Abstract
Nano-hydroxyapatite/polyamide 66 (nHA/PA66) composite with good bioactivity and osteoconductivity was employed to develop a novel porous membrane with asymmetric structure for guided bone regeneration (GBR). In order to test material cytotoxicity and to investigate surface-dependent responses of bone-forming cells, the morphology, proliferation, and cell cycle of bone marrow stromal cells (BMSCs) of rats cultured on the prepared membrane were determined. The polygonal and fusiform shape of BMSCs was observed by scanning electronic microscopy (SEM). The proliferation of BMSCs cultured on nHA/PA66 membrane tested by the MTT method (MTT: [3-{4,5-dimethylthiazol-2yl}-2,5-diphenyl-2H-tetrazoliumbromide]) was higher than that of negative control groups for 1 and 4 days’ incubation and had no significant difference for 7 and 11 days’ culture. The results of cell cycle also suggested that the membrane has no negative influence on cell division. The nHA/PA66 membranes were then implanted into subcutaneous sites of nine Sprague Dawley rats. The wounds and implant sites were free from suppuration and necrosis in all periods. All nHA/PA66 membranes were surrounded by a fibrous capsule with decreasing thickness 1 to 8 weeks postoperatively. In conclusion, the results of the in vitro and in vivo studies reveal that nHA/PA66 membrane has excellent biocompatibility and indicate its use in guided tissue regeneration (GTR) or GBR.
Introduction
Guided tissue regeneration (GTR) using barrier membranes has been proven as an effective modality in periodontal therapy.Citation1 The barrier membrane technique was employed to guide bone regeneration in the bone defect site and given the name of “guided bone regeneration (GBR)” by some researchers.Citation2 Criteria for ideal barrier membranes include biocompatibility, cell occlusiveness, space making, tissue integration and clinical manageability. In the past twenty years, nonabsorbableCitation3–Citation6 and absorbable membranesCitation7–Citation11 had been studied and applied to GTR or GBR techniques. However, both the absorbable and nonabsorbable barrier membranes have their shortcomings and the available products are limited.Citation12–Citation14
The main disadvantage for absorbable membranes is unexpected absorption ahead of sufficient bone forming,Citation6 while the main disadvantage for nonabsorbable membranes is the need for a second surgery.Citation15
The nano-hydroxyapatite/polyamide 66 (nHA/PA66) composite developed by our research group is a biomimetic and bioactive material for bone repair engineering.Citation16 The nHA/PA66 composite dramatically resembles natural bone in its composition, structure, and mechanical properties, which is responsible for its good biocompatibility, osteoconductivity, and bioactivity.Citation17–Citation19 Our previous study revealed that the incorporation of nano-hydroxyapatite (nHA) in a polyamide 66 (PA66) matrix could improve properties of the membrane substantially. The elongation at break and the tensile strength suggest that the composite membrane (with 40 wt% of nHA) has good strength and toughness.Citation20
In order to overcome the drawbacks of the currently-used barrier membranes and to provide an optimal alternative, our hypothesis is to develop a novel nHA/PA66 membrane which is nonabsorbable in nature and does not require retrieval.
This study assesses the in vitro cytotoxicity and in vivo biocompatibility of the novel membrane. The morphology, proliferation, and cell cycle of bone marrow stromal cells (BMSCs) of rats cultured on the prepared membrane were determined to test the material’s cytotoxicity. In vivo biocompatibility was investigated in healthy Sprague-Dawley rats.
Materials and methods
Materials
PA66 with a viscosity-average molecular weight (Mv) of 18 kDa was obtained from BASF, (Ludwigshafen, Germany). The slurry of nano-hydroxyapatite used for the composite was prepared by our laboratory using the methods of wet synthesis and hydrothermal treatment.Citation21,Citation22
Fabrication of the nHA/PA66 membrane
The nHA/PA66 (4:6 in wt%) composite slurry was prepared according to previous work.Citation19 PA66 was completely dissolved in ethanol solution at 70°C. The nHA slurry was gradually added to the PA66/ethanol solution with vigorous stirring for 2 h. The composite slurry was left standing for at least 4 h at room temperature to remove the bubbles. The slurry was poured onto a glass plate to form an even liquid film, which was then evaporated at room temperature for 24 h to form a membrane, and washed repeatedly with deionized water.
Membrane characterization
The microstructure of the nHA/PA66 membrane was observed under scanning electron microscope (SEM). The membranes were carefully sectioned with a razor blade and mounted onto copper stubs. Prior to examination, each sample was coated with gold. A Hitachi S-450 SEM microscope at 20 kV was used to perform image analysis.
Cell culture
BMSCs were obtained from the tibiae and femora of young Sprague-Dawley rats and cultured in α-MEM culture medium supplemented with 20% fetal FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 0.219 mg/mL L-glutamine, 100 mM HEPES buffer (Gibco, USA) in a humidified incubator with 5% CO2 at 37°C. The culture media was changed every other day. The fourth passage BMSCs were used in the experiments.
Cell seeding
The nHA/PA66 membrane was prepared in a square form (2 cm × 3 cm), sterilized by autoclaving, placed into 6-well culture plate, and seeded at a density of 3 × 106 cells/well in 2 mL supplemented medium. The cell/membrane constructs were cultured in a humidified incubator at 37°C with 5% CO2 for 7 days. The medium was changed every other day. Cells cultured without membranes were assigned as control.
Cellular morphology
An inverted phase contrast microscope and a scanning electron microscope were used to determine cellular morphology.
BMSCs cultured on nHA/PA66 membrane were rinsed with phosphate buffered saline (PBS), fixed with 1% paraformaldehyde, subjected to graded alcohol dehydrations, rinsed with PBS, sputter-coated with gold, and examined with a scanning electron microscope (JSM-5900LV, Hitachi). The growth of cells on the scaffolds was observed by SEM at 24 h and 96 h.
Analysis of proliferation of the BMSCs
The proliferation of BMSCs cells cultured on the nHA/PA66 membrane was determined by the MTT assay using an HTS 7000 plus Bio Assay Reader (Perkin Elmer, USA). The medium was removed and 2 mL of MTT solution (5 mg/mL) was added to each well. Following incubation at 37°C for 4 h in a fully-humidified atmosphere at 5% CO2 in air, MTT was taken up by active cells and reduced in the mitochondria to insoluble purple formazan granules. Subsequently, the medium was discarded and the precipitated formazan was dissolved in dimethyl sulfoxide (DMSO) (150 mL/well). The optical density of the solution was evaluated using a microplate spectrophotometer at a wavelength of 490 nm.
Analysis of cell cycle
At 1, 4, 7 days, cultures were trypsinized and centrifuged at 1000 rpm for 8 min, then resuspended in 1 mL of 70% ethanol. Cell cycle was determined using a flow cytometer (EPLCS® ELITE, Coulter, USA).
In vivo biocompatibility – subcutaneous implant test
Nine Sprague-Dawley rats were anesthetized by an introabdominal injection of pentobarbital (Nembutal 3.5 mg/100 g BW) to undergo bilaterally dorsal subcutaneous implantation. Four subcutaneous pouches were created in the back of one rat, where membranes were implanted. After blunt dissection through the subcutaneous tissues, each rat received four pieces of membrane in the back. Skin incision was closed by simple interrupted sutures of monofilament nylon 4-0. The rats were randomly assigned into three groups with three in each group representing three different time points. The composites and surrounding tissue were obtained and processed for histological analysis at 1, 4, and 8 weeks after implantation. All samples were fixed in 10% buffered formalin, decalcified (K-CX solution, Falma Co., Tokyo, Japan), dehydrated, embedded in paraffin, and stained with hematoxylin and eosin. These samples were observed by optical microscope (Olympus, IX 70, Japan).
Statistical analysis
Statistical analysis was performed with SPSS 11.5. For all experiments, the results are expressed as mean ± standard deviation, ‘n’ value was 5, and independent experiments were performed three times. Analysis of variance (ANOVA) was performed, followed by Student-Newman-Keuls multiple comparison test. When a difference between groups was identified by ANOVA, a comparison of group means was performed using a Student’s t-test. The value of P < 0.05 was considered statistically significant.
Results and discussion
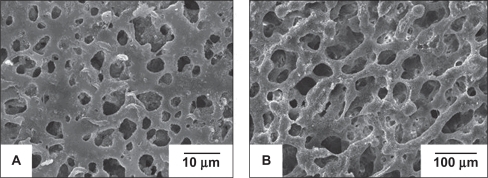
The microstructure of the membrane
shows that the membrane has an asymmetric porous structure, in which pores less than 10 μm are distributed on one side {microporous layer ()}, while pores ranging from 30 μm to 200 μm are located on the other side {macroporous layer ()}. The microporous layer of the membrane can prevent the fibrous connective tissue from migrating into the bony defect, while being able to permeate sufficient nutrients for the regenerated tissue. Meanwhile the spongy structure of the membrane can promote ingrowth of progenitor bone cells, leading to direct bonding between the original and regenerated tissue.Citation23 This particular layout features in the commercially available collagen bilayer membranes Bio-Gide®. Its dense and smooth outer surface is covered by a particularly dense film, aiming at preventing the invasion of undesirable gingival epithelium and connective tissue cells into the membrane-protected area. The inner rough side of Bio-Gide® promotes the ingrowth of periodontal ligament (PDL) cells, bone cells, and cementoblasts.Citation24
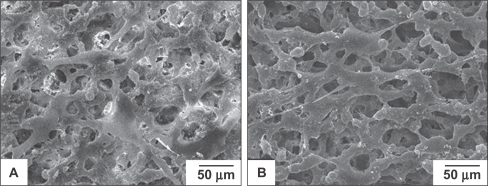
The growth of cells on the surface of porous nHA/PA66
The morphology of BMSCs anchored on nHA/PA66 as observed by SEM is shown in . The polygonal and fusiform BMSCs were well distributed over the membranes at various incubation periods. Cells cultured on the membrane for 96 h had dramatically reproduced and aggregated with each other to form cell layers. More filamentous fibers were formed on the surface of the cells, and cells penetrated into the pores of the membrane. This shows that the nHA/PA66 membrane is favorable for BMSCs attachment and spreading.
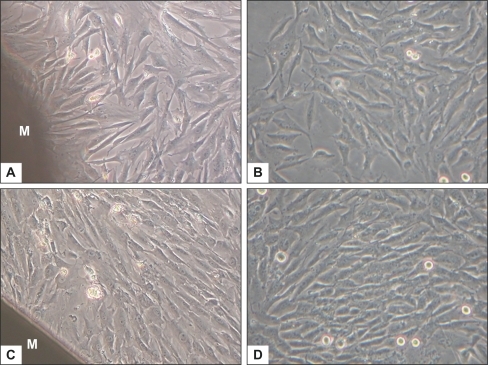
Observation under the inverted phase contrast microscope
shows representative phase-contrast micrographs of BMSCs cultured with or without the membrane, at day 4 and day 7. Comparing with , or with , cells beside the membrane exhibited the same morphologic characteristics – polygonal and spindle-like shapes – as in the control group. Cellular densities in both groups were similar at the same indicated period, with a marked increase of cell population at day 7. It shows that the nHA/PA66 membrane has no deleterious or cytotoxic effect on the morphology and proliferation of BMSCs.
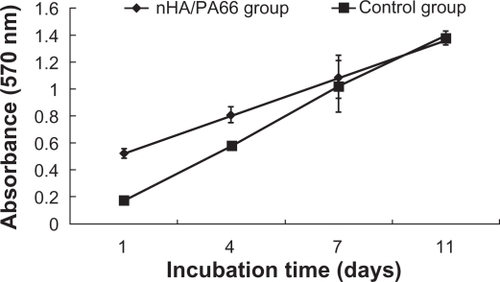
Effect of nHA/PA66 membranes on viability of the BMSCs
The proliferation of BMSCs cultured on the nHA/PA66 membrane was evaluated by MTT test (). The cell number increased with the culture time on both the tested group and control group. The proliferation of BMSCs cultured on the nHA/PA66 membrane was higher than that of the negative control group for 1 and 4 days’ incubation (P < 0.05). There was no significant difference of cell number between the two groups for 7 and 11 days’ culture (P > 0.05) (). This ascendant tendency of cell population demonstrates that nHA/PA66 membrane imposes little influence on BMSCs proliferation. PA66 is a polar compound containing –COOH, –NH2 and –NH–C(O)– groups, which can promote adsorption of proteins such as fibronectin from the medium to enhance the cells’ attachment.Citation25 The addition of nHA in the polymer matrix can also increase adsorption of proteinsCitation26 to facilitate cell attachment, spreading, and proliferation. The good hydrophilicity may be another reason for the excellent cell affinity of nHA/PA66 membrane.Citation20
Analysis of cell cycle
The distribution of different phases of BMSCs cultured with or without nHA/PA66 membrane is reported in . The cell cycle of BMSCs cultured with nHA/PA66 was not affected in comparison with the control group. Although most cells were blocked in the G0G1 phase due to contact inhibitation resulting from high cell density for 4 and 7 days’ incubation in both groups, cells in the S and G2M phases of the test group were higher than in the control group. The results suggest that the proliferation of BMSCs cultured on nHA/PA66 porous membrane is promoted. The results of in vitro experiments indicate that nHA/PA66 membrane has good cell affinity and excellent biocompatibility.
Table 1 Distribution in the different phases of BMSCs (control) and BMSCs cultured with nHA/PA66 membrane
Biocompatibility in vivo
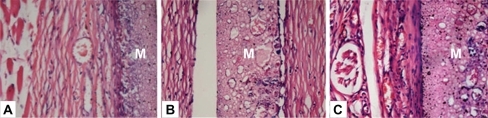
A subcutaneous implantation test is an important step to explore host response to foreign materials. Three time points were selected to investigate the tissue reaction at acute (one week), transitional (four weeks), and chronic phases (eight weeks) respectively. The wounds and implantation sites were free from suppuration and necrosis after subcutaneous implantation in all periods. Histological study revealed a fibrous capsid with inflammatory cellular infiltration around the membrane at 1 week (). It indicated that the implant induced a mild acute inflammation, which was characterized by the infiltration of inflammatory cells – mainly polymorphonuclear cells and lymphocytes – at the interface between the implant and tissue (). Observation at 4 weeks showed a reduced inflammatory infiltrate. Sparse neovascularization could be observed within the porous structure of the membrane. The capsule became better organized. At 8 weeks, the fibrous encapsulation was well-defined, thinner and more mature with pronounced vascularization around the membrane (). No severe inflammation, hemorrhage or necrosis was induced around the implants. Over the time, the surrounding connective tissues displayed a reduced inflammation process and the membrane was present at all evaluation points with no signs of resorption. Foreign body reaction, which is indicated by multinucleated giant cells, was not observed throughout the study period. Absence of a foreign body reaction negates the necessity of additional surgery for removal of the device.Citation27
Figure 5 Hematoxylin/eosin-stained sections of subcutaneously-implanted nHA/PA66 membrane and surrounding tissue, which were harvested at 1 (A), 4 (B), and 8 (C) weeks post-implantation (magnification:400×). In the photos, M denotes the nHA/PA66 membrane.
In our previous study, nano-hydroxyapatite/polyamide (nHA/PA) composite scaffolds were implanted in rabbit mandibles. At 8 weeks post-implantation, the boundary between material and host bone became unclear due to the sufficient formation of mature bone tissues which had ingrown into the pores of the artificial scaffold and bonded tightly with the material. With the implantation period prolonged, new bone regenerated and penetrated through the interconnective pores to the center of the scaffolds, increasing the quantity and density of the defective area. At 12 weeks, the interface between material and host bone was hardly detectable and formed a close union without any gap.Citation17 The previous study confirmed the extensive osteoconductivity and bone regeneration potential of the nano-hydroxyapatite/polyamide (nHA/PA) composite scaffolds.
As a novel membrane designed for GBR, the effect of nHA/PA66 membrane will be further investigated in our subsequent GBR experimental study. Within the limitation of the present study, the results indicate that the nHA/PA66 composite has desirable in vivo biocompatibility. Since the body didn’t exclude the membrane and the composite incorporated with surrounding tissue harmoniously, it is possible to maintain the membrane in situ without retrieval.
Conclusions
The interest in developing an ideal barrier membrane for GTR or GBR drives us to focus on preparing the novel nano-hydroxyapatite/polyamide 66 membrane. The results demonstrate that the n-HA/PA66 membrane is a gradient porous 3-D structure with a microporous dense layer on one side and a macroporous spongy layer on the other side. In vitro experiments show that nHA/PA66 membrane has good affinity for BMSCs attachment, and no negative effects on cell viability and proliferation. Subsequently, a subcutaneous implantation test was employed for in vivo evaluation of the bio-compatibility of the membrane. Macroscopic and histological observation revealed no foreign body reaction and a reduced inflammation process. The good biocompatibility of the membrane can meet the requirement of GBR. The nHA/PA66 membrane has the potential to be employed as a novel barrier membrane.
Acknowledgments and disclosures
This work was supported by China 973 fund (No. 2007-CB936102) and a grant from the State Key Laboratory of Oral Diseases (free application Project No. SKLODP007). The authors report no conflicts of interest in this work.
References
- KostopoulosLKarringTAugmentation of the rat mandible using guided tissue regenerationClin Oral Implants Res1994575827918912
- DahlinCSennerbyLLekholmULindeANymanSGeneration of new bone around titanium implants using a membrane technique: an experimental study in rabbitsInt J Oral Maxillofac Implants1989419252599578
- ZitzmannNUNaefRScharerPResorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regenerationInt J Oral Maxillofac Implants1997128448529425767
- SchenkRKBuserDHardwickWRDahlinCHealing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandibleInt J Oral Maxillofac Implants1994913298150509
- WangDMLinFCChenLYLaiJYApplication of asymmetric TPX membranes to transdermal delivery of nitroglycerinJ Control Release1998501871959685885
- NasserNJFriedmanAFriedmanMMoorEMosheiffRGuided bone regeneration in the treatment of segmental diaphyseal defects: a comparison between resorbable and non-resorbable membranesInjury2005361460146616243336
- ZhangYZSuBVenugopalJRamakrishnaSLimCTBiomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibersInt J Nanomedicine2007262363818203429
- HongHWeiJLiuCSDevelopment of asymmetric gradational-changed porous chitosan membrane for guided periodontal tissue regenerationCompos Part B Eng200738311316
- OwenGRJacksonJChehroudiBBurtHBrunetteDMA PLGA membrane controlling cell behaviour for promoting tissue regenerationBiomaterials2005267447745616039709
- HammerleCHLangNPSingle stage surgery combining transmucosal implant placement with guided bone regeneration and bioresorbable materialsClin Oral Implants Res20011291811168266
- KimKHJeongLParkHNBiological efficacy of silk fibroin nanofiber membranes for guided bone regenerationJ Biotechnol200512032733916150508
- LundgrenAKSennerbyLLundgrenDGuided jaw-bone regeneration using an experimental rabbit modelInt J Oral Max Surg199827135140
- NowzariHSlotsJMicrobiologic and clinical study of polytetrafluoroethylene membranes for guided bone regeneration around implantsInt J Oral Maxillofac Implants19951067737615319
- MachteiEEThe effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysisJ Periodontol20017251251611338304
- NocitiFHJrMachadoMAStefaniCMSallumEASallumAWAbsorbable versus nonabsorbable membranes and bone grafts in the treatment of ligature-induced peri-implantitis defects in dogs. Part I. A clinical investigationClin Oral Implants Res20011211512011251660
- WangXLiYWeiJde GrootKDevelopment of biomimetic nano-hydroxyapatite/poly(hexamethylene adipamide) compositesBiomaterials2002234787479112361617
- WangHLiYZuoYLiJMaSChengLBiocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineeringBiomaterials2007283338334817481726
- ZhangLLiYBWangXJWeiJPengXLStudies on the porous scaffold made of the nano-HA/PA66 compositeJ Mater Sci200540107110
- ZhangXLiYBZuoYLvGYMuYHLiHMorphology, hydrogen-bonding and crystallinity of nano-hydroxyapatite/polyamide 66 biocompositesCompos Part A Appl Sci Manuf200738843848
- LiJDZuoYChengXMYangWHWangHNLiYBPreparation and characterization of nano-hydroxyapatite/polyamide 66 composite GBR membrane with asymmetric porous structureJ Mater Sci Mater Med2009201031103819115093
- LiYBde WijnJKleinCPATvan de MeerSde GrootKPreparation and characterization of nanograde osteoapatite-like rod crystalsJ Mater Sci Mater Med19945252255
- SmithIOMcCabeLRBaumannMJMC3T3-E1 osteoblast attachment and proliferation on porous hydroxyapatite scaffolds fabricated with nanophase powderInt J Nanomedicine2006118919417722535
- CerroniLFilocamoRFabbriMPiconiCCaropresoSCondoSGGrowth of osteoblast-like cells on porous hydroxyapatite ceramics: an in vitro studyBiomol Eng20021911912412202171
- HillmannGSteinkamp-ZuchtAGeurtsenWGrossGHoffmannACulture of primary human gingival fibroblasts on biodegradable membranesBiomaterials2002231461146911829442
- HynesROIntegrins: versatility, modulation, and signaling in cell adhesionCell19926911251555235
- WeiGMaPXStructure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineeringBiomaterials2004254749475715120521
- LindeAAlberiusPDahlinCBjurstamKSundinYOsteopromotion: a soft-tissue exclusion principle using a membrane for bone healing and bone neogenesisJ Periodontol199364Suppl 11111611288295100