Abstract
Thrombotic complications figure among the most frequent causes of mortality in diabetic ketoacidosis (DKA) and hyperosmolar state. We report the case of a 55-year-old woman presenting with DKA whereby a newly discovered patent foramen ovale was found due in part to the observation of bilateral deep vein thrombosis in legs, bilateral multiple pulmonary embolisms, and left subclavian acute artery thrombosis. Diabetes is known as a hypercoagulability state, and DKA is rising as a risk factor for vascular events. The importance of prophylactic anticoagulation should be emphasized in this setting.
Case presentation
A 55-year-old nonsmoking unmedicated black woman without past surgical or medical history apart from mild untreated hypertension presented with deteriorated general condition, lethargy, and confusion. There was no personal or family history of thrombosis. A few weeks prior to admission, she had polyuria, polydipsia, and polyphagia. Laboratory findings mainly showed diabetic ketoacidosis (DKA) in the context of a new-onset diabetes with a pH of 7.22, partial pressure of carbon dioxide (pCO2) 12 mmHg, bicarbonates 5 mmol/L, random glucose 56.8 mmol/L, urinary ketones 7.8 mmol/L, sodium 158 mmol/L, chloride 111 mmol/L, potassium 7.9 mmol/L, urea 24.0 mmol/L, creatinine 302 µmol/L, international normalized ratio (INR) 1.16, partial thromboplastin time 25.5 s, and fibrinogen 5.92 g/L. The calculated osmolarity of 396.8 mOsm and intensity of the hyperglycemia evoked mixed features of hyperglycemic hyperosmolar state (HHS). A DKA protocol composed of intravenous insulin and normal saline fluid was started, and the latter rapidly changed to a quarter-normal saline and then dextrose 5% in water due to initial hypernatremia. Cerebral computed tomography scan showed no intracranial hemorrhage or other acute problems. No thromboprophylaxis was given. One of the two blood cultures returned positive for Gram-positive cocci in clusters 29.8 hours after admission for which vancomycin was started. Institutional Review Board approval as well as informed, written consent was not deemed necessary for the following retrospective review. All principles outlined in the Declaration of Helsinki were followed.
A peripherally inserted central catheter (PICC) line was installed on the left side on day 3 to facilitate fluid administration. The patient’s level of consciousness improved, as she was no longer confused. With regard to thromboprophylaxis, compression stockings were preferred to heparin because of thrombocytopenia (platelets at 199×109/L at arrival to 49 on day 4) with a normal coagulogram. Thrombotic thrombocytopenic purpura was contemplated, but no schistocyte was seen that day or on the two subsequent blood films. On day 4, the INR was slightly elevated at 1.33 (prothrombin time of 16.2 s) with a normal activated partial thromboplastin time of 27.5 s, a marginally low fibrinogen of 1.98 g/L, elevated D-dimers at >2 mg/L, and lactate dehydrogenase (LDH) of 334 U/L, revealing some extent of disseminated intravascular coagulation. On day 5, those values were stable, and the patient reported mild catheter-related discomfort that was relieved by acetaminophen. On day 6, platelet count was at 29×109/L and LDH was 848 U/L, and the patient was found to have dyspnea and tachypnea without chest pain, and left arm pain from shoulder to wrist as well as erythema without induration or edema. The patient had a blood pressure of 115/81 mmHg, heart rate 104/min, oxygen saturation 97%, respiratory rate 40/min, and temperature 36.7°C. On the left arm, no exudate was seen at the PICC line site. Radial pulse was nearly absent, and capillary refill was prolonged. There was hand numbness and motor function impairment from the elbow to the distal hand extremity. Arterial thrombosis and pulmonary emboli were suspected.
The vascular surgery team was contacted, and the patient immediately underwent a Doppler ultrasonography and angioscan which showed bilateral deep vein thrombosis (DVT) in legs, bilateral multiple pulmonary embolisms, and left subclavian artery thrombosis with significant extension in descending aorta. The concomitant events raised suspicions of paradoxical embolus through a patent foramen ovale (PFO). There was no evidence of cerebral involvement. A left subclavian thrombectomy was performed. The venous aspect of the clot clinically heightened the probability of a PFO. This was confirmed by transesophageal echocardiography (TOE) which showed left-to-right and spontaneous right-to-left shunts through a PFO but no thrombus. Unfractionated heparin was given as treatment for pulmonary embolisms as the patient was hemodynamically stable. Platelet transfusions were given as platelets count reached 25×109/L. Bacteria in the previously positive blood culture was identified as Micrococcus sp., a commensal organism considered nonpathogenic, so vancomycin was discontinued. Over 2 days, creatine kinase went from a high of 25,367 U/L to 6,331 U/L. Factor V (506) Leiden mutation and prothrombin 20210A mutation were ruled out. At discharge, the patient had regained most of her left arm’s motricity and had scheduled physiotherapy appointments. Warfarin bridging was done using tinzaparin until therapeutic INRs of 2–3 were reached. Long-term – possibly lifelong – anticoagulation was discussed, while PFO closure was not promoted.
Discussion
Diabetes is known as a hypercoagulability state, making it a risk factor for stroke and heart disease, mainly because of endothelial abnormalities, coagulation activation, hypofibrinolysis, and chronic platelet hyperactivity.Citation1 Acute hyperglycemia is believed to boost coagulation by its positive impact on factor VII and factor VIII activity as well as on tissue factor pathway inhibitor levels. Hyperinsulinemia also contributes as it increases plasminogen-activator inhibitor type 1 level, a substance isolated in disproportionate quantity in atheromatous material extracted from diabetic patients.Citation1
Moreover, thrombotic complications, for example, myocardial infarctions, thrombosis, and disseminated intravascular coagulation, are among the most frequent causes of mortality in DKA.Citation2 As a consequence, DKA is rising as a possible risk factor for vascular event, although no large study is currently available.
The prothrombotic state in DKA could be explained by paradoxical platelet behavior,Citation3,Citation4 coagulation,Citation3–Citation5 endothelium activation,Citation6 and diminution of the anticoagulation system.Citation6–Citation9 Treatment could normalize protein C activity and von Wille-brand factor concentration.Citation6 Global activation of the fibrinolytic system is also present in DKA. Altogether, these studies imply that diabetes is a hypercoagulability state and that DKA generates further hemostatic abnormalities ().
Table 1 Hematological parameters during diabetic ketoacidosis and after treatment
Is it probable that coagulation abnormalities related to DKA translate into vascular events? In a population-based case–control study, neither diabetes nor its complications (retinopathy, nephropathy, neuropathy, DKA) were considered as independent risk factors for DVT when controlling for nursing home confinement and hospitalization for major surgery or acute medical illness.Citation10 However, this study was not powerful enough to demonstrate a difference in DKA patients. An association was made by Keenan et al between uncomplicated diabetes, DKA or HHS, and in-hospital DVT or readmission for DVT in the subsequent 3 months.Citation11 Compared to patients with diabetes without complications, patients with DKA were not found to be at higher risk, while the HHS group did (hazard ratio =3.0).Citation11 It has been hypothesized that this difference is due to some risk factors of thrombosis as dehydration, hyperosmolarity, and hyperglycemia being possibly more pronounced in HHS. Therefore, although we know that DKA modifies coagulation dynamics, experimental design does not allow the identification of a direct link between DKA and vascular events. What about the risk of DVT associated with central venous catheter (CVC)? Two retrospective pediatric studies found an increase in the incidence of CVC-associated DVT in DKA patients.Citation7,Citation12
At the moment, DVT prophylaxis is not officially indicated as part of the management protocol of DKA, although some care centers have implemented DVT prophylaxis in DKA with signs of HHS.Citation13 HHS is recognized as a risk factor for DVT, and the Oxford Handbook of Clinical Medicine of 2014 advises using DVT thromboprophylaxis in case of hyperosmolar nonketotic diabetic coma, while it only mentions thrombosis as a complication of DKA without recommending prevention.Citation14,Citation15 As we previously saw, even with aggressive hydration, the hypercoagulability state persisted until resolution of DKA and sometimes after. Some cases of thrombosis in DKA occurred in patients receiving prophylactic anticoagulation, but it is thought that it can at least lower the risk in a context of accumulating cases of vascular events in DKA, for example, pulmonary embolism as initial presentation or within 72 hours.Citation16–Citation18 In this context, we agree that our relatively healthy patient would have benefited from DVT prophylaxis at arrival due to her DKA and complete immobility for 6 days. Anyhow, the HHS traits identified could have been stated as an indication for prophylaxis as there is evidence that this condition increases the risk of thrombosis, adding to our patient’s burden at arrival. It should also be emphasized that asymptomatic thrombocytopenia was a strong red flag of the platelet consumption due to the underlying developing thrombosis. It is thought that the patient developed her DVTs in the context of the DKA, which then traveled directly to the lungs, and via the PFO, to the left arm (). It might be argued that this supplementary thrombosis was part of a “thrombotic crisis” as venous and arterial thromboses are being studied as a continuous spectrum.Citation19 In situ arterial thrombosis surely remains a reasonable explanation to acknowledge and could be precipitated by DKA with mixed HHS features. However, the sudden onset in a previously asymptomatic patient and the proximity in timing with the venous thrombosis oriented us in another direction.
Figure 1 Schematic representation of deep venous thromboembolisms in the legs, bilateral pulmonary embolisms, and a paradoxical embolus in the left subclavian artery through a patent foramen ovale.
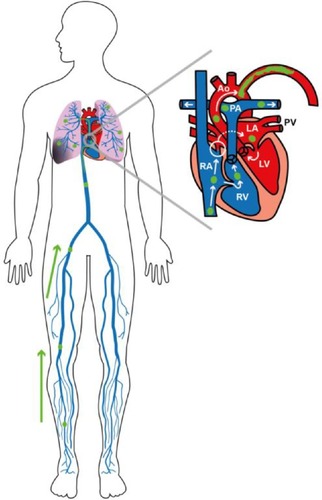
Suspicion for PFO was raised by the context of concomitant venous and arterial thrombosis and by the venous aspect of the clot noted during the thrombectomy. This visual constatation was based on the macroscopic particularities described between an arterial thrombus and a venous thrombus due to their composition differences. The first is usually depicted as a firm and white material made of platelets and fibrin, while the latter tends to be spongy with ill-defined layers of red material rich in erythrocytes, which is what was seen in this case.Citation20,Citation21 No histopathological examination of the thrombus was done, but it might be interesting to compare the clinical classification with the images of the angioscan as this technique appears to give satisfactory results for estimating the proportion of erythrocytes in a thrombus.Citation22 Thus, the combination of DVTs, pulmonary emboli, acute arterial thrombosis containing what was said to be a venous thrombus, and a PFO with a spontaneous right-to-left shunt certainly qualifies as a presumptive case of paradoxical embolus, while a definitive diagnosis would have required a thrombus to be seen during TOE. Medical treatment was chosen over surgical management as the added benefit to PFO closure remained unclear in this case. Studies about secondary prevention of embolic events among patients with PFO did not offer convincing evidence, although they mainly addressed cryptogenic stroke instead of peripheral thromboembolism.Citation23–Citation25
Conclusion
Recognizing the possible link between DKA and thromboembolism should prompt high suspicion for thrombosis, especially in a context of thrombocytopenia. It should raise awareness about the importance of prophylactic anticoagulation, since pulmonary embolism is a serious condition and approximately 25% of people have a PFO, making it possible for paradoxical embolism to happen as seen in this case of ischemic limb. In the light of the literature and our experience, a patient with DKA should be considered at increased risk of thrombosis until proven otherwise and benefit from thromboprophylaxis from admission if there is no contraindication.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- CarrMEDiabetes mellitus: a hypercoagulable stateJ Diabetes Complications200115445411259926
- HamblinPSToplissDJChosichNLordingDWStockigtJRDeaths associated with diabetic ketoacidosis and hyperosmolar coma. 1973–1988Med J Aust19891514142
- BuyukasikYIleriNSHaznedarogluICEnhanced subclinical coagulation activation during diabetic ketoacidosisDiabetes Care1998218688709589259
- IleriNSBuyukasikYKaraahmetogluSEvaluation of the haemostatic system during ketoacidotic deterioration of diabetes mellitusHaemostasis19992931832510844405
- BiliciMTavilBDogruODavutogluMBosnakMDiabetic ketoasidosis is associated with prothrombotic tendency in childrenPediatr Hematol Oncol20112841842421615248
- CarlGFHoffmanWHPassmoreGGDiabetic ketoacidosis promotes a prothrombotic stateEndocr Res200329738212665320
- WorlyJMFortenberryJDHansenIChamblissCRStockwellJDeep venous thrombosis in children with diabetic ketoacidosis and femoral central venous cathetersPediatrics2004113e57e6014702496
- SdogouTKossivaLKakleasKDeep vein thrombosis and pulmonary embolism in a child with diabetic ketoacidosis and protein s deficiency: a case reportHorm Res Paediatr20137911411823306559
- AlfredRWright-PascoeRAcute limb ischaemia in a septic patient with diabetic ketoacidosisWest Indian Med J20116021421621942130
- HeitJALeibsonCLAshraniAAPettersonTMBaileyKRMeltonLJ3rdIs diabetes mellitus an independent risk factor for venous thromboembolism?: a population-based case-control studyArterioscler Thromb Vasc Biol2009291399140519542020
- KeenanCRMurinSWhiteRHHigh risk for venous thromboembolism in diabetics with hyperosmolar state: comparison with other acute medical illnessesJ Thromb Haemost200751185119017403099
- GutierrezJABagatellRSamsonMPTheodorouAABergRAFemoral central venous catheter-associated deep venous thrombosis in children with diabetic ketoacidosisCrit Care Med200331808312544997
- BurzynskiJDKA and thrombosisCMAJ2005173132 author reply – 316027420
- LongmoreJMWilkinsonIBaldwinAWallinEOxford Handbook of Clinical Medicine9th edOxfordOxford University Press2014842844
- KianKEigerGAnticoagulant therapy in hyperosmolar non-ketotic diabetic comaDiabet Med20032060312823246
- QuigleyRLCurranRDStaglRDAlexanderJCJrManagement of massive pulmonary thromboembolism complicating diabetic ketoacidosisAnn Thorac Surg199457132213248179409
- El GhousseinHHegaziMAlajmiMDiabetic ketoacidosis presenting with saddle pulmonary embolismActa Endo (Buc)20095117120
- ShujaatASJMassive pulmonary embolism in diabetic ketoacidosis and non-ketotic hyperosmolar state: case series and review of the literatureClin Intensive Care2004157377
- Jerjes-SanchezCVenous and arterial thrombosis: a continuous spectrum of the same disease?Eur Heart J20052613415615791
- RumbautREThiagarajanPArterial, venous and microvascular hemostasis/thrombosisPlatelet-Vessel Wall Interactions in Hemostasis and Thrombosis Chap 6San Rafael, CAMorgan and Claypool Life Sciences20103542
- LippiGFranchiniMTargherGArterial thrombus formation in cardiovascular diseaseNat Rev Cardiol20118950251221727917
- KirchhofKWelzelTMeckeCZoubaaSSartorKDifferentiation of white, mixed, and red thrombi: value of CT in estimation of the prognosis of thrombolysis phantom studyRadiology2003228112613012728185
- FurlanAJReismanMMassaroJCLOSURE I InvestigatorsClosure or medical therapy for cryptogenic stroke with patent foramen ovaleN Engl J Med20123661199199922417252
- MeierBKalesanBMattleHPPC Trial InvestigatorsPercutaneous closure of patent foramen ovale in cryptogenic embolismN Engl J Med2013368121083109123514285
- CarrollJDSaverJLThalerDERESPECT InvestigatorsClosure of patent foramen ovale versus medical therapy after cryptogenic strokeN Engl J Med2013368121092110023514286
