Abstract
Globally, approximately 170 million people (representing approximately 3% of the population worldwide), are infected with hepatitis C virus (HCV) and at risk of serious liver disease, including chronic hepatitis. We propose a new quantum dots (QDs)-supported RNA oligonucleotide approach for the specific and sensitive detection of viral protein using a biochip. This method was developed by immobilizing a HCV nonstructural protein 5B (NS5B) on the surface of a glass chip via the formation of a covalent bond between an amine protein group and a ProLinker™ glass chip. The QDs-supported RNA oligonucleotide was conjugated via an amide formation reaction from coupling of a 5′-end-amine-modified RNA oligonucleotide on the surface of QDs displaying carboxyl groups via standard EDC coupling. The QDs-conjugated RNA oligonucleotide was interacted to immobilized viral protein NS5B on the biochip. The detection is based on the variation of signal of QDs-supported RNA oligonucleotide bound on an immobilized biochip. It was demonstrated that the value of the signal has a linear relationship with concentrations of the HCV NS5B viral protein in the 1 μg mL−1 to 1 ng mL−1 range with a detection limit of 1 ng mL−1. The major advantages of this RNA-oligonucleotide nanoparticle assay are its good specificity, ease of performance, and ability to perform one-spot monitoring. The proposed method could be used as a general method of HCV detection and is expected to be applicable to other types of diseases as well.
Introduction
Hepatitis C virus (HCV) is a major human health problem worldwide which can result in acute and chronic hepatitis, cirrhosis, and/or development of hepatocellular carcinoma. Citation1,Citation2 HCV is a single-stranded positive sense RNA virus, the 9.6 kb genome of which is organized to contain a single, large translational open-reading frame that encodes a large polyprotein precursor (3010–3030 amino acids).Citation3,Citation4 The genome is replicated via a negative-stranded intermediate by the nonstructural protein 5B (NS5B), that is viral RNA-dependent RNA polymerase, a crucial and unique component of viral replication machinery.Citation5–Citation15 Because of its essential role in viral replication, HCV NS5B viral protein is regarded as a prime target for antiviral therapy.
There has been considerable interest in the development of simple and reliable methods for detection of HCV for applications in diagnostic medicine. Presently, the most widely used method for diagnosing HCV is the detection of anti-HCV antibodies using a screening enzyme-linked immunosorbent assay (ELISA), based on recombinant proteins from the genome of HCV.Citation16,Citation17 Although it is highly specific, this assay has limitations. For example, it cannot detect viruses during the early stage of infection, at a time when antibodies against HCV antigens are not yet produced. The ELISA method sometimes generates false-positive or false-negative results.Citation18 In addition, antibody use is temperature-sensitive, has specific reaction conditions, and requires a secondary antibody conjugated with enzyme and fluorescent dyes.
Quantum dots (QDs), which are colloidal nanoparticles of semiconductor materials with a CdSe/ZnS core shell, have attracted considerable attention in the fields of nanotechnology and biotechnology, especially influorescence-based biological imaging applications.Citation19–Citation23 Semiconductor QDs possess remarkable optical characteristics compared with conventional organic fluorophores in terms of being bright, tunable, and having narrow fluorescence emission, as well as broad absorption spectra.Citation24–Citation26
Recently, there have been many studies on the development of fluorescence-based assays for HCV RNA detection.Citation27,Citation28 Furthermore, a variety of assay methods have been developed for the qualitative and quantitative detection of HCV. Among the developed methods, biosensors based on RNA-oligonucleotide nanoparticles have attracted significant attention and are a promising method for specific detection of oligonucleotide because of their high sensitivity, low cost, rapid response, portability (compatibility for miniaturization), and low labor requirement. Several papers have been published on RNA biosensors for HCV detection,Citation29,Citation30 however, to date, little has been reported on HCV viral protein detection using QDs-supported RNA oligonucleotide. Driven by the need to detect the presence of the HCV disease, herein it was demonstrated for the first time that RNA oligonucleotide with functional signal sequence could be used for screening and quantifying HCV viral protein with selectivity and sensitivity. It is shown here that QDs-conjugated RNA oligonucleotide could be used as a probe for the detection of HCV viral protein using biochip.
Materials and methods
Materials
EDC (N-(3-dimethylaminopropyl)-N’ ethylcarbodiimide hydrochloride), bovine serum albumin (BSA) and kanamycin were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Quantum dots (QDs) was purchased from Invitrogen Corporation (Carlsbad, CA). The Prolinker™- terminated glass slide was purchased from Proteogen (Seoul, Republic of Korea). All other chemicals were of the highest grade.
Conjugation of quantum dots and RNA oligonucleotide
An amine group with a terminal modification of NS5B RNA oligonucleotide (NS5Bnor) and NS5B RNA oligonucleotide mutant (NS5Bmut) were synthesized by BIONEER Co. Ltd. (Seoul, Republic of Korea) and carboxylterminated QDs525 was purchased from Invitrogen (Cals-bad, CA). The amino group of NS5B RNA oligonucleotide (NS5Bnor: H2N-5′-GGCCACAUUGUGAGGGGCUC-3′) and unspecific oligonucleotide (NS5Bmut: H2N-5′- CCCCACAUUCUCACCCCCUC-3′) used as a mutant of NS5B RNA oligonucleotide were first covalently conjugated onto the surface of the carboxyl-terminated QDs (10 pM, 1.25 μL). That is, 10 pM of QDs were conjugated with 400 pM of oligonucleotide with EDC 40 nM, 1 μL to activate amide bond formation to produce QDs-conjugated oligonucleotide (QDs-NS5B oligonucleotide) at a QDs:RNA oligonucleotide molar ratio of 1:40 for one hour at room temperature. There-after, QDs-oligonucleotide conjugate was collected using a centrifugal filtration at 15,000 rpm for 30 minutes, followed by several washing steps with a Tris buffer (50 mM Tris-HCl pH 7.4, 5 mM KCl, 100 mM NaCl, 1 mM MgCl2, and 0.1% NaN3). After centrifugal filtration and washing, the pellet of QDs-conjugated RNA oligonucleotide was dispersed by brief sonication (22 kHz, amplitude 12 μm, and sonication time 120 seconds) using a sonic dismembrator (Model F60 Sonic Dismembrator; Fisher Scientific, Fair Lawn, NJ).
Subcloning, expression, and purification of viral protein
The gene was amplified by a PCR with the primer set, sense: 5′-CGCGAATTCATGTCCTACACATGGACAGG- 3′; antisense: 5′-TTTCTCGAGTCGGTTGGGGAGCAGGTA- 3′, containing restriction enzyme sites of EcoRI/XhoI. PCR was run with the following conditions on a thermal cycler: denaturation at 94° for one minute, annealing at 62° for 30 seconds, and extension at 72° for 2.5 minutes. The sequence was repeated 35 times followed by a seven-minute final extension step at 72°. The PCR product was digested with EcoRI/XhoI, and then ligated into a EcoRI/XhoI digested expression vector pET 28a+ (Novagen, Madison, WI), and transformed into Escherichia coli DH5α (Stratagene, La Jolla, CA). The correct colony transformed with an insert gene, and was then transformed into E. coli BL21 (DE3) (Stratagene, La Jolla, CA) and plated on Luria-Bertani (LB) agar containing 50 μg mL−1 kanamycin. The transformant was grown in a 250 mL flask containing 50 mL LB medium supplemented with 50 μg mL−1 of kanamycin at 37° until the cell concentration reached an OD600 nm of 0.6, and isopropyl- thio-β-D-galactopyranoside (IPTG) at a final concentration of 0.1 mM, followed by overnight growth at 25°C with shaking at 180 rpm. Cells were harvested by centrifugation at 4000 rpm for 30 minutes at 4°C and resuspended in 100 mM potassium phosphate buffer (pH 7.5) containing 1 mM phenylmethylsulfonyl fluoride (PMSF). Cells were lysed by sonic dismembrator. The cell debris was removed by centrifugation at 15,000 rpm for 30 minutes. The supernatant was collected and the recombinant protein was purified using a Ni-nitrilotriacetic acid (Ni-NTA) affinity chromatography column (Qiagen, Hilden, Germany). The supernatant was equilibrated with buffer A (10 mM Tri-HCl, 500 mM NaCl, 5 mM imidazole, pH 8.0). The bound protein was eluted with buffer B (10 mM Tris-HCl, 500 mM NaCl, 500 mM imidazole, pH 8.0) at 4°. Purity of the protein was estimated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in the eluted fractions, using 10% polyacrylamide running gels.Citation31 The purity of the enzyme was estimated by SDS-PAGE. The protein concentration was determined as described by BradfordCitation32 with a BSA as standard. Enzyme samples were supplemented with 50% glycerol and stored at −20°C until use.
Electron microscopy
The free QDs and QD-conjugated RNA oligonucleotide were dried on a carbon Formvar-coated 200-mesh nickel grid, and were analyzed by transmission electron microscopy (TEM) (JEM-1400, JEOL Tokyo, Japan). The analysis of TEM was done at 80 kV.
Fluorescence microscopy
The recombinant HCV viral protein was immobilized directly onto the functional ProLinker-terminated surface. For the targeting of the specific RNA oligonucleotide, the QDs-conjugated RNA oligonucleotide was facilitated by spotting on immobilized HCV viral protein glass chip. After incubation for one hour at 25°C, the glass chip was then washed three times with the phosphate buffer (pH 7.2) for one minute. The glass chip was analyzed by a confocal laser scanning microscope LSM 510 META (Carl Zeiss, Jena, Germany). The signal intensity was determined by software for the LSM510 (LSM Image Browser). A histogram of the intensity was obtained from the region of the spotted chip. The value of the signal intensity was obtained by calculating and expressing it as the mean intensity.
Results and discussion
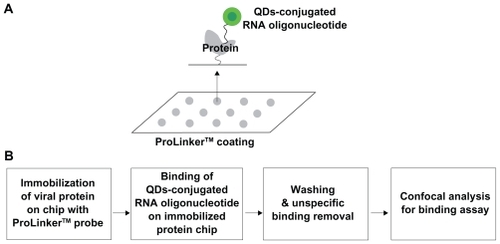
Schematic outline of the biochip
The biochip, a solid-phase assay method to investigate protein-RNA interaction, is becoming an attractive tool in biotechnology today for diagnostic and therapeutic purposes as well as for basic research. We designed a highly sensitive biochip using a ProteoChip coated with ProLinker, a novel calixcrown derivative with a bifunctional coupling property that permits efficient immobilization of captured viral protein on solid matrices and makes a simple analysis of protein-RNA interactions possible.Citation33 Specific detection of the viral protein was demonstrated by QDs-conjugated RNA oligonucleotide on the biochip. The full procedure for detection on biochip is as follows. First, the viral protein was spotted on a glass chip with carboxyl as the functional group. Second, the QDs-conjugated RNA oligonucleotide was bound on an immobilized chip. Third, the bound chip was washed to remove nonspecific binding. Fourth, it was analyzed to identify the specific detection of viral protein on the biochip. The targeting process for effective monitoring of the viral protein on the biochip is illustrated in . Feasibility of targeting and imaging was achieved by using QDs525 conjugates containing RNA oligonucleotide for specific NS5B viral protein.
QDs-conjugated RNA oligonucleotide
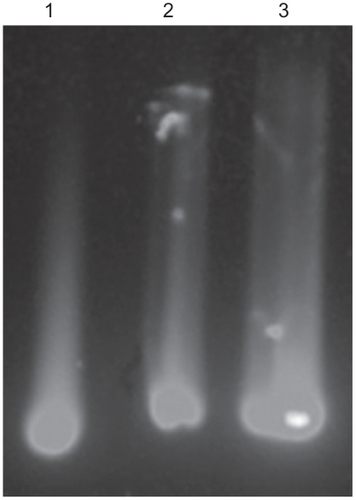
The QDs-supported RNA oligonucleotide was conjugated via amide formation from coupling of the 5′-end-amine- modified RNA oligonucleotide at the surface of QDs displaying carboxyl groups via standard EDC coupling. Formation of the QDs-conjugated RNA oligonucleotide was confirmed on 2% agarose gel at 100 V in Tris-acetate-EDTA buffer. Agarose gel electrophoresis showed a distinct mobile band pattern between the free QDs and the QDs-conjugated RNA oligonucleotide, confirming the formation of QDs-conjugated RNA oligonucleotide. The mobility shifts are compared in . On agarose gel, both the QDs-conjugated NS5Bnor RNA oligonucleotide and the NS5Bmut oligonucleotide showed less mobility shift than free QDs, thereby demonstrating conjugation by amide formation between the QDs and RNA oligonucleotide.
Figure 2 The conjugation pattern on 2% agarose gel electrophoresis was examined at UV excitation wavelength (345 nm), free QDs (lane 1), QDs-NS5Bnor RNA oligonucleotide (lane 2), and QDs-NS5Bmut RNA oligonucleotide (lane 3). Carboxyl-terminated QDs are conjugated with RNA oligonucleotide by an amine-carboxyl reaction.
Abbreviations: NS5B, nonstructural protein 5B; QDs, quantum dots; UV, ultraviolet.
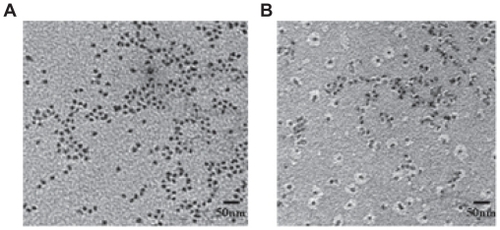
By TEM, both the QDs and the QDs-conjugated RNA oligonucleotide appeared spherical and fairly monodispersed (). In , only the free QDs nanoparticles are visible. In addition, the nanoparticles appeared to be evenly distributed on the surface. Both the QDs and the RNA oligonucleotide were conjugated in the EDC coupling reaction. The QDs (dark spot) was surrounded by a white disk of conjugated RNA oligonucleotide ().
Detection of viral protein on biochip
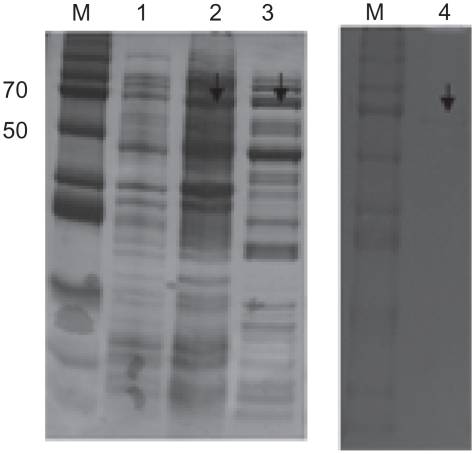
The recombinant HCV NS5B viral protein in the E. coli expression system was expressed and purified by Ni-NTA affinity chromatography. In the purification step, the protein was eluted in 1 mL fractions with 250 mM imidazole buffer. To verify the purity and homogeneity of the eluted NS5B, aliquots of eluted fractions were analyzed by Coomassie blue staining on SDS-PAGE. Eluates with a purity of 95% were pooled, dialyzed and stored with 50% glycerol in aliquots at −80°C. The HCV NS5B viral protein was purified by a single chromatography step on a Ni2+ affinity column. The C-terminally his-tagged HCV NS5B was visualized with a molecular mass of approximately 66 kDa on SDS-PAGE ().
Figure 4 Purification of HCV-NS5B viral protein. The SDS-PAGE 10% gel showing NS5B protein with his-tag. M, protein marker; lane 1, before induction form of NS5B; lane 2, total form of NS5B; lane 3, soluble form of NS5B; lane 4, his-Tag form of NS5B.
Abbreviations: HCV, hepatitis C virus; NS5B, non-structural protein 5B; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
We demonstrated the specific interaction between QDs-conjugated RNA oligonucleotide and immobilized HCV NS5B viral protein on a chip. As illustrated in , the QDs-NS5Bnor RNA oligonucleotide-treated conjugates showed high fluorescent signals on the chip. In , no fluorescence signal was detected with a NS5Bmut RNA oligonucleotide, because of its lack of affinity. The signal of QDs-conjugated NS5Bmut RNA oligonucleotide for protein-binding affinity was very similar to the background signal. shows that QDs-conjugated RNA oligonucleotide is selective for HCV NS5B viral protein at varying protein concentrations. The binding affinity by confocal assay with varying concentrations of the NS5B viral protein was examined, and it was observed that the signal intensity of the QDs-conjugated RNA oligonucleotide could be detected even at the lowest concentration (1 ng mL−1). The signal intensity was found to increase gradually by up to 1ng mL−1 of protein concentration. The insert figure in shows that the detection limit of this method was at the 1 ng mL−1 level.
Figure 5 A, B) Selective specificity of HCV-NS5B viral protein by QDs-conjugated RNA oligonucleotide. The NS5Bnor and NS5Bmut RNA oligonucleotides were bound on an immobilized viral protein chip, respectively. C, D) Effect of HCV-NS5B viral protein concentrations on binding assay.
Abbreviations: HCV, hepatitis C virus; NS5B, non-structural protein 5B; NS5Bnor, normal NS5B; NS5Bmut, mutant NS5B; QDS, quantum dots.
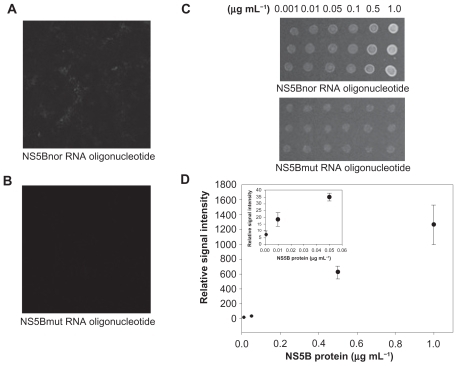
These results suggest the NS5B RNA oligonucleotide could specifically target the HCV NS5B viral protein on an immobilized chip. These results show that the QDs-RNA oligonucleotide could be effective for the quantification of HCV NS5B viral protein. To confirm a competition reaction of specific binding, BSA was used as a negative control protein. No fluorescence signal was detected with BSA (data not shown). Using the QDs-RNA oligonucleotide, we developed a HCV biosensor prototype biochip that could detect and quantify the HCV NS5B viral protein. Here, the specific RNA oligonucleotide was examined for binding to the HCV NS5B viral protein by using a QDs-conjugated imaging system. HCV-specific targeting and imaging using a QDs-conjugated RNA oligonucleotide agent was demonstrated. Preferential binding of QDs-conjugated RNA oligonucleotide for detection of HCV NS5B viral protein was determined by analyzing fluorescence intensity measured by confocal microscopy. The immobilized protein chip showed no binding to NS5Bmut RNA oligonucleotide, thus demonstrating selectivity for HCV NS5B viral protein. Imaging by semiconductor nanocrystal QDs allows for monitoring with intense light emission and accuracy.
During the last decade, there has been an increasing interest in using nanoparticles in biologic applications. The availability of colloidal semiconductor QDs with highly controlled optical properties in the nanometer size range has created widespread interest in the use of biotechnologic systems as a diagnostic platform and for biologic imaging. In this study, we report a QDs-conjugated RNA oligonucleotide probe for screening of HCV with high sensitivity and selectivity.
A RNA oligonucleotide sensor system that could quantify HCV NS5B viral protein using conjugated QDs-RNA oligonucleotide could provide an efficient strategy and a promising new platform for monitoring applications.
In summary, we have shown that QDs-RNA oligonucleotide can specifically recognize the HCV viral protein, and have demonstrated that this conjugated QDs-RNA oligonucleotide can interact on a designed biochip specifically and sensitively. This device could generate a QDs-conjugated biosensor prototype biochip for HCV diagnosis. The present visual HCV detection technique may avoid the limitations with its reported methods, namely, its high sensitivity, good specificity, simplicity, speed, and cost-effectiveness. This technique has potential applications in many fields, especially in multiple virus detection chip coupled with nanoparticle will find applications in clinic.
Conclusions
The results of this study indicate that the QDs-conjugated RNA oligonucleotide can recognize the HCV viral protein with a detection limit of 1 ng mL−1 specifically and sensitively on a designed biochip. This device could generate a nanoparticle-conjugated prototype biochip for HCV diagnosis.
Acknowledgments
We thank Professor Dr Myung Heejoon and Professor Dr Seong-Wook Lee for their very kind donation of plasmid pCite. This work was supported by SNU-KAERI Degree and Research Center for Radiation Convergence Sciences from the Korea Research Council of Foundation (KRCF) Science and Technology, South Korea.
Disclosure
The authors report no conflicts of interest in this work.
References
- WeissUHepatitisNature2005436929
- JameelSSiddiquiAHuKQGitnickGThe molecular biology of hepatitis virusPrinciples and Practice of Gastroenterology and HepatologyNew York, NYElsevier1994743757
- PawlotskyJMHepatitis C virus population dynamics during infectionCurr Top Microbiol Immunol200629926128416568902
- ReedKERiceCMOverview of hepatitis C virus genome structure, polyprotein processing, and protein propertiesCurr Top Microbiol Immunol2000242558410592656
- De FrancescoRTomeiLAltamuraSApproaching a new era for hepatitis C virus therapy: Inhibitors of the NS3-4A serine protease and the NS5B RNA-dependent RNA polymeraseAntiviral Res20035811612719002
- BehrensSETomeiLDe FrancescoRIdentification and properties of the RNA-dependent RNA polymerase of hepatitis C virusEMBO J19961512228598194
- AdachiTAgoHHabukaNThe essential role of C-terminal residues in regulating the activity of hepatitis C virus RNA-dependent RNA polymeraseBiochim Biophys Acta20021601384812429501
- FerrariEWright-MinogueJFangJWCharacterization of soluble hepatitis C virus RNA-dependent RNA polymerase expressed in Escherichia coliJ Virol199973164916549882374
- YamashitaTKanekoSShirotaYRNA-dependent RNA polymerase activity of the soluble recombinant hepatitis C virus NS5B protein truncated at the C-terminal regionJ Biol Chem199827315479154869624134
- IshiiKTanakaYYapCCExpression of hepatitis C virus NS5B protein: Characterization of its RNA polymerase activity and RNA bindingHepatology1999291227123510094969
- AgoHAdachiTYoshidaACrystal structure of the RNA- dependent RNA-polymerase of hepatitis C virusStructure1999714171412610574802
- BressanelliSTomeiLRousselACrystal structure of the RNAdependent RNA-polymerase of hepatitis C virusProc Natl Acad Sci U S A199996130341303910557268
- LohmannVOvertonHBartenschlagerRSelective stimulation of hepatitis C virus and pestivirus NS5B RNA polymerase activity by GTPJ Biol Chem1999274108071081510196156
- DhanakDDuffyKJJohnstonVKIdentification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNAdependent RNA-polymeraseJ Biol Chem2002277383223832712167642
- NeerjaKBAlainBWTanajiTTIdentification and characterization of coumestans as novel HCV NS5B polymerase inhibitorsNucl Acids Res2008361482149618203743
- CullenBRImmunology. Outwitted by viral RNAsScience200731732933017641188
- GriffinJSinghAKSenapatiDSize- and distance-dependent nanoparticle surface-energy transfer (NSET) method for selective sensing of hepatitis C virus RNAChemistry20091534235119035615
- GaudyCThevenasCTichetJUsefulness of the hepatitis C virus core antigen assay for screening of a population undergoing routine medical checkupJ Clin Microbiol2005431722172615814991
- AlivisatosPThe use of nanocrystals in biological detectionNat Biotechnol200422475214704706
- FortinaPKrickaLJSurreySNanobiotechnology: The promise and reality of new approaches to molecular recognitionTrends Biotechnol20052316817315780707
- PinaudFKingDMooreHPBioactivation and cell targeting of semiconductor CdSe/ZnS nanocrystals with phytochelatin-related peptidesJ Am Chem Soc20041266115612315137777
- BijuVItohTAnasASemiconductor quantum dots and metal nanoparticles: Syntheses, optical properties, and biological applicationsAnal Bioanal Chem20083912469249518548237
- GhasemiYPeymaniPAfifiSQuantum dot: Magic nanoparticle for imaging, detection and targetingActa Biomed20098015616519848055
- JunTBochuWLiancaiZQuantum Dots: A novel tool to discoveryPak J Biol Sci20069917922
- WilliamWYChangEDrezekRWater-soluble quantum dots for biomedical applicationsBiochem Biophys Res Comm200634878178616904647
- MedintzILMattoussiHClappARPotential clinical applications of quantum dotsInt J Nanomedicine2008315116718686776
- SchröterSFeuchtMZöllnerBQuantitative detection of hepatitis C virus RNA by light cycler PCR and comparison with two different PCR assaysJ Clin Microbiol2001393056305911526128
- BartoloméJLópez-AlcorochoJMCastilloIUltracentrifugation of serum samples allows detection of hepatitis C virus RNA in patients with occult hepatitis CJ Virol2007817710771517475654
- RiccardiCSDahmoucheKSantilliCVImmobilization of streptavidin in sol-gel films: Application on the diagnosis of hepatitis C virusTalanta20067063764318970820
- RiccardiCSKranzCKowalikJLabel-free DNA detection of hepatitis C virus based on modified conducting polypyrrole films at microelectrodes and atomic force microscopy Tip-integrated electrodesAnal Chem20088023724518034460
- LaemmliUKCleavage of structural proteins during the assembly of the head of bacteriophage T4Nature19702276806855432063
- BradfordMA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bindingAnal Biochem197672248254942051
- LeeYLeeEKChoYWProteoChip: A highly sensitive protein microarray prepared by a novel method of protein immobilization for application of protein-protein interaction studiesProteomics200332289230414673779