a region of interest in vivo, thus giving information regarding the metabolic processes within that region. Several investigators have found consistent abnormalities in the MRS data from patients with HE.Citation60
Other imaging modalities, such as PET, have been used in one case series,Citation29 but have not been replicated by other groups. Similarly, techniques such as magnetoencephalography have been described in the research setting,Citation61 but worldwide, very few centers have this equipment available.
Treatment of hepatic encephalopathy
No truly specific treatment for HE exists, as the exact pathogenesis is unknown. The pharmacological treatments that are currently used in clinical practice are directed toward reducing the production and absorption of gut-derived ammonia.
Antibiotics
Antibiotics have been used to treat HE since the 1950s.Citation62 The nonabsorbable antibiotic, neomycin, reduces bacterial ammonia production in the colon, by altering the normal gut flora. Neomycin is considered to be nonabsorbable, but a small percentage is absorbed, causing ototoxic and nephrotoxic side effects.Citation63 Neomycin is now rarely used as rifaximin, which lacks these side effects, has become available more recently.
Rifaximin is a nonabsorbable derivative of rifamycin with a broad spectrum of activity against aerobic and anaerobic gram-positive and gram-negative organisms. It has a bioavailability of <0.4%, and ~97% of the drug is excreted in feces unchanged.Citation64 These pharmacokinetic properties make it an attractive option for the treatment of HE. Clinical trials have compared rifaximin to lactulose or neomycin for treatment of HE. Rifaximin demonstrates a general trend toward better efficacy, compared with lactulose or neomycin, but long-term safety data are lacking.Citation65 More recent papers have found rifaximin to be highly effective in the management of HE for the treatment, maintenance of remission, and reduction in hospitalizations of chronic liver disease patients.Citation66,Citation67 However, the recent EASL/AASLD guidelines only recommend its use as an add-on therapy to lactulose, not as a first-line or sole treatment agent.Citation1,Citation2
Lactulose
Lactulose is a synthetic disaccharide taken orally, primarily used to treat constipation. It has been used to treat HE since the 1960s.Citation68 When it reaches the colon, lactulose is metabolized by bacteria, predominantly to lactic acid. The fecal pH drops, favoring growth of lactose-fermenting organisms and suppressing organisms such as Bacteroides spp., which are ammonia formers. Additionally, it is thought that fecal acidity reduces ionization and subsequent absorption of ammonia. It has been demonstrated that lactulose at least doubles the colonic output of bacterial mass and soluble nitrogen, which can then no longer be absorbed.Citation69 Side effects of treatment include flatulence, diarrhea, and abdominal pain.
l-Ornithine l-aspartate
l-Ornithine l-aspartate (LOLA) is a treatment directed at removal of circulating ammonia. Ornithine promotes hepatic removal of ammonia by stimulating residual hepatic urea cycle activity through action of ornithine carbamoyltransferase and carbamoylphosphate synthetase. Additionally, ornithine and aspartate are both substrates for the urea cycle. In perivenous hepatocytes, ornithine and aspartate combine with α-ketoglutarate to produce GLU. GLU is used by skeletal muscle and brain to use ammonia, via the action of GS to produce GLN, reducing the amount of circulating ammonia.Citation70 One study has demonstrated that intravenous LOLA ameliorates deleterious psychometric effects of GLN in Child’s grade B and C patients.Citation71 A study of overt HE patients randomized to receive either oral LOLA or lactulose for 2 weeks found that while both agents reduced circulating ammonia levels, use of LOLA was also associated with significant improvements in mental status, psychometric parameters, and EEG activity.Citation72 A meta-analysis of three randomized, controlled studies of the LOLA use in 212 patients with HE concluded that LOLA is of benefit in overt HE, but the currently available data do not support its use in mHE.Citation73
Nutritional therapies
Vegetable-based protein
Contrary to previous reports, patients with HE should not be protein restricted. They are often in a catabolic state and require 1–1.5 g/kg of protein per day. Vegetable protein tends to be better tolerated than meat protein. Additionally, due to their high fiber content, vegetable proteins increase colonic motility, as well as improve fecal nitrogen output.Citation74,Citation75 However, the clinical utility of a vegetable protein-based diet is limited by poor patient compliance and is not used routinely in many centers.
Prebiotics, probiotics, and symbiotics
Prebiotics are nondigestible food ingredients, stimulating growth of select colonic bacteria to improve host health, whereas probiotics are live microbial food supplement improving host gut microbial balance. Symbiotics are a combination of the two. A meta-analysis demonstrated that supplementing the diet with pre-, pro-, and symbiotics can lead to significant improvements in patients with MHE, suggesting that their inclusion may have clinical benefits. Furthermore, the supplements were well tolerated.Citation76
Branched-chain amino acids
Administration of preparations high in BCAAs and low in aromatic amino acids (AAAs) is based on reducing transmission of AAAs across the blood–brain barrier, thus reducing production of false neurotransmitters, which can contribute to encephalopathy.Citation75 Although BCAAs have been shown to improve both recovery and duration of hospital admissions,Citation77 a Cochrane systematic review found that BCAAs do not have significant beneficial effects on patients with HE. Studies involving BCAAs tend to be limited by short follow-up periods and poor methodological quality.Citation78 Higher quality studies are required in this area.
Orthotopic liver transplantation
Orthotopic liver transplantation (OLT) is the definitive treatment for HE arising in both acute liver failure (ALF) and chronic liver disease (CLD). However, its urgency depends on the cause and nature of liver failure. In patients with ALF who are progressing to cerebral edema and HE, an OLT is crucial to survival and urgent discussion with a liver transplant center is required to prevent deterioration to cerebral coning and brain death. In patients with CLD, HE tends to be chronic and fluctuating. It is considered to be one of the cardinal manifestations of decompensating liver disease. OLT in this group of patients tends to be determined according to need and prospective survival via established CLD criteria.Citation79
Conclusion
HE is a neuropsychiatric syndrome, with symptoms existing on a continuum. Early recognition and management is imperative in optimizing outcome, particularly in the context of the timing of OLT. With the right treatment, most patients with overt HE can lead relatively normal lives with reasonable neuropsychological function. Stability of underlying liver function and prompt treatment of precipitating factors, such as variceal bleeding and infections, is crucial to this. However, it should be remembered that in the acute setting, HE is the hallmark of acute liver failure and that urgent OLT may be required.
Acknowledgments
All the authors acknowledge the support of the National Institute for Health Research Biomedical Research Centre at Imperial College London for infrastructure support.
Disclosure
The authors report no conflicts of interest in this work.
References
- American Association for the Study of Liver, European Association for the Study of the LiverHepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the study of the liver and the American Association for the study of liver diseasesJ Hepatol201461364265925015420
- VilstrupHAmodioPBajajJHepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the study of liver diseases and the European Association for the study of the liverHepatology201460271573525042402
- HäussingerDSchliessFPathogenetic mechanisms of hepatic encephalopathyGut20085781156116518628377
- MontagneseSAmodioPMorganMYMethods for diagnosing hepatic encephalopathy in patients with cirrhosis: a multidimensional approachMetab Brain Dis200419328131215554423
- ButterworthRFPathophysiology of hepatic encephalopathy: a new look at ammoniaMetab Brain Dis200217422122712602499
- ShawcrossDJalanRThe pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammationCell Mol Life Sci200562192295230416158192
- HahnMMassenONenckiMPavlovIDie Eck’sche fistel zwischen der unteren hohlvene und der pfortader und ihre folgen fur den organismusArch Exp Pathol Pharm189332161210
- NenckiMPawlowJPZalenskiJUeber den ammoniakgehalt des blutes und der organe und die harnstoff- bildung bei den saugethieren [On the subject of ammonia content of blood and organs and urea formation in mammals]Archiv Fuer Experimentelle Pathologie und Pharmakologie1896372651 German
- GabuzdaGPhillipsGDavidsonCReversible toxic manifestations in patients with cirrhosis of the liver given cation-exchange resinsN Engl J Med1952246412413014890821
- ButterworthRGiguèreJMichaudJLavoieJLayrarguesGAmmonia: key factor in the pathogenesis of hepatic encephalopathyNeurochem Pathol198761–21123306479
- GregoriosJMozesLNorenbergMMorphologic effects of ammonia on primary astrocyte cultures. II. Electron microscopic studiesJ Neuropathol Exp Neurol19854444044144040156
- ChenSMahadevanVZieveLVolatile fatty acids in the breath of patients with cirrhosis of the liverJ Lab Clin Med19707546226275444347
- ZieveLDoizakiWMZieveJSynergism between mercaptans and ammonia or fatty acids in the production of coma: a possible role for mercaptans in the pathogenesis of hepatic comaJ Lab Clin Med197483116284808653
- MorganMBoltonCMorrisJReadAMedium chain triglycerides and hepatic encephalopathyGut19741531801844841275
- FischerJYoshimuraNAguirreAPlasma amino acids in patients with hepatic encephalopathyAm J Surg1974127140474808685
- IberFLRosenHLevensonSMChalmersTCThe plasma amino acids in patients with liver failureJ Lab Clin Med195750341742513463457
- SoetersPWeirGEbeidAFischerJInsulin, glucagon, portal systemic shunting, and hepatic failure in the dogJ Surg Res1977233183188886853
- CurzonGKantamaneniBFernandoJWoodsMCavanaghJEffects of chronic porto-caval anastomosis on brain tryptophan, tyrosine and 5-hydroxytryptamineJ Neurochem1975245106510701141890
- MoroniFLombardiGMonetiGCortesiniCThe release and neosynthesis of glutamic acid are increased in experimental models of hepatic encephalopathyJ Neurochem19834038508546131108
- RaabeWSynaptic transmission in ammonia intoxicationNeurochem Pathol198761–21451662819792
- HouraniBCerebrospinal fluid glutamine as a measure of hepatic encephalopathyArch Intern Med1971127610335578559
- BasileAJonesEAmmonia and GABA-ergic neurotransmission: interrelated factors in the pathogenesis of hepatic encephalopathyHepatology1997256130313059185743
- LevyLJLeekJLosowskyMSEvidence for gamma-aminobutyric acid as the inhibitor of gamma-aminobutyric acid binding in the plasma of humans with liver disease and hepatic encephalopathyClin Sci (Lond)19877355315343677559
- SherlockSDooleyJDiseases of the Liver and Biliary System11 edOxfordBlackwell Science2002
- AnholtRRPedersenPLDe SouzaEBSnyderSHThe peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membraneJ Biol Chem198626125765833001071
- BanatiRVisualising microglial activation in vivoGlia200240220621712379908
- ItzhakYNorenbergMAmmonia-induced upregulation of peripheral-type benzodiazepine receptors in cultured astrocytes labeled with [3H] PK 11195Neurosci Lett19941771–235387824177
- LavoieJLayrarguesGButterworthRIncreased densities of peripheral-type benzodiazepine receptors in brain autopsy samples from cirrhotic patients with hepatic encephalopathyHepatology19901158748782161396
- CagninATaylor-RobinsonSDFortonDMBanatiRBIn vivo imaging of cerebral “peripheral benzodiazepine binding sites” in patients with hepatic encephalopathyGut200655454755316210399
- ShawcrossDWrightGOlde DaminkSJalanRRole of ammonia and inflammation in minimal hepatic encephalopathyMetab Brain Dis200722112513817260161
- LiuQDuanZHaDBengmarkSKurtovicJRiordanSSynbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosisHepatology20043951441144915122774
- HäussingerDKircheisGFischerRSchliessFDahlSHepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema?J Hepatol20003261035103810898326
- HäussingerDLaubenbergerJvom DahlSProton magnetic resonance spectroscopy studies on human brain Myo-inositol in hypoosmolarity and hepatic encephalopathyGastroenterology19941075147514807926510
- Parsons-SmithBSummerskillWDawsonASherlockSThe electroencephalograph in liver diseaseLancet1957270700186787113482229
- ConnHOLeevyCMVlahcevicZRComparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trialGastroenterology1977724 pt 157358314049
- ZeegenRDrinkwaterJDawsonAMethod for measuring cerebral dysfunction in patients with liver diseaseBMJ1970257106336365429105
- RikkersLJenkoPRudmanDFreidesDSubclinical hepatic encephalopathy: detection, prevalence, and relationship to nitrogen metabolismGastroenterology1978753462469680502
- TarterREHegedusAMVan ThielDHSchadeRRGavalerJSStarzlTENonalcoholic cirrhosis associated with neuropsychological dysfunction in the absence of overt evidence of hepatic encephalopathyGastroenterology1984866142114276714571
- FerenciPHepatic encephalopathy – definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998Hepatology200235371672111870389
- GroenewegMQueroJCDe BruijnISubclinical hepatic encephalopathy impairs daily functioningHepatology199828145499657095
- BergnerMBobbittRCarterWGilsonBThe sickness impact profile: development and final revision of a health status measureMed Care19811987878057278416
- SchomerusHHamsterWQuality of life in cirrhotics with minimal hepatic encephalopathyMetab Brain Dis2001161–2374111726087
- WeinCKochHPoppBOehlerGSchauderPMinimal hepatic encephalopathy impairs fitness to driveHepatology200439373974514999692
- BajajJHafeezullahMHoffmannRSaeianKMinimal hepatic encephalopathy: a vehicle for accidents and traffic violationsAm J Gastroenterol200710291903190917640323
- GroenewegMMoerlandWQueroJHopWKrabbePSchalmSScreening of subclinical hepatic encephalopathyJ Hepatol200032574875310845661
- SoodGKSarinSKMahaptraJBroorSLComparative efficacy of psychometric tests in detection of subclinical hepatic encephalopathy in nonalcoholic cirrhotics: search for a rational approachAm J Gastroenterol19898421561592916526
- MarchesiniGZoliMDondiCPrevalence of subclinical hepatic encephalopathy in cirrhotics and relationship to plasma amino acid imbalanceDigest Dis Sci198025107637687428585
- WeissenbornKEnnenJSchomerusHRückertNHeckerHNeuropsychological characterization of hepatic encephalopathyJ Hepatol200134576877311434627
- ReitanRThe relation of the trail making test to organic brain damageJ Consult Psychol195519539339413263471
- WechslerDWechsler Adult Intelligence Scale – RevisedNew YorkPsychological Corporation1981
- Romero GómezMCórdobaJJoverRNormality tables in the spanish population for psychometric tests used in the diagnosis of minimal hepatic encephalopathyMed Clin (Barc)2006127724624916942726
- AmodioPCampagnaFOlianasSDetection of minimal hepatic encephalopathy: normalization and optimization of the psychometric hepatic encephalopathy score. A neuropsychological and quantified EEG studyJ Hepatol200849334635318602716
- LearmonthYDlugonskiDPiluttiLSandroffBKlarenRMotlRPsychometric properties of the fatigue severity scale and the modified fatigue impact scaleJ Neurol Sci20133311–210210723791482
- PrcicAAganovicDHadziosmanovicOSickness impact profile (SIP) score, a good alternative instrument for measuring quality of life in patients with ileal urinary diversionsActa Informatica Medica201321316024167383
- TreanorCDonnellyMA methodological review of the short form health survey 36 (SF-36) and its derivatives among breast cancer survivorsQual Life Res201524233936225139502
- SchatzPBrowndykeJApplications of computer-based neuropsychological assessmentJ Head Trauma Rehabil200217539541012802251
- TartaglioneEVDerlethMYuLIoannouGNCan computerized brain training games be used to identify early cognitive impairment in cirrhosis?Am J Gastroenterol2014109331632324594947
- FoleyJMWatsonCWAdamsRDSignificance of the electroencephalographic changes in hepatic comaTrans Am Neurol Assoc19505116116514788100
- KircheisGCritical flicker frequency for quantification of low-grade hepatic encephalopathyHepatology200235235736611826409
- GroverVPDresnerMAFortonDMCurrent and future applications of magnetic resonance imaging and spectroscopy of the brain in hepatic encephalopathyWorld J Gastroenterol200612192969297816718775
- TimmermannLButzMGrossJImpaired cerebral oscillatory processing in hepatic encephalopathyNeurophysiol Clin20081192265272
- DawsonANeomycin in the treatment of hepatic comaLancet1957270700812631268
- FerenciPHernethASteindlPNewer approaches to therapy of hepatic encephalopathySemin Liver Dis199616033293388989818
- AdachiJDuPontHRifaximin: a novel nonabsorbed rifamycin for gastrointestinal disordersClin Infect Dis200642454154716421799
- de MeloRCharneskiLHilasORifaximin for the treatment of hepatic encephalopathyAm J Health-Syst Pharm200865981882218436728
- BassNMMullenKDSanyalARifaximin treatment in hepatic encephalopathyN Engl J Med2010362121071108120335583
- KimerNKragAMollerSBendtsenFGluudLLSystematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathyAliment Pharmacol Ther201440212313224849268
- BircherJMüllerJGuggenheimPHaemmerliUTreatment of chronic portal-systemic encephalopathy with lactuloseLancet196628774438908934159616
- WeberFLJrBanwellJGFresardKMCummingsJHNitrogen in fecal bacterial, fiber, and soluble fractions of patients with cirrhosis: effects of lactulose and lactulose plus neomycinJ Lab Clin Med198711032592633611949
- ButterworthRRoseCQuackGKircheisGl-Ornithine-l-aspartate in experimental portal-systemic encephalopathy: therapeutic efficacy and mechanism of actionJ Hepatol199828709537866
- ReesCEffect of l-ornithine-l-aspartate on patients with and without TIPS undergoing glutamine challenge: a double blind, placebo controlled trialGut200047457157410986219
- PooJLGóngoraJSánchez-AvilaFEfficacy and safety of l-ornithine-l-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathyAnn Hepatol20065428128817151582
- JiangQJiangXZhengMChenYl-Ornithine-l-aspartate in the management of hepatic encephalopathy: a meta-analysisJ Gastroenterol Hepatol200924191418823442
- PrakashRMullenKMechanisms, diagnosis and management of hepatic encephalopathyNat Rev Gastroenterol Hepatol20107951552520703237
- RiordanSMWilliamsRTreatment of hepatic encephalopathyN Engl J Med199733774734799250851
- ShuklaSShuklaAMehboobSGuhaSMeta-analysis: the effects of gut flora modulation using prebiotics, probiotics and synbiotics on minimal hepatic encephalopathyAliment Pharmacol Ther201133666267121251030
- MutoYSatoSWatanabeAEffects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosisClin Gastroenterol Hepatol20053770571316206505
- Als-NielsenBKoretzRLKjaergardLLGluudCBranched-chain amino acids for hepatic encephalopathyCochrane Database Syst Rev20032CD00193912804416
- NeubergerJGimsonADaviesMSelection of patients for liver transplantation and allocation of donated livers in the UKGut200757225225717895356