 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Diseases caused by bacterial and fungal pathogens are among the major health problems in the world. Newer antimicrobial therapies based on novel molecules urgently need to be developed, and this includes the antimicrobial peptides. In spite of the potential of antimicrobial peptides, very few of them were able to be successfully developed into therapeutics. The major problems they present are molecule stability, toxicity in host cells, and production costs. A novel strategy to overcome these obstacles is conjugation to nanomaterial preparations. The antimicrobial activity of different types of nanoparticles has been previously demonstrated. Specifically, magnetic nanoparticles have been widely studied in biomedicine due to their physicochemical properties. The citric acid-modified manganese ferrite nanoparticles used in this study were characterized by high-resolution transmission electron microscopy, which confirmed the formation of nanocrystals of approximately 5 nm diameter. These nanoparticles were able to inhibit Candida albicans growth in vitro. The minimal inhibitory concentration was 250 µg/mL. However, the nanoparticles were not capable of inhibiting Gram-negative bacteria (Escherichia coli) or Gram-positive bacteria (Staphylococcus aureus). Finally, an antifungal peptide (Cm-p5) from the sea animal Cenchritis muricatus (Gastropoda: Littorinidae) was conjugated to the modified manganese ferrite nanoparticles. The antifungal activity of the conjugated nanoparticles was higher than their bulk counterparts, showing a minimal inhibitory concentration of 100 µg/mL. This conjugate proved to be nontoxic to a macrophage cell line at concentrations that showed antimicrobial activity.
Introduction
In recent decades, several new infectious diseases have emerged or reemerged. In many cases, they have been the origin for entirely new and life-threatening infectious emergencies.Citation1,Citation2 The absence of effective antibiotics for the treatment of certain illnesses combined with the appearance of multidrug-resistance in related strains has prompted even greater urgency for innovative approaches in the control of infections.Citation3 Antimicrobial peptides (AMPs) form part of the first line of defense against pathogens of many organisms. Their use as anti-infective tools represents a novel approach for the development of antimicrobial therapies.Citation4,Citation5 Even though such peptides have been shown to be both broad spectrum (having antimicrobial, anticancer, immunomodulatory, wound healing and angiogenesis properties) and significantly potent in preclinical studies, this success has not been transmitted to clinical practice yet.Citation6 The major disadvantages of AMPs have been low stability, cytotoxicity, biodistribution, and high production costs.Citation4,Citation7
Development of nanoparticles for delivery or conjugation of AMPs could represent an alternative to bypass the abovementioned clinical obstacles.Citation8 In fact, the antimicrobial activity of different types of nanoparticles has been demonstrated. Some metals like silver, zinc, and copper exhibit antibacterial properties themselves, increasing the effect of these metals at nanoscale dimensions.Citation9 Magnetic nanoparticles (MNPs) could be a suitable carrier for AMPs due to their wide spectrum of activities (anticancer, antimicrobial, drug delivery, diagnosis) and their low toxicity demonstrated in vivo.Citation10–Citation12 The potential applications of manganese ferrite nanoparticles (MnFe2O4-NPs) for bioanalytical applications have been described, and it is perfectly possible to conjugate such nanoparticles to an anti-Sticholysin II monoclonal antibody, while preserving their antigenic recognition.Citation13 The isostructural substitution of Fe(II) by Mn(II) in the magnetite structure provides the possibility of an enhanced peroxidase-like activity and a better material ability to participate in redox reactions, because the manganese ion has a large variety of available oxidation states, for example, Mn(III), Mn(IV), Mn(V), Mn(VII).
Furthermore, iron oxide (eg, magnetite and maghemite) is not antibacterial in its bulk form but may exhibit antibacterial properties in the form of nanoparticles.Citation14 Such behavior is ascribed to the availability of a large fraction of the particle atoms at surface positions with an unsaturated coordination environment, and from this, enhanced reactivity. For example, magnetite nanoparticles coated with quaternary ammonium were bactericidal against Escherichia coli.Citation15 Additionally, bacitracin-conjugated iron oxide (Fe3O4) nanoparticles have shown higher antimicrobial activity against both Gram-positive and Gram-negative organisms, in comparison with the bacitracin peptide.Citation16 Additionally, ceragenin CSA-13-coated MNPs (MNP-CSA-13) exhibited strong antibacterial activity and the ability to prevent bacterial biofilm formation in different body fluids, with a significant decrease in its hemolytic activity when the molecule was immobilized on the nanoparticle surface.Citation17 In contrast, it has been reported that iron-containing nanoparticles may influence the ability of bacteria to absorb iron for growth, increase virulence, and inhibit AMP function.Citation18
Here, we present the intrinsic antifungal activity of the citric acid-coated MnFe2O4-NPs and the improvement of their antifungal activity when these nanoparticles were conjugated with a 12 aa peptide named Cm-p5.Citation19 This peptide is derived from Cm-p1, which is an MS-MS sequence found in a tryptic chromatographic fraction from the whole animal extract (Cenchritis muricatus [Gastropoda: Littorinidae], Linnaeus, 1758), which was positive for antimicrobial activity. We previously demonstrated that such sequence belongs to the original invertebrate, but we do not know if the corresponding peptide naturally acts as an AMP or if it is part of a major protein potentially undergoing cleavage by induction or not.Citation20 From this sequence we obtained, by bioinformatics, the sequence and corresponding synthetized peptide Cm-p5 with an increased antifungal activity. Therefore, we have based our thinking on previous results in which Cm-p5 showed a minimal loss of activity after conjugation with our manganese ferrite nanoparticles, taking into account that the nonimmunogenic small size of the peptide and its hydrophilicity are probably working together in accordance with our purposes.
Materials and methods
Nanoparticles (modified MnFe2O4)
Modified MnFe2O4-NPs used in this work were supplied by the Institute of Materials Science and Technology, University of Havana, Havana, Cuba. These nanoparticles were part of a batch characterized by X-ray diffraction (XRD), Mössbauer spectroscopy, and Fourier-transform infrared (IR) spectroscopy by Figueroa-Espi et al.Citation13 In brief, XRD patterns were recorded in the Bragg–Brentano geometry using Kα1 radiation. Mössbauer spectrum was collected at room temperature (RT) in the transmission geometry using a 57Co/Rh radiation source and provided information on the substitution of Fe(II) by Mn(II) in the spinel-type structure of the host magnetite framework.
Characterization of MNPs by transmission electron microscopy
The size of the MNPs was assessed by transmission electron microscopy (TEM). TEM images were obtained using a Jeol Jem 2010 F30 microscope (JEOL USA, Inc., Peabody, MA, USA) operated at 200 kV. Samples for TEM were prepared by letting a drop of the samples dispersed in ethanol evaporate on top of a carbon-coated copper grid of 200 meshes. Micrographs were taken by a Gatan Orius SC200 high-speed digital camera (Gatan, Inc., Pleasanton, CA, USA).
Dynamic light scattering measurements
Dynamic light scattering (DLS) measurements were made on a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) for particle size determination. The temperature of the sample cell was controlled to 20°C by a thermostating system. A He–Ne laser with λ =633 nm was used to measure data at 173° noninvasive backscattering using PCS8501 glass cuvette with round aperture. The experiments were performed using the nanoparticles in Tris-HCl buffer, pH 8.0. The final concentrations of the nanoparticles were 2 mg/mL.
Peptide synthesis
Cm-p5 was obtained by solid-phase synthesis using 9-fluorenyl-methoxycarbonyl chemistry,Citation21 purified by reversed-phase high-performance liquid chromatography to >98% purity using an acetonitrile/H2O-trifluoroacetic acid gradient. Purity was evaluated by ion-spray mass spectrometry (Micromass, Manchester, UK).
Determination of peptide concentration
Peptide concentrations were estimated by ultraviolet absorption at 205, 215, and 225 nm (Abs205, Abs215, Abs225) using the following equations:Citation22
Conjugation of Cm-p5 with modified MnFe2O4-NPs
MnFe2O4-NPs 4 mg were activated by 1-ethyl-3-(dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide solution (0.5 and 0.7 mg/mL), respectively, in phosphate-buffered saline (PBS) for 30 minutes at RT. The activated nanoparticles were incubated with 0.4 mg of peptide resuspended in 1 mL PBS and mixed at RT for 2 hours. The nanoparticles were washed twice in PBS and in 0.1 M Tris, pH 8.0.
Antibacterial bioassays
The antibacterial bioassays were evaluated for Staphylococcus aureus (ATCC29213) and E. coli (ATCC8739) by a colorimetric microreader (BioTek Instruments, Winooski, VT, USA) using 96-well microplates according to Hetru and Bulet’s methodology.Citation23 Bioassay analyses against bacteria were performed in Luria-Bertani medium (pH 7.0). Previously, a growth curve of the original culture was established to determine the relationship between colony-forming units and optical density. For antimicrobial activity evaluation, 0.1 mL inoculum was cultured in 4 mL Luria-Bertani medium for 3 hours until it reached the mid-exponential phase. An aliquot corresponding to 5×105 colony-forming units/mL was then added to Luria-Bertani medium to produce a final volume of 0.1 mL in the microplate wells. Nanoparticles were added to reach a final concentration of 250 µg/mL. Chloramphenicol at a concentration of 40 µg/mL and distilled water were used as positive and negative controls, respectively. Microplates were incubated at 37°C, and bacterial growth was monitored at 620 nm every hour. The percentage of survival was determined as the ratio of optical density of normal bacterial growth (100%) and the growth under nanoparticle action. All bioassays were performed in triplicate. To avoid potential optical interference during measurements of the growing cultures caused by the light-scattering properties of the nanoparticles, Luria-Bertani medium without microorganisms but containing the same concentration of nanoparticles cultured under the same conditions was used as a blank control.
Antifungal bioassay
Bioassays against yeast were performed using the broth microdilution method according to the Clinical and Laboratory Standards Institute guidelines.Citation24 The bioassays against the clinically isolated strain of Candida albicans (01U) were performed in Roswell Park Memorial Institute (RPMI)-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA). Amphotericin-B (30 µg/mL) and sterile distilled water were used as positive and negative controls, respectively. Microplates were incubated at 30°C and yeast growth was monitored at 620 nm every hour. The percent (%) of survival was determined as the ratio of optical density of normal fungal growth (100%) and the growth under peptide action. All tests were conducted in triplicate. The background (turbidity due to RPMI medium and MNPs suspension) was eliminated by taking blank readings. The citric acid-modified MnFe2O4-NPs concentrations for the antifungal bioassay were 100 and 250 µg/mL. The concentration of the citric acid-modified MnFe2O4-NPs conjugated with Cm-p5 was 100 µg/mL in comparison with Cm-p5 at 12 µg/mL.
Cytotoxicity assay
Testing with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St Louis, MO, USA) assay was performed according to Pasupuleti et al.Citation25 RAW 264.7 murine macrophage-like cells (Rio de Janeiro Cell Bank, Rio de Janeiro, Brazil) were plated at a concentration of 1×105 cells per well in supplemented Dulbecco’s Modified Eagle’s Medium (4 mM glutamine, 10% fetal calf serum, and 100 units/mL penicillin/streptomycin) and incubated with different concentrations of nanoparticles (0–750 µg/mL). After overnight incubation, 10 mL of the MTT solution (5 mg/mL in PBS) was added to each well. Plates were incubated for 4 hours in 5% CO2 at 37°C. The generated blue formazan product was dissolved by the addition of 100 µL of 100% dimethyl sulfoxide (Mallinckrodt Chemical, Paris, KY, USA) per well. The absorbance was monitored at 575 nm in an enzyme-linked immunosorbent assay plate reader (BioTek, Winooski, VT, USA). The citric acid-modified MnFe2O4-NPs concentrations for the viability and proliferation assay were 250, 500, and 750 µg/mL.
Interaction of modified MnFe2O4-NPs with C. albicans cells visualized by optical microscopy
C. albicans cells were cultivated in the same way as described in the section of ‘Antifungal bioassay’. Nanoparticles (250 µg/mL) were incubated for 1 hour with C. albicans (105 cells/mL) in RPMI-1640 medium at 30°C. Observations were performed with an AxioPhot epifluorescence microscope equipped with an Axiocam MRc camera and complemented with capture software (AxioVision; Carl Zeiss GmbH, Jena, Germany).
Results and discussion
The MnFe2O4-NPs used in this study were prepared by microemulsion method.Citation26 These nanoparticles were characterized by XRD, Mössbauer spectroscopy and high-resolution TEM (HR-TEM) as previously described.Citation13 XRD powder pattern corresponds to the face-centered cubic unit cell expected for manganese ferrite, which crystallizes with spinel-type structure. shows TEM micrographies, XRD pattern, and IR spectrum of manganese ferrite nanoparticles under study. Also, no evidence of accompanying phases was found. With the purpose of stabilizing the nanoparticles and creating reactive groups suitable for further conjugation procedures, MnFe2O4-NPs were modified with citric acid. As a sensing tool for the coating process, IR spectroscopy was used. The recorded IR spectra show several absorption bands characteristic of citrate groups ().Citation13
Figure 1 This material crystallizes with the face-centered cubic unit cell typical of spinel 3d metal oxides.
Notes: Their crystallite size, according to XRD and TEM images, remains below 12 nm (see ). The IR spectrum of citric acid-coated nanoparticles is conclusive regarding the anchoring of the organic molecule on the nanoparticles’ surface. (A–C) HR-TEM micrographs at different resolutions from manganese ferrite nanocrystals. (D) XRD pattern. (E) IR spectrum of Mn-ferrite nanoparticles under study.
Abbreviations: IR, infrared; MNPs, magnetic nanoparticles; HR-TEM, high resolution transmission electron microscopy; XRD, X-ray diffraction.
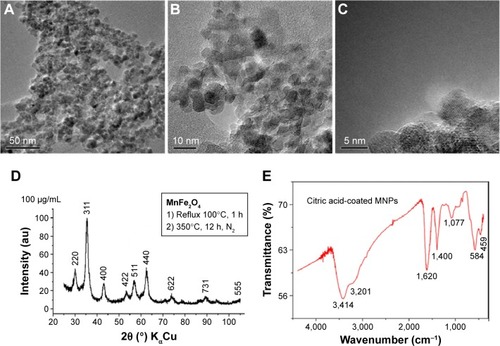
In order to study the crystalline nature of the modified MnFe2O4-NPs, HR-TEM images were obtained (). We observed well-aligned atomic planes, demonstrating the uniformity of the obtained nanocrystals. The HR-TEM images confirm the formation of MnFe2O4 nanocrystals of about 5 nm diameter. This result is in agreement with Vestal et al, who used the same synthesis method and reported a nanoparticle diameter below 15 nm.Citation27 In the same sense, comparable diameter sizes of 4 and 5 nm for manganese ferrite nanoparticles prepared by thermal decomposition have been reported.Citation28,Citation29
The measurements of the diameter of nanoparticles by DLS differ from HR-TEM analysis. The analysis by DLS shows that 93% of the NPs present a diameter range of 24–51 nm. Observed differences between both techniques are related with the fact that MNPs behave in solvents as a dispersion, in contrast to HR-TEM in which nanoparticles are analyzed as a dried sample. Similar results have been obtained by Nunes et al with citrate-coated manganese ferrite nanoparticles.Citation30
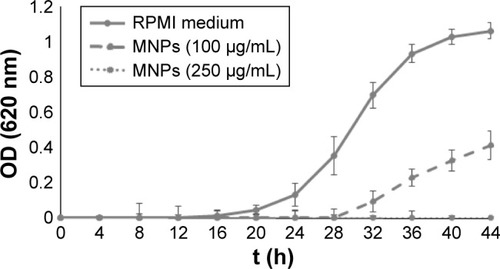
The antimicrobial activity of different coated MNPs has been demonstrated.Citation9,Citation14 To this end, the citric acid-modified MnFe2O4-NPs were evaluated for their antimicrobial activity. These nanoparticles showed a significant antifungal activity against C. albicans at different concentrations (). The minimal inhibitory concentration (MIC) of the MNPs was reached at 250 µg/mL in the RPMI medium. The antifungal activity of the nanoparticles was not fungicidal (data not shown). On the other hand, nanoparticles were not effective against the Gram-positive S. aureus and the Gram-negative E. coli bacteria (data not shown).
Figure 2 Candida albicans growth curves in RPMI-1640 medium using different concentrations of modified MnFe2O4-NPs in a final volume of 200 µL/well.
Notes: Each value represents the mean of three replicates in three different experiments. Bars represent the standard deviation.
Abbreviations: MNPs, magnetic nanoparticles; OD, optical density; RPMI, Roswell Park Memorial Institute.
The antifungal activity of nanoparticles had been recently demonstrated for magnetic binary nanocomposites of iron oxide (magnetite and maghemite) and silver nanoparticles.Citation31 These nanoparticles showed very significant antibacterial and antifungal activities against ten tested bacterial strains (MIC from 15.6 to 125 µg/mL) and four Candida species (MIC from 1.9 to 31.3 µg/mL). In this case, the inherent antimicrobial activity of silver can be taken into account, but on the other hand, some attempts to inhibit Candida biofilm formation succeeded by coating MNPs with plant essential oils applied to different surfaces as dressings and medical devices.Citation32–Citation34 Nevertheless, no direct antifungal activity was demonstrated for these nanoparticles. In fact, no antimicrobial activity has been reported for “naked” MNPs. MnFe2O4-NPs have semiconductor behavior with a relatively low band gap, in the visible region, and for such reason, this nanoparticle system is unable to oxidize the water molecule producing radicals. In contrast, for instance, TiO2 and ZnO nanoparticles have a band gap in the ultraviolet region and the light absorption creates holes in the valence region, which are responsible for oxidizing the water and forming radicals, which are highly reactive species. In our work, it is probable that the antimicrobial activity of citric acid-modified MnFe2O4-NPs is related to such coating.
Indeed, the antibacterial activity of citric acid solutions has been previously reported.Citation35–Citation37 For example, the conjugation of citric acid to ciprofloxacin significantly improved the antibacterial activity of the antibiotic.Citation38 However, the nanoparticles described in this paper were not effective against bacteria. It is possible that this effect could be related to the electrostatic repulsion between citrate groups on the surface of nanoparticles and the transmembrane potential of bacteria. In this sense, it has been reported that positively charged MNPs coated with a chitosan polymeric matrix have the ability to bind to the bacteria cell surface, exerting antimicrobial activity.Citation39 Taking into account the electrostatic differences between prokaryotic bacterial and eukaryotic fungal plasma membrane,Citation40 the selectivity of citric acid-modified MnFe2O4-NPs could be explained in such terms. Indeed, the interaction of citric acid-adsorbed CoFe2O4 nanoparticles with artificial lipid membranes (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) was demonstrated.Citation41 This result is relevant in this context since phosphatidylcholine is the most abundant phospholipid in yeast membrane. The mechanism of action for most of the cationic AMPs (and also extendable to our manganese ferrite nanoparticles) depends on the interaction with the microbial membrane to promote membrane destabilization, and these agents can function in vivo but are not toxic to host cells. One explanation is that AMPs produced by mammals will not act on eukaryotic cells because of the cholesterol content of eukaryotic membranes. In contrast, microbial cell membranes are free of cholesterol. Because cholesterol is known to cause condensation of phospholipid bilayers, it might prevent these agents from penetrating into the cytoplasmic membrane of eukaryotic cells. Besides cholesterol, the asymmetric distribution of phospholipids in the cytoplasmic membrane of mammalian cells might also contribute to the insensitivity of eukaryotic cells to this attack.Citation42 On the other hand, C. albicans plasma membrane presents ergosterol instead of cholesterol and a combination of phosphatidylcholine, phosphatidylinositol, phosphatidylserine, and phosphatidylethanolamine, not present in mammalian cells; this could favor the interaction with the Mn-ferrite nanoparticles and subsequent destabilization.
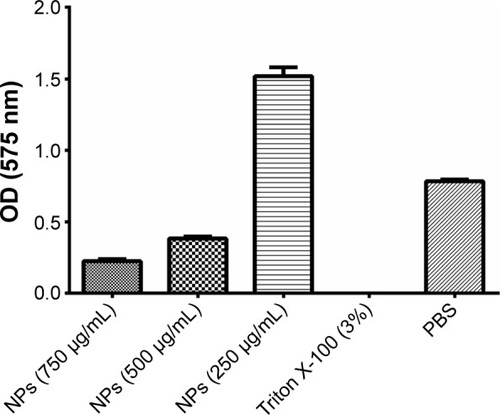
With the purpose of discarding any toxic activity of modified MnFe2O4-NPs against animal cells, a viability test was conducted. A dose-dependent influence on cell damage was found. Nanoparticles were seen to be nontoxic to a macrophage cell line at concentrations that showed antifungal activity (≤250 µg/mL). Otherwise, higher concentrations (≥500 µg/mL) affected the viability of such cells (). As for our antifungal peptide Cm-p5, when it is in contact with C. albicans cells at a sub-MIC concentration, instead of causing disturbing the cell membrane, the orientation and concentration of peptide in the membrane stimulate a higher yeast growth than the control with only cells and medium. For this reason, nanoparticles at 250 µg/mL, like AMPs under MIC concentrations, could favor the cell’s uptake of nutrients. This argument needs to be more thoroughly explored.
Figure 3 Viability and proliferation of the RAW 264.7 cells in RPMI-1640 medium at different concentrations of citric acid-modified MnFe2O4-NPs.
Notes: Triton X-100 (3%) was used as a positive control for toxicity, and PBS was used as a negative control. Each column represents the mean of three replicates. Bars represent the standard deviation.
Abbreviations: MnFe2O4-NPs, manganese ferrite nanoparticles; NPs, nanoparticles; OD, optical density; PBS, phosphate-buffered saline; RPMI, Roswell Park Memorial Institute.
In agreement with our results, manganese ferrite nanoparticles functionalized with citric acid, with a mean particle size of 8 nm, demonstrated dose-dependent cytotoxic effects from a concentration of 50 µg/mL, as seen in the MTT assay using the mouse Balb/3T3 fibroblast cell line.Citation43
Additionally, citric acid-coated iron oxide nanoparticles showed cytotoxicity on SK-MEL-37 human melanoma cell line with an IC50 (half-maximal inhibitory concentration) value of 433 µg Fe/mL.Citation44 Indeed, COOH-modified iron oxide nanoparticles have shown some toxicity in human epithelial carcinoma cell lines at a concentration of 10 µg/mL.Citation45,Citation46 This behavior can be explained by the charge on the surface of iron oxide nanoparticles, which could play an important role in the intracellular uptake.Citation47 In this sense, there was no observable uptake of citrate-coated MNPs at concentrations of up to 0.1 mg/mL for murine macrophage cells (RAW 264.7) and Jurkat cell line (clone E6-1). At concentrations until 0.6 mg/mL, a relevant cellular uptake was observed, but the uptake of nanoparticles did not affect the viability and proliferation of the cells. At higher concentrations, cell viability decreased markedly.Citation48
Furthermore, after exposing endothelial cells to citric acid-coated iron oxide nanoparticles, dose-dependent effects on human umbilical vein endothelial cells’ viability, cytoskeleton, and function were demonstrated.Citation49 On the other hand, cationic polymeric manganese ferrite nanoparticles exhibited higher cytotoxicity in macrophages (RAW 264.7 cells) and lower cellular membrane rigidity than anionic and nonionic polymeric manganese ferrite nanoparticles.Citation50 In general, the toxicity of magnetic particles may depend on different factors, including the concentration, composition, size, structure, solubility, and surface chemistry.Citation51
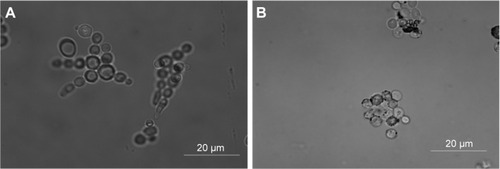
In order to explore the antifungal effect of citric acid-modified MnFe2O4-NPs, microscopy studies of C. albicans cells grown in RPMI medium in the presence of modified nanoparticles were performed. As shown in , C. albicans growth inhibition by MNPs may have occurred due to the aggregation of yeast cells. More experiments are necessary to study such an interaction further.
Figure 4 Interaction of modified MnFe2O4-NPs with Candida albicans cells visualized by optical microscopy.
Notes: (A) C. albicans cells (105 cells/mL) in RPMI-1640 medium at 30°C. (B) C. albicans cells (105 cells/mL) in RPMI-1640 medium at 30°C with citric acid-modified MnFe2O4-NPs (250 µg/mL).
Abbreviations: MnFe2O4-NPs, manganese ferrite nanoparticles; RPMI, Roswell Park Memorial Institute.
In agreement with our results, MNPs coated with aminated silica were able to interact with the surface of the fungus Fusarium oxysporum. In this case, no antifungal action was detected.Citation52
Taking into account the intrinsic antifungal activity found for the citric acid-functionalized manganese ferrites, we explored the antimicrobial activity of these nanoparticles conjugated with the antifungal peptide Cm-p5. The efficiency of the carbodiimide-based conjugation was above 80%. This type of coupling chemistry has been used for immobilizing AMPs by their amino groups.Citation53,Citation54 Significantly, the antifungal activity of the conjugated nanoparticles was higher than that of their bulk counterparts (). In fact, the MIC of Cm-p5 conjugated to MnFe2O4-NPs was 100 µg/mL, which was 2.5 times lower than the MIC of nonconjugated nanoparticles (). On the other hand, with 7.5 times less Cm-p5 in the conjugate regarding the soluble peptide, complete C. albicans growth inhibition was achieved ().
Figure 5 Antifungal activity assays.
Notes: (A) Candida albicans growth curves in RPMI-1640 medium of modified MnFe2O4-NPs and Cm-p5-conjugated MnFe2O4-NPs. Each value represents the mean of three replicates in three different experiments. Bars represent the standard deviation. (B) C. albicans growth curves in RPMI-1640 medium of the Cm-p5 peptide and Cm-p5-conjugated MnFe2O4-NPs. Each value represents the mean of three replicates in three different experiments. Bars represent the standard deviation.
Abbreviations: MNPs, magnetic nanoparticles; MnFe2O4-NPs, manganese ferrite nanoparticles; OD, optical density; RPMI, Roswell Park Memorial Institute.
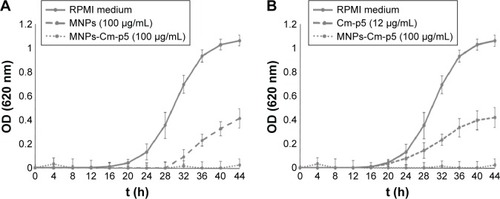
These results differ from the work of other authors, who reported that the activity of AMPs decreased upon conjugation to different supports.Citation55–Citation58 Indeed, Borcherding et al reported that iron-containing nanoparticles affect the activity of soluble AMPs, probably influenced by the ability of bacteria to scavenge iron for growth from nanoparticles and the adsorption of peptides onto nanoparticle surfaces.Citation18 It is obvious that AMPs with different physicochemical properties can be affected differently by immobilization reactions.Citation59
In this sense, Cm-p5 is not an amphipathic peptide, and its antifungal activity does not depend on its amino-terminal group.Citation19 Also, the mechanism of action of this peptide is related to interactions with the fungal cell surface; therefore, it is possible to have a synergistic antifungal action in the conjugate between the peptide and MNPs. In the same way, the immobilization of the AMP nisin to multiwalled carbon nanotubes improved the antibacterial and antibiofilm properties of noncoated carbon nanotubes.Citation60 Furthermore, bacitracin-conjugated iron oxide nanoparticles showed higher antibacterial activity than bacitracin alone. Because of this improved activity, conjugated MNPs allow lower dosages and fewer collateral effects of the antibiotic.Citation16 Finally, Cm-p5-conjugated MnFe2O4-NPs showed a similar pattern of toxicity in the cell line RAW 264.7 as found for citric acid-modified MnFe2O4-NPs (data not shown), but less of the conjugate was needed to completely inhibit C. albicans growth.
Conclusion
These results contribute to marking the importance of MNPs in biomedicine, specifically in the antimicrobials field. Herein, the intrinsic antifungal activity of citric acid-coated MnFe2O4-NPs was demonstrated. In addition, the improvement of their antifungal activity was achieved when these nanoparticles were conjugated with the AMP Cm-p5. Nowadays, strategies are required to reduce the toxicity and enhance the efficacy and biodistribution of AMPs. MNPs could be an excellent alternative due to their broad spectrum of activities (antimicrobial, drug delivery, diagnosis) and their low toxicity.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors thank FAPDF (Support Research of the Federal District Foundation, Brasilia DF, Brazil), FUNDECT (Support Foundation The Education Development, Science and Technology state of Mato Grosso do Sul, Brazil), and CAPES-MES (Higher Education Personnel Improvement Coordination-Ministry of Higher Education), Brazil; International Foundation of Science, Sweden (F 4614 and F/5199 projects).
References
- KruijshaarMEWatsonJMDrobniewskiFIncreasing antituberculosis drug resistance in the United Kingdom: analysis of National Surveillance DataBMJ200833676551231123418456593
- SnellNJExamining unmet needs in infectious diseaseDrug Discov Today200381223012546988
- AriasCAMurrayBEAntibiotic-resistant bugs in the 21st century – a clinical super-challengeN Engl J Med2009360543944319179312
- AfacanNJYeungATPenaOMHancockRETherapeutic potential of host defense peptides in antibiotic-resistant infectionsCurr Pharm Des20121880781922236127
- RiedlSZweytickDLohnerKMembrane-active host defense peptides – challenges and perspectives for the development of novel anticancer drugsChem Phys Lipids201116476678121945565
- EckertRRoad to clinical efficacy: challenges and novel strategies for antimicrobial peptide developmentFuture Microbiol20116663565121707311
- BrogdenNKBrogdenKAWill new generations of modified antimicrobial peptides improve their potential as pharmaceuticals?Int J Antimicrob Agents201138321722521733662
- BrandelliANanostructures as promising tools for delivery of antimicrobial peptidesMini Rev Med Chem20121273174122512554
- Lopez-AbarrateguiCOtero-GonzalezAJAlba-MenendezARegueraEFrancoOLNanoparticles as a promise for host defense peptides therapeuticsProkopovichPBiological and Pharmaceutical Applications of NanomaterialsBoca Raton, FLCRC Press2015129147
- Lopez-AbarrateguiCFigueroa-EspiVReyes-AcostaORegueraEOtero-GonzalezAJMagnetic nanoparticles: new players in antimicrobial peptide therapeuticsCurr Protein Pept Sci201314759560623968342
- SabaleSJadhavVKhotVZhuXXinMChenHSuperparamagnetic MFe2O4 (M = Ni, Co, Zn, Mn) nanoparticles: synthesis, characterization, induction heating and cell viability studies for cancer hyperthermia applicationsJ Mater Sci Mater Med20152635466
- ZottisADBeltrameJMLaraLRPheomelanin-coated iron oxide magnetic nanoparticles: a promising candidate for negative T2 contrast enhancement in magnetic resonance imagingChem Commun (Camb)20155156111941119726073290
- Figueroa-EspiVAlvarez-PanequeATorrensMOtero-GonzalezAJRegueraEConjugation of manganese ferrite nanoparticles to an anti Sticholysin monoclonal antibody and conjugate applicationsColloid Surf A2011387118124
- LiakosIGrumezescuAMHolbanAMMagnetite nanostructures as novel strategies for anti-infectious therapyMolecules2014198127101272625140449
- DongHHuangJKoepselRRYePRussellAJMatyjaszewskiKRecyclable antibacterial magnetic nanoparticles grafted with quaternized poly(2-(dimethylamino)ethyl methacrylate) brushesBiomacromolecules20111241305131121384911
- ZhangWShiXHuangJZhangYWuZXianYBacitracin-conjugated superparamagnetic iron oxide nanoparticles: synthesis, characterization and antibacterial activityChem Phys Chem201213143388339622753190
- NiemirowiczKSurelUWilczewskaAZBactericidal activity and biocompatibility of ceragenin-coated magnetic nanoparticlesJ Nanobiotechnology2015133225929281
- BorcherdingJBaltrusaitisJChenHIron oxide nanoparticles induce growth, induce biofilm formation, and inhibit antimicrobial peptide functionEnviron Sci Nano20141212313225221673
- Lopez-AbarrateguiCMcBethCMandalSMCm-p5: an antifungal hydrophilic peptide derived from the coastal mollusk Cenchritis muricatus (Gastropoda: Littorinidae)FASEB J2015293315332525921828
- Lopez-AbarrateguiCAlbaASilvaONFunctional characterization of a synthetic hydrophilic antifungal peptide derived from the marine snail Cenchritis muricatusBiochimie201294496897422210491
- MerrifieldBSolid phase synthesisScience198623247483413473961484
- MurphyJKiesMNote on the spectrophotometric determination of proteins in dilute solutionsBiochim Biophys Acta19603382384
- HetruCBuletPStrategies for the isolation and characterization of antimicrobial peptides of invertebratesMethods Mol Biol19977835499276295
- CLSIReference method for broth dilution antifungal susceptibility testing of yeastsApproved StandardEighth EditionWayne, PAClinical and Laboratory Standards Institute2008
- PasupuletiMSchmidtchenAChalupkaARingstadLMalmstenMEnd-tagging of ultra-short antimicrobial peptides by W/F stretches to facilitate bacterial killingPLoS One200944e528519381271
- Alvarez-PanequeADíazSSantiago-JacintoPRegueraESíntesis y caracterización de nanopartículas magnéticas basadas en la MnFe2O4 tipo espinela [Synthesis and characterization of magnetic nanoparticles based on spinel type manganese ferrite MnFe2O4]Rev Cub Física2008252B117122
- VestalCRZhangZJAtom transfer radical polymerization synthesis and magnetic characterization of MnFe2O4/polystyrene core/shell nanoparticlesJ Am Chem Soc200212448143121431312452698
- HyunSWHongSCKimSJKimCSEffect of proton irradiation on the magnetic properties of manganese ferriteJ Nanosci Nanotechnol20111176241624422121693
- PalMRakshitRMandalKSurface modification of MnFe(2)O(4) nanoparticles to impart intrinsic multiple fluorescence and novel photocatalytic propertiesACS Appl Mater Interfaces2014674903491024621387
- NunesADRamalhoLSSouzaAPManganese ferrite-based nanoparticles induce ex vivo, but not in vivo, cardiovascular effectsInt J Nanomedicine201493299331225031535
- PrucekRTucekJKilianovaMThe targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticlesBiomaterials201132214704471321507482
- AnghelIGrumezescuAMHolbanAMFicaiAAnghelAGChifiriucMCBiohybrid nanostructured iron oxide nanoparticles and Satureja hortensis to prevent fungal biofilm developmentInt J Mol Sci201314181101812324009022
- ChifiriucCGrumezescuVGrumezescuAMSaviucCLazarVAndronescuEHybrid magnetite nanoparticles/Rosmarinus officinalis essential oil nanobiosystem with antibiofilm activityNanoscale Res Lett2012720922490675
- GrumezescuAMChifiriucMCSaviucCHybrid nanomaterial for stabilizing the antibiofilm activity of Eugenia carryophyllata essential oilIEEE Trans Nanobioscience201211436036522949098
- Arias-MolizMTFerrer-LuqueCMEspigares-RodriguezELiebana-UrenaJEspigares-GarciaMBactericidal activity of phosphoric acid, citric acid, and EDTA solutions against Enterococcus faecalisOral Surg Oral Med Oral Pathol Oral Radiol Endod20081062e84e8918554953
- KimSARheeMSSynergistic antimicrobial activity of caprylic acid in combination with citric acid against both Escherichia coli O157:H7 and indigenous microflora in carrot juiceFood Microbiol20154916617225846927
- KoKYMendoncaAFAhnDUInfluence of zinc, sodium bicarbonate, and citric acid on the antibacterial activity of ovotransferrin against Escherichia coli O157:H7 and Listeria monocytogenes in model systems and hamPoult Sci200887122660267019038824
- MilnerSJSnellingAMKerrKGProbing linker design in citric acid-ciprofloxacin conjugatesBioorg Med Chem201422164499450524794750
- ChifiriucCMGrumezescuAMSaviucCCroitoruCMihaiescuDELazarVImproved antibacterial activity of cephalosporins loaded in magnetic chitosan microspheresInt J Pharm201243620120522732671
- MatsuzakiKControl of cell selectivity of antimicrobial peptidesBiochim Biophys Acta2008178881687169218952049
- DraslerBDrobneDNovakSEffects of magnetic cobalt ferrite nanoparticles on biological and artificial lipid membranesInt J Nanomedicine201491559158124741305
- LaiYGalloRLAMPed up immunity: how antimicrobial peptides have multiple roles in immune defenseTrends Immunol20093013114119217824
- BellusciMLa BarberaAPadellaFBiodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical processInt J Nanomedicine201491919192924790434
- de FreitasERSoaresPRSantosRPIn vitro biological activities of anionic gamma-Fe2O3 nanoparticles on human melanoma cellsJ Nanosci Nanotechnol2008852385239118572653
- SharmaGKodaliVGaffreyMIron oxide nanoparticle agglomeration influences dose rates and modulates oxidative stress-mediated dose-response profiles in vitroNanotoxicology2014866367523837572
- ShenMCaiHWangXFacile one-pot preparation, surface functionalization, and toxicity assay of APTS-coated iron oxide nanoparticlesNanotechnology2012231010560122349004
- PatilUSAdireddySJaiswalAMandavaSLeeBRChriseyDBIn vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticlesInt J Mol Sci20151610244172445026501258
- SrivastavaSAwasthiRGajbhiyeNSInnovative synthesis of citrate-coated superparamagnetic Fe3O4 nanoparticles and its preliminary applicationsJ Colloid Interface Sci2011359110411121513942
- WuXTanYMaoHZhangMToxic effects of iron oxide nanoparticles on human umbilical vein endothelial cellsInt J Nanomedicine2010538539920957160
- YangSHHeoDParkJRole of surface charge in cytotoxicity of charged manganese ferrite nanoparticles towards macrophagesNanotechnology2012235050570223164999
- ReddyLHAriasJLNicolasJCouvreurPMagnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applicationsChem Rev2012112115818587823043508
- RispailNDe MatteisLSantosRQuantum dot and superparamagnetic nanoparticle interaction with pathogenic fungi: internalization and toxicity profileACS Appl Mater Interfaces20146129100911024853082
- ChenGZhouMChenSLvGYaoJNanolayer biofilm coated on magnetic nanoparticles by using a dielectric barrier discharge glow plasma fluidized bed for immobilizing an antimicrobial peptideNanotechnology2009204646570619847021
- CostaFCarvalhoIFMontelaroRCGomesPMartinsMCCovalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfacesActa Biomater2011741431144021056701
- BagheriMBeyermannMDatheMImmobilization reduces the activity of surface-bound cationic antimicrobial peptides with no influence upon the activity spectrumAntimicrob Agents Chemother20095331132114119104020
- ChoWMJoshiBPChoHLeeKHDesign and synthesis of novel antibacterial peptide-resin conjugatesBioorg Med Chem Lett200717215772577617827001
- HaynieSLCrumGADoeleBAAntimicrobial activities of amphiphilic peptides covalently bonded to a water-insoluble resinAntimicrob Agents Chemother19953923013077726486
- HilpertKElliottMJenssenHScreening and characterization of surface-tethered cationic peptides for antimicrobial activityChem Biol2009161586919171306
- VriesRAndradeCABakuzisAFMandalSMFrancoOLNext-generation nanoantibacterial tools developed from peptidesNanomedicine (Lond)201510101643166126008197
- QiXPoernomoGWangKCovalent immobilization of nisin on multi-walled carbon nanotubes: superior antimicrobial and anti-biofilm propertiesNanoscale2011341874188021431164
