 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A thorough understanding of interactions occurring at the interface between nanocarriers and biological systems is crucial to predict and interpret their biodistribution, targeting, and efficacy, and thus design more effective drug delivery systems. Upon intravenous injection, nanoparticles are coated by a protein corona (PC). This confers a new biological identity on the particles that largely determines their biological fate. Liposomes have great pharmaceutical versatility, so, as proof of concept, their PC has recently been implicated in the mechanism and efficiency of their internalization into the cell. In an attempt to better understand the interactions between nanocarriers and biological systems, we analyzed the plasma proteins adsorbed on the surface of multicomponent liposomes. Specifically, we analyzed the physical properties and ultrastructure of liposome/PC complexes and the aggregation process that occurs when liposomes are dispersed in plasma. The results of combined confocal microscopy and flow cytometry experiments demonstrated that the PC favors liposome internalization by both macrophages and tumor cells. This work provides insights into the effects of the PC on liposomes’ physical properties and, consequently, liposome–liposome and liposome–cell interactions.
Introduction
In the last few decades, nanoparticle (NP)-based delivery platforms have improved the therapeutic index of many drugs. In fact, by increasing their pharmaceutical bioavailability and targeting, NPs avoid systemic toxicity while retaining pharmacological activity.Citation1 After intravenous injection, NPs adsorb a multitude of biological molecules (proteins, lipids, glycans, and metabolites) from plasma on their surface. These biomolecules continuously recycleCitation2,Citation3 on the NP surface, thereby forming a dynamic shell known as a “biomolecular corona.”Citation4 Most research studying the biomolecular corona has focused on protein composition characterization:Citation5,Citation6 therefore, the biomolecular corona is generally called the “protein corona” (PC).Citation7,Citation8 This natural coating provides NPs with a new biological identity and significantly affects their fate, pharmacokinetics, and cellular interactions.
In terms of NP targeting abilities, the PC can 1) hinder the function of targeting ligands attached to the NP surface, thereby reducing their accumulation in the targeted tissue,Citation3,Citation9,Citation10 and 2) modify the carrier surface, facilitating cell internalization.Citation11 The PC can also modulate immune response.Citation12 Several studies have characterized PC structure, function, and formation dynamics.Citation13,Citation14 Structurally, proteins that are weakly bound to the NP surface form the “soft corona,” while proteins strongly bound to the NP surface form the “hard corona.”Citation15,Citation16 Functionally, the PC is characterized by the presence of opsonins (complement proteins), immunoglobulins, and lectins,Citation17 which match to specific receptors on leukocyte membranes. Until recently, NP opsonization was considered part of the body’s defense mechanism by inducing NP clearance from circulation and consequent accumulation in the organs of the mononuclear phagocytic system (mainly spleen and liver).Citation18 However, in a recent study, unfolded proteins that promote macrophage uptake or their shielding by other proteins in the PC were found to reduce in vitro particle uptake by RAW264.7 macrophages.Citation19,Citation20 The particular context, therefore, needs to be discussed when considering cellular uptake. Although the biological identity of NPs largely determines their biological fate, their physical identity (ie, shape, size, and surface charge) strictly regulates NP–cell interactions. Indeed, large NPs (>300 nm) with a higher positive or negative surface charge (< −15 mV or > +15 mV) exhibited increased macrophage uptake, in vitro and in vivo.Citation21,Citation22
The scope of this work was to shed light on the interactions occurring at the interface between nanocarriers and biological systems. To this aim, we evaluated liposomes as proof-of-concept NPs, given their widespread use in the pharmaceutical field and their high versatility. While most research into the PC has studied inorganic NPs, only recently the PC of liposomes is emerging as a fundamental entity regulating the interactions of liposomes with living systems.Citation23 The PC is believed to have a potentially key impact on targeted delivery in nanomedicine, as it could be manipulated by using different liposomal formulations. Herein, we selected multicomponent choline-based liposomes as a model system to study the PC’s effects in targeted drug delivery applications.Citation24 We studied the correlation between the biological and physical identity of liposomes. To investigate the physical changes occurring in liposomes after PC formation, we characterized NP–PC complexes and used proteomics to analyze the PC composition. Lastly, in an attempt to reproduce the environment surrounding NPs upon systemic injection, we investigated the interactions between liposome–PC complexes and two cell lines in vitro. We studied the impact of the PC composition on liposome uptake by the main cell population responsible for clearance of foreign materials (macrophages J774 cells) and by cancer cells (breast cancer 4T1 cells).
Materials and methods
Liposome assembly
Liposomes were prepared with the thin-layer evaporation method. Briefly, dipalmitoylphosphatidylcholine, dioleoylphosphatidylcholine, and cholesterol (Avanti Polar Lipids, Alabaster, AL, USA) in 6:3:1 molar ratio were dissolved in chloroform/methanol (3:1, v/v). This choline-based lipid composition provides safe and not immunogenic liposomes, relevant for drug delivery application.Citation25 This allowed us to study the formation of the PC focusing on the physical identity of the particles, reducing the effects of secondary factors due to the intrinsic immunogenic features of liposome components. A thin lipid film was prepared with a rotary evaporator (BUCHI Labortechnik AG, Flawil, Switzerland). The film was then hydrated with ultrapure distilled water to obtain a lipid concentration of 20 mM. Liposomes were extruded through 200-nm pore cellulose acetate membranes (Whatman, Schleicher & Schuell, Maidstone, UK) at 45°C. The unilamellar liposomes obtained were stored at 4°C until use. The same procedure was used to assemble rhodamine-labeled liposomes for cellular uptake studies. For those particles, Rhod-DOPE was also added to the lipid mixture; the entire procedure was performed in the dark.
Plasma collection
The study was conducted according to the Guide for the Care and Use of Laboratory Animals and was approved by the Houston Methodist Institutional Animal Care and Use Committee. An aseptic technique was used to withdraw blood from healthy BALB/C mice (Charles River Laboratories International, Inc., Wilmington, MA, USA) when they were sedated. Within 30 minutes after collection, using 50 mM ethylenediaminetetraacetic acid as an anticoagulant, plasma was isolated by centrifugation for 15 minutes at 1,000× g. Plasma was aliquoted and stored at −80°C until use. When required, aliquots were thawed at 4°C and then centrifuged at room temperature for 5 minutes at 15,000× g to eliminate protein aggregates. The blood volume withdrawn from the mice was calculated according to the Lee and Blaufox equation:
Formation of liposome–PC complexes
Liposome–PC complexes were obtained by incubating NPs with plasma. Liposome formulation was diluted 20 times (1 mM final lipid concentration) with ultrapure water and then incubated in plasma at 1:1 liposome/plasma ratio (v/v) in order to mimic the protein concentration in vivo (50% plasma in blood).Citation27 The incubation was done in murine plasma for 1 hour at 37°C under continuous agitation. The liposome–PC complexes were recovered after 10 minutes centrifugation at 15,000× g, and then washed three times in cold phosphate buffered saline to remove unbound proteins. We conducted a Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA) on the washing solution to ensure that no additional proteins were eluted from the liposomes. The tubes were changed after each washing step to minimize contamination with plasma proteins bound to the tubes. A plasma aliquot not incubated with liposomes was subjected to the same procedure, as a control, to verify the absence of protein precipitation. All experiments were performed in triplicate.
Size and zeta potential experiment
A Multisizer 4 Coulter counter (Beckman Coulter Inc., Miami, FL, USA) and ZetaSizer Nano ZS (Malvern Instruments, Malvern, UK) were employed to measure the size and the zeta potential, respectively. Samples were diluted 50× with distilled water at 25°C. The results are presented as the average of five measurements.
Cryo-electron microscopy analysis
Lipid vesicles were plunge-frozen on holey film grids (R2x2 Quantifoil®; Micro Tools GmbH, Jena, Germany). A 626 cryo-specimen holder (Gatan, Inc., Pleasanton, CA) was used for imaging. Data were collected on a JEOL 2100 electron microscope (JEOL Ltd., Tokyo, Japan). Images were recorded under low electron dose conditions (5–20 electrons/Å2) using a 4,096×4,096 pixel CCD camera (UltraScan 895, GATAN, Inc., Pleasanton, CA, USA) at a nominal magnification of 20,000×.
Atomic force microscopy
Atomic force microscopy (AFM) images of the liposome and the liposome–PC complex were collected in the Scan Asyst® mode on a Multimode (Bruker Corporation, Santa Barbara, CA, USA) microscope using single-beam silicon cantilever probes (Bruker MLCT at a resonance frequency of 10 kHz, a nominal tip curvature radius of 10 nm, and a force constant of 0.04 N/m). All measurements were subjected to first-order flattening. Particle size was calculated using the Nanoscope 6.13R1 software (Digital Instruments, NY, USA). Mean values from 40 random particles in three independent experiments are reported. Quantitative AFM force mapping analysis was performed to evaluate the relative elasticity of particles in order to obtain a complete elastic property map of heterogeneous samples. We prepared samples by coating the mica surface sample holder with 0.1% (3-Aminopropyl) triethoxysilane (APTES) to stabilize the NPs (to prevent their collapse on the mica surface), and then performed the AFM analysis. Young’s modulus was calculated for six different samples corresponding to 512×512 force–separation curves obtained over a 10×10 μm area. Young’s modulus was calculated using the following equation, as reported elsewhere:Citation28
Protein separation by one-dimensional gel electrophoresis and in situ digestion
Proteins adsorbed on the liposomes were separated by one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained by Coomassie Brilliant Blue (Thermo Fisher Scientific, Waltham, MA, USA). Plasma proteins on the surface of liposomes were quantified by densitometric analysis with the ImageJ software Version 1.45 (imagej.en.softonic.com/). The most abundant protein bands were cut and treated as previously described.Citation29,Citation30 Briefly, each band was de-stained by washing in acetonitrile and 50 mM ammonium bicarbonate. Then, cysteine residues of the proteins were reduced in 10 mM dithiothreitol for 1 hour at 57°C, and alkylated with 55 mM iodoacetamide for 45 minutes at room temperature in the dark. Proteins were in situ digested using Porcine Trypsin Sequencing Grade (Promega Corporation, Fitchburg, WI, USA) overnight at 37°C at an enzyme/substrate ratio of 1:50. The next day, the supernatant was collected and larger peptides were extracted by adding acetonitrile to the bands. Enzyme activity was stopped by adding 0.5% trifluoroacetic acid. Peptides were recovered from the supernatant after centrifugation at 18,000× g for 10 minutes. The resulting peptides were extracted and identified by liquid chromatography-tandem mass spectrometry.
Mass spectrometry and identification of proteins
We used a NanoAcquity UPLC system (Waters Corporation, Milford, MA, USA) coupled inline with a Synapt HDMS (G1) mass spectrometer with an electrospray source to analyze proteins. The liquid chromatography system consisted of a 180 μm ×20 mm Symmetry C18 (5 μm particle) trapping column, and a 75 μm ×250 mm BEH130 C18 (1.7 μm particle) analytical column. The peptide mixture was resolved as previously described.Citation31 The ProteinLynx Global Server (PLGS v2.4; Waters Corporation) was used to identify proteins. The Uniprot 2013_03 (“reviewed”) mouse proteome (16,614 entries) served as the target database. Only proteins with a minimum of two peptides, 95% confidence interval, precursor ion mass error tolerance ≤5 ppm, and fragment ion mass tolerance ≤15 ppm were considered. The following criteria were used in the database search strategy: one trypsin missed cleavage was also allowed, together with oxidation of methionines as variable modification. The only allowed fixed modification was, instead, the carbamidomethylation of cysteines. Quantitative values were normalized using 100 fmol/sample of yeast alcohol dehydrogenase as internal standard and analyzed with the Identity E algorithm.Citation32
Cell culture
The J774 mouse macrophage cell line (ATCC® TIB-67™) and the 4T1 mouse mammary tumor cell line (ATCC® CRL2539™) were used. Cells were cultured using Dulbecco’s Modified Eagle’s Medium containing 10% (v/v) fetal bovine serum and 1% (v/v) penicillin/streptomycin solution. They were maintained in humidified incubators under 5% CO2, at 37°C and passaged at 70%–80% of confluence.
Flow cytometry
To evaluate cellular uptake of liposomes by flow cytometry, 200,000 cells/well were seeded in a twelve-well tissue culture plate. After 24 hours of cell culture expansion, cells were incubated in serum-free conditions with 0.15 mM of rhodamine-labeled bare liposomes and rhodamine-labeled liposome–PC complexes. Incubation occurred for 3 hours at 37°C. After treatment, the cells were washed with phosphate buffered saline, harvested using trypsin, and centrifuged for 10 minutes at 1,500 rpm. A solution of trypan blue (0.01%) was added to the tubes before acquisition to quench the surface fluorescence.Citation33 The analysis was performed using a Becton Dickinson LSR II instrument equipped with Diva software. Each sample was evaluated with 10,000 events. Untreated cells were used as controls. Results are presented as averages of three independent experiments.
Confocal fluorescence microscopy
Cells were seeded onto an eight-well chamber slide (10,000 cells/well) and treated with the particles as described earlier. At the end of the experiment, cells were thoroughly washed and fixed in 4% paraformaldehyde solution. Nuclei were stained using DAPI (blue), and cell membranes were stained using WGA-AlexaFluor 488 conjugate (green). Slides were imaged using a Nikon A1 confocal imaging system and analyzed via NIS Elements AR. Images are presented as a maximum projection or as a 3D side projection of a chosen z section. For statistical analysis, images (n=3) were acquired for each sample at five different areas. For each micrograph, the area fraction for the particles signal (red) was calculated and divided by the number of nuclei counted (blue). All results are presented as mean ± SD in a graph of area fraction/cell in the different treatments.
Results and discussion
Characterization of liposomes before and after PC formation
Liposomes were incubated in plasma for 1 hour to allow liposome–PC complex formation. This incubation time was chosen because it is known that a stable PC forms over a period of 1 hour.Citation34 Moreover, previous studies reported that 1 hour is the liposomes’ saturation point in terms of proteins adsorbed during incubation in plasma.Citation35
Samples were analyzed before and after incubation in plasma by dynamic light scattering (DLS), zeta potential, and cryo-electron microscopy analyses to investigate their size (and size homogeneity: polydispersity index, PDI), their surface charge, and their structure, respectively. Control liposomes had a very low PDI (0.07), which is typical of homogeneous formulations, and a hydrodynamic diameter (HD) of ~140 nm (), while the liposome–PC complexes had a wide size distribution (PDI 0.21). In particular, three main populations were detected in the liposome–PC complexes sample (): 1) a population of ~60 nm in HD attributable to plasma proteins and plasma protein aggregates and/or to liposomes that had shrunk due to the elastic properties of their lipid bilayer;Citation36,Citation37 2) a population with an HD slightly larger (≈180 nm) than control liposomes (ie, liposomes before incubation in plasma) ascribable to PC formation; and 3) a population of particles from two to three times larger than control liposomes (HD =400 nm), which are most likely due to PC-induced liposome aggregation.
Table 1 DLS analysis of liposomes before and after incubation in plasma
As given in , control liposomes had a slightly negatively charged surface (ζ-potential =−5 mV). The ζ-potential decreased to =−16 mV after the formation of the PC on the liposome surface, which is similar to the charge of plasma proteins (ζ-potential =−18 mV, data not shown).
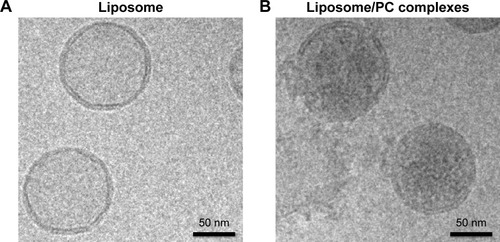
We used cryo-electron microscopy to evaluate the morphology of the liposome–PC complex. Single-vesicle analysis of control liposomes and liposome–PC complexes revealed that liposomes preserved their structure and shape after incubation in plasma (). In addition, unlike control liposomes (), additional mass adsorbed on liposome surfaces attributable to a compact PC was observed ().
Figure 1 Liposome and liposome/PC complex characterization by cryo-electron microscopy.
Notes: High-magnification cryo-EM images of liposomes before (A) and after incubation in plasma (B). Cryo-EM analysis reveals a spherical and unilamellar shape for both samples. Liposomes retained their shape and structure after incubation with plasma. Note the significant difference in electron density on the particle surface after plasma incubation, which indicates the presence of the PC (B).
Abbreviations: PC, protein corona; cryo-EM, cryo-electron microscopy.
We used AFM to identify changes in the mechanical properties of liposomes after PC formation. As shown in , liposome–PC complexes were larger than control liposomes (142–171 nm), which confirms our DLS results. Control liposomes resulted in smaller, homogeneous particles, while the liposome–PC complexes are relatively larger and heterogeneous. Size distribution analysis showed a large variation in particle size ranging from smaller values of 180–820 nm.
Figure 2 AFM showing the impact of the PC on the mechanical properties of liposomes.
Notes: Size distribution of liposomes and liposome–PCs (A). Young’s modulus property quantification (B). Young’s modulus property map of representative liposome (C) and liposome–PC (D) samples with AFM. Schematic representation of liposome aggregation process following exposure to plasma (E).
Abbreviations: AFM, atomic force microscopy; PC, protein corona.
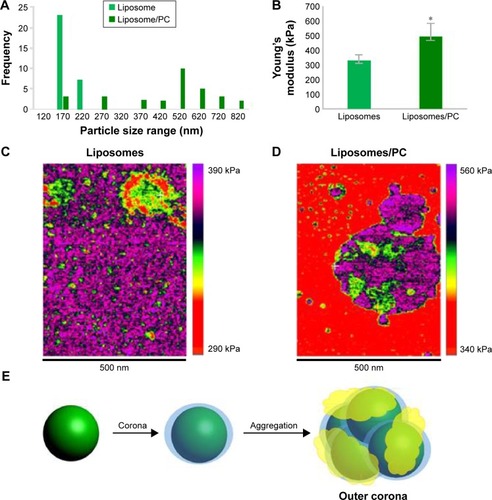
Quantitative AFM analysis () revealed that liposomes after incubation with plasma were significantly stiffer (P<0.005) than bare liposomes (470 kPa vs 310 kPa). The increased elastic modulus is most likely induced by the presence of particle aggregates with PC deposition. Bare liposomes contained a stiffer central area corresponding to the highest particle zone, while a more elastic region (the external ring in red) corresponded to the particle edges (). The liposome–PC aggregates map, instead, showed a heterogeneous elastic modulus variation over the large surface ().
Taken together, these data suggest that the formation of particle aggregates could be a two-step process (). As mentioned earlier, PC formation is a dynamic process.Citation38 Our analysis showed a heterogeneous scenario as a snapshot of the overall mechanism. The smaller particles found in the liposome–PC complex sample represent single particle–PC complexes. AFM analysis revealed that these individual liposome/PC complexes were stiffer than bare liposomes but softer than liposome–PC aggregates (). According to this finding, we speculate that once each liposome is covered by the first PC layer, it becomes adhesive, thereby inducing aggregation into larger clusters.Citation39 Moreover, the analysis of the elastic map revealed another protein adsorption process on the particle cluster that we call the “outer corona” (). In addition, although the cluster itself is stiffer than bare liposomes, there are some areas underneath the outer corona that are less stiff (). However, these softer areas remain stiffer than the bare liposomes (green) and are, instead, very similar in value to those of single liposome–PC complexes (). Therefore, we hypothesize a nonhomogeneous presence of the outer corona due to this difference in topographical arrangement. We speculate that formation of the outer corona might be triggered by the size of the PC rather than by its composition ().
Identification of proteins in the corona
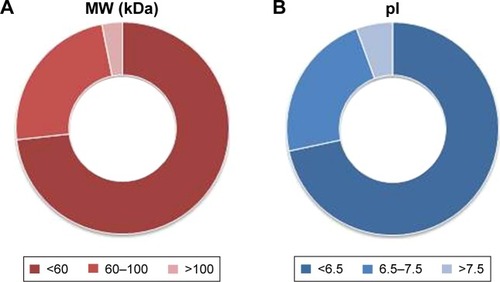
As reported in the section Characterization of liposomes before and after PC formation, liposomes were incubated in plasma for 1 hour to allow liposome–PC complex formation. Our aim was to define proteins constituting the PC in order to understand their potential biological effect on liposomes. To this aim, the adsorbed proteins (PCs) were eluted from the liposomes, separated by 1D SDS PAGE (), and identified by mass spectrometry. The most abundant proteins, selected by manual visualization and densitometric analysis (), were excised and hydrolyzed with trypsin. The 20 most abundant proteins adsorbed on the liposome surface after exposure to murine plasma are shown in , while the mass spectrometry profiles of the identified proteins are listed in . Overall, most PC components were small, negatively charged proteins (>70% with molecular weight [MW] <60 kDa, , and >70% with isoeletric point [pI] <6.5, ). A previous study showed that negatively charged particles preferentially attract positively charged proteins.Citation40,Citation41 Contrary to our expectation, negatively charged proteins preferentially adsorbed on our negatively charged liposomes. A similar trend was reported by others,Citation42,Citation43 and it supports the idea that predictions of PC composition exclusively based on NP charge are too simplistic. Electrostatic interactions are not the only force that drives NP–PC interactions. Many other factors (eg, protein abundance in plasma, the presence of a functional group on the NP surface, plasma type, and incubation time) affect PC composition. On the other hand, neutral and positive proteins are also present in the PC, though in small percents (). Considering our observation discussed above on the formation of a nonhomogenous corona, we believe that it is possible that these proteins create small positive charges or neutral patches on the particles’ surface, which facilitates aggregation by electrostatic or hydrophobic interactions.
Figure 3 Proteomic analysis of the PC of liposomes.
Notes: Liposomes were incubated for 1 hour at 37°C in plasma under agitation (A). NPs were centrifuged and extensively washed, and the PC was eluted in SDS loading buffer. SDS-PAGE (12% acrylamide) was carried out at 120 V for 90 minutes. Plasma was loaded as a control. Gels were Coomassie-stained. Plasma proteins on the surface of liposomes were quantified by densitometric analysis. The top five most abundant proteins are labeled by number in the graph (B). The top 20 most abundant proteins in the liposomes PC (C). Bands were selected and identified by LC-MS.
Abbreviations: PC, protein corona; NP, nanoparticle; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; LC-MS, liquid chromatography-mass spectrometry; MW, molecular weight.
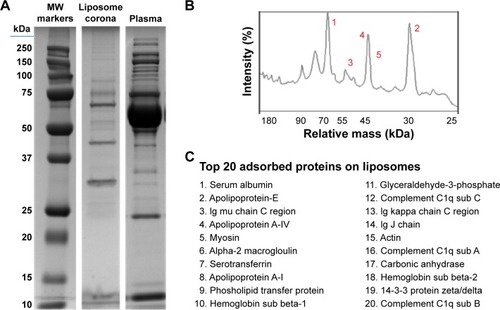
Figure 4 Clusterization of proteins in the corona.
Notes: Classification of proteins according to the MW (A) and pI (B) shows that 73.2% of proteins are smaller than 60 kDa and 71.6% of proteins have a pI <6.5, indicating that their overall charge in plasma (pH 7.4) is negative. Values reported represent molar percentages.
Abbreviations: MW, molecular weight; pI, isoelectric point.
In our study, albumin, apolipoproteins, and IgGs were the main PC components (), which confirms data obtained with other vesicle types.Citation35 To better understand the biological impact of the PC on particle–cell interactions, we investigated liposome and liposome–PC complex uptake by macrophages and breast cancer cells. We discuss the results in the next section.
Cellular uptake of liposomes before and after PCformation
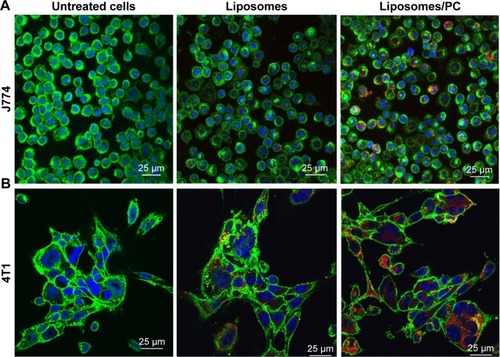
It is well recognized that the PC affects the fate of particles in the bloodstream.Citation44 Notably, the PC can affect particle clearance by immune cells,Citation8 thereby affecting their biodistribution properties. To obtain an overview of what could happen in the context of in vivo cancer therapy when therapeutic NPs are systemically injected, we studied their uptake by macrophages, which are responsible for particle clearance, and their internalization by cancer cells, which is essential for pharmacological efficacy. We used mouse J774 macrophages () and mouse 4T1 breast cancer cells () as proof-of-concept models for this in vitro study. We treated both cell lines with fluorescent particles, and then examined their interactions with NPs using confocal microscopy. The uptake of liposomes with a PC was greater than that of bare liposomes in both cell lines. Moreover, large particle aggregates formed in PC-endowed liposomes but not in bare liposomes (white arrows in ).
Figure 5 Uptake of liposomes by macrophages and cancer cells in the absence and presence of a PC.
Notes: Confocal images of J774 (A) and 4T1 (B) treated for 3 hours with bare liposomes and liposomes with PC. Cell membranes are stained by using WGA-Alexa fluor 488 (green), nuclei are DAPI-stained (blue), and particles are in red. The images reveal that the PC increased liposome uptake by both analyzed cell lines.
Abbreviation: PC, protein corona.
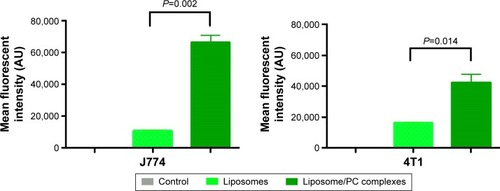
To determine the fraction of particles internalized by J774 and 4T1 cells, we carried out a flow cytometry analysis, in which cells were quenched with trypan blue to obscure the fluorescence associated with the cell surface. As shown in , the quantitative analysis confirmed the increased uptake of liposomes with a PC. These results concur with studies of NP uptake by immuneCitation45 and tumor cells.Citation46
Figure 6 Flow cytometry.
Notes: Histograms show the average fluorescent values of three experiments ± SD. Student’s t-test. Liposomes with a PC had a significantly higher uptake by J774 and 4T1 cells than bare liposomes. Naïve cells were used as controls.
Abbreviations: AU, arbitrary units; PC, protein corona; SD, standard deviation.
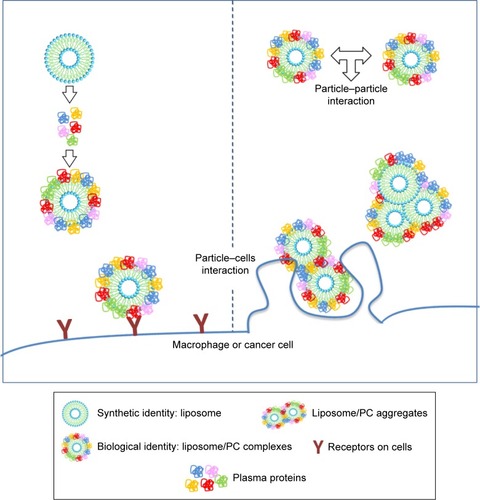
Data on the impact of the PC on the biological fate of NPs are controversial. Since PC formation is a highly dynamic process, it is difficult to envisage a unique explanation of the interfacial events that occur between the PC and biological components. For several decades, cell opsonization was considered the body’s first defense mechanism against foreign entities,Citation47 but recent studies have shown that the PC can shield particles from the action of adsorbed serum components (IgG and complement factors) that normally favor particle clearance.Citation19,Citation20 Here we report increased liposome uptake by both J774 and 4T1 cells after plasma incubation. We hypothesize that complement factors and apolipoproteins present in the PC are mainly responsible for this effect. Complement proteins, together with immunoglobulins and coagulation factors, mediate particle uptake through interactions with complement Fc and Toll-like receptors on the macrophage surface.Citation47 On the other hand, apolipoproteins belong to the family of lipoproteins, which are key players in the cholesterol metabolism process, through which they stimulate cancer cell growth.Citation48 Cholesterol availability is crucial for the proliferation of rapidly dividing cancer cells. Breast cancer cells, like other cancer cells, express B-class scavenger receptors on their surface,Citation49 which are intermediate in lipid transfer between lipoproteins and cells.Citation50 We believe that the functional motifs of these proteins are exposed on the PC, in the correct orientation to perform their function and to be recognized by their specific cell receptors, allowing PC-mediated cell internalization. These findings explain the impact of the PC, ie, the liposome’s new biological identity, on cellular uptake. On the other hand, our DLS, cryo-EM, and AFM experiments conducted to characterize liposomes provide insights into the impact of the PC also on the liposome’s new physical identity. We found that PC formation induces liposome aggregation, which implies the presence of clusters of NPs (, ) that coexist with single liposome–PC complexes, consistent with what has been demonstrated elsewhere.Citation51,Citation52 This means that cells interact not only with single particles surrounded by a PC through a receptor-mediated pathway but also with particles in the form of larger aggregates. The latter are likely to be internalized via phagocytosis, particularly in the case of macrophages, which would explain why we observed a major uptake by cells ().
Figure 7 Cellular uptake of liposome/PC complexes.
Notes: The incubation of liposomes with plasma resulted in liposome/PC complexes. The latter are heterogeneous and can be single vesicles surrounded by a PC and clusters of up to three vesicles (each of them surrounded by a PC) surrounded also by an outer corona. The cells (macrophage or cancer cells) can interact with single particles through a receptor-mediated pathway, and with larger aggregates via phagocytosis.
Abbreviation: PC, protein corona.
Conclusion
In the last decade, the biological components of synthetic NPs have attracted the attention of the scientific community because of the fundamental role they play in the interaction between particles and cells. Several assertions have been made to describe the PC’s composition and correlate it to NP uptake by immune and cancer cells. Thus far, the results have been controversial due to the variability of the experimental settings (particle chemical composition, plasma source, plasma concentration, particle incubation time, cell lines, etc).Citation8 In this study, we reported the structure, composition, and biological effect of PCs adsorbed on multicomponent liposomes. We found that the formation of a PC on liposomes makes their surface adhesive, thus inducing the formation of particle clusters that coexist with single vesicles. We also showed that internalization of liposome–PC complexes by both macrophages and cancer cells was greater than that of control liposomes. The presence of a PC strongly affects the NP’s biological response. This can be due to 1) the presence of molecules (ie, immunoglobulins and apolipoproteins) in the PC that recognize specific receptors on the surface of immune and cancer cells, and/or 2) the formation of NP cluster that induce phagocytosis by the cells, or 3) a combination of these effects.
We shed light on the impact of the PC first on particle–particle and then on particle–cell interactions. Our observations suggest that to predict the fate of a NP in vivo, one must not only know the PC composition and eventually the orientation of proteins, but should also carefully consider the physical changes (size, surface, and charge) that the NP undergoes when surrounded by a PC and the consequent impact on particle–particle interactions. This study shows that the efficacy of liposomal formulations must be reinterpreted, as it is strongly affected by PC formation in plasma. Classical characterization of nanodelivery systems should include studies on PC formation and be combined with experiments that simulate the tendency of NPs to aggregate after exposure to plasma proteins. We believe that such studies will hopefully help us interpret and predict in vivo data and to design more effective therapeutic carriers.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors would like to thank Kemi Cui and HMRI Advanced Cellular and Tissue Microscope Core Facility for traditional confocal scanning services, David Haviland and the HMRI Flow Cytometry Core Facility for flow cytometry setup and acquisition, and DA Engler, RK Matsunami, and the HMRI Proteomics Programmatic Core Laboratory for mass spectrometry analyses. The authors acknowledge the Sealy Center for Structural Biology and Molecular Biophysics at the University of Texas Medical Branch at Galveston for providing research resources. We thank Jean Ann Gilder (Scientific Communication srl. Naples, Italy) and Megan Livingston for editing the text. We thank Associazione Bianca Garavaglia, Via C Cattaneo 8, 21052 Busto Arsizio, Varese, Italy. This work was supported by grants RF-2010-2318372 and RF-2010-2305526 from Italian Ministry of Health, and by grants 1R21CA173579-01A1 from NIH/NCI, 5U54CA143837 PSOC Pilot project from NIH/NCI, W81XWH-12-10414 BCRP Innovator Expansion from Department of Defense, William Randolph Hearst Foundation, and The Regenerative Medicine Program Cullen Trust for Health Care to ET and by POR Campania FSE 2007–2013 Project DIAINTECH, Italy (to FS).
Supplementary materials
Figure S1 AFM elastic map of a single liposome/PC complex.
Note: The single liposome/PC vesicles are stiffer than bare liposomes, but softer than liposome/PC aggregates.
Abbreviations: AFM, atomic force microscopy; PC, protein corona.
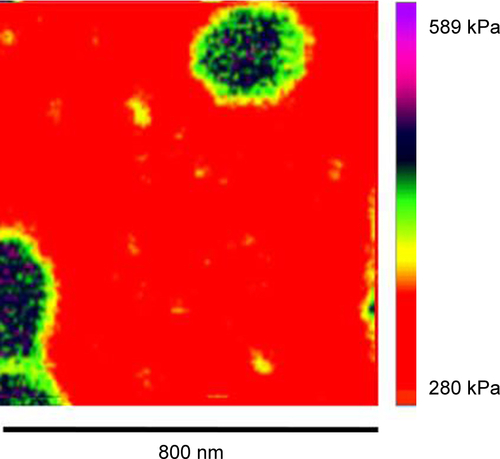
Figure S2 AFM analysis of the height of a liposome/PC complex.
Notes: Offline image processing of an AFM image of a representative liposome/PC complex. The original scan of this single spherical liposome was obtained in height mode. Using offline Nanoscope software, the liposome was magnified further, and then a section analysis was performed. Along the whole particle profile, there are regions at different heights (differences =5 nm).
Abbreviations: AFM, atomic force microscopy; PC, protein corona.
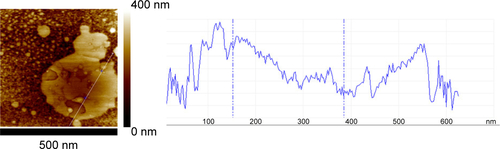
Figure S3 AFM elastic map of another area of the liposome/PC samples.
Notes: The image reveals a more complete picture of the mechanical properties of the particle aggregates. In detail, the image shows three different regions imputed to the presence of a cluster of three particles (yellow dotted line).
Abbreviations: AFM, atomic force microscopy; PC, protein corona.
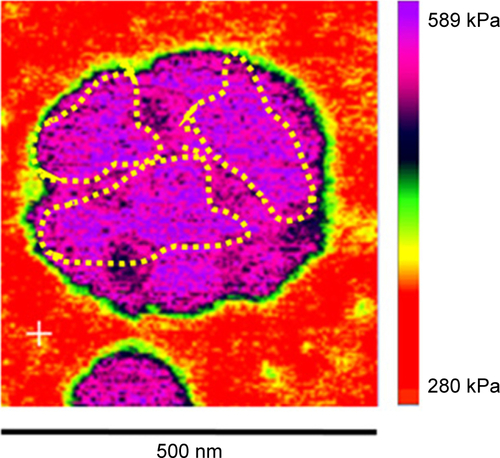
Figure S4 Confocal images of macrophages and cancer cells treated with liposomes in the absence and presence of a PC.
Note: The increased uptake and the presence of particles’ cluster (white arrows) in both J774 and 4T1 cells in the liposomes after incubation in plasma.
Abbreviation: PC, protein corona.
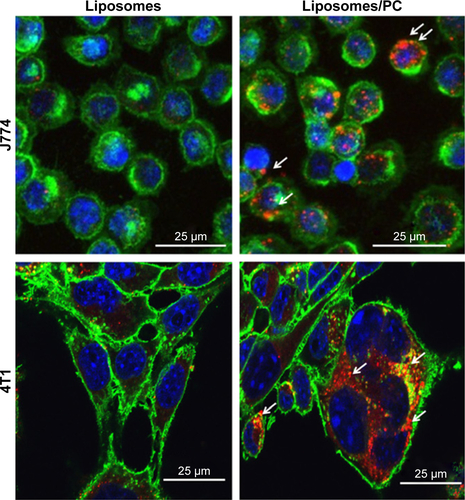
Table S1 Mass spectrometry details of the proteins identified in the corona
References
- SinghRLillardJWNanoparticle-based targeted drug deliveryExp Mol Pathol200986321522319186176
- LundqvistMNanoparticles: tracking protein corona over timeNat Nanotechnol201381070170224056903
- TenzerSDocterDKuharevJRapid formation of plasma protein corona critically affects nanoparticle pathophysiologyNat Nanotechnol201381077278124056901
- LynchIDawsonKAProtein-nanoparticle interactionsNano Today2008314047
- MirsadeghiSDinarvandRGhahremaniMHProtein corona composition of gold nanoparticles/nanorods affects amyloid beta fibrillation processNanoscale20157115004501325695421
- O’ConnellDBombelliFBPitekAMonopoliMCahillDDawsonKACharacterization of the bionano interface and mapping extrinsic interactions of the corona of nanomaterialsNanoscale2015737152681527626324751
- ZanganehSSpitlerRErfanzadehMAlkilanyAMMahmoudiMProtein corona: opportunities and challengesInt J Biochem Cell Biol20167514314726783938
- CorboCMolinaroRParodiAToledano FurmanNESalvatoreFTasciottiEThe impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug deliveryNanomedicine20161118110026653875
- SalvatiAPitekASMonopoliMPTransferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surfaceNat Nanotechnol20138213714323334168
- MirshafieeVMahmoudiMLouKChengJKraftMLProtein corona significantly reduces active targeting yieldChem Commun (Camb)201349252557255923423192
- QiuYLiuYWangLSurface chemistry and aspect ratio mediated cellular uptake of Au nanorodsBiomaterials201031307606761920656344
- GeCDuJZhaoLBinding of blood proteins to carbon nanotubes reduces cytotoxicityProc Natl Acad Sci U S A201110841169681697321969544
- GeCTianJZhaoYChenCZhouRChaiZTowards understanding of nanoparticle–protein coronaArch Toxicol201589451953925637415
- SahnehFDScoglioCMMonteiro-RiviereNARiviereJEPredicting the impact of biocorona formation kinetics on interspecies extrapolations of nanoparticle biodistribution modelingNanomedicine (Lond)2015101253325032980
- MilaniSBaldelli BombelliFPitekASDawsonKARädlerJReversible versus irreversible binding of transferrin to polystyrene nanoparticles: soft and hard coronaACS Nano2012632532254122356488
- LiuWRoseJPlantevinSAuffanMBotteroJ-YVidaudCProtein corona formation for nanomaterials and proteins of a similar size: hard or soft corona?Nanoscale2013541658166823334428
- KarmaliPPSimbergDInteractions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systemsExpert Opin Drug Deliv20118334335721291354
- MoghimiSMSzebeniJStealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding propertiesProg Lipid Res200342646347814559067
- MirshafieeVKimRParkSMahmoudiMKraftMLImpact of protein pre-coating on the protein corona composition and nanoparticle cellular uptakeBiomaterials20167529530426513421
- CaraccioloGPalchettiSColapicchioniVStealth effect of biomolecular corona on nanoparticle uptake by immune cellsLangmuir20153139107641077326378619
- HeCHuYYinLTangCYinCEffects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticlesBiomaterials201031133657366620138662
- KulkarniSAFengS-SEffects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug deliveryPharm Res201330102512252223314933
- CaraccioloGThe protein corona effect for targeted drug deliveryBioinspired Biomimetic Nanobiomater2013215457
- MahonESalvatiABombelliFBLynchIDawsonKADesigning the nanoparticle–biomolecule interface for “targeting and therapeutic delivery”J Control Release2012161216417422516097
- Van RooijenNvan NieuwmegenRLiposomes in immunology: multilamellar phosphatidylcholine liposomes as a simple, biodegradable and harmless adjuvant without any immunogenic activity of its ownImmunol Commun1980932432567399568
- LeeHBlaufoxMBlood volume in the ratJ Nucl Med198526172763965655
- MonopoliMPWalczykDCampbellAPhysical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticlesJ Am Chem Soc201113382525253421288025
- MaugisDContact, Adhesion and Rupture of Elastic Solids130BerlinSpringer Science & Business Media2013
- CorboCOrrùSGemeiMProtein cross-talk in CD133+ colon cancer cells indicates activation of the Wnt pathway and upregulation of SRp20 that is potentially involved in tumorigenicityProteomics201212122045205922623141
- CaterinoMCorboCImperliniEDifferential proteomic analysis in human cells subjected to ribosomal stressProteomics20131371220122723412928
- CorboCParodiAEvangelopoulosMProteomic profiling of a biomimetic drug delivery platformCurr Drug Targets201516131540154725382209
- LiG-ZVissersJPSilvaJCGolickDGorensteinMVGeromanosSJDatabase searching and accounting of multiplexed precursor and product ion spectra from the data independent analysis of simple and complex peptide mixturesProteomics2009961696171919294629
- NuutilaJLiliusEMFlow cytometric quantitative determination of ingestion by phagocytes needs the distinguishing of overlapping populations of binding and ingesting cellsCytometry A20056529310215825183
- WalczykDBombelliFBMonopoliMPLynchIDawsonKAWhat the cell “sees” in bionanoscienceJ Am Chem Soc2010132165761576820356039
- Barrán-BerdónALPozziDCaraccioloGTime evolution of nanoparticle–protein corona in human plasma: relevance for targeted drug deliveryLangmuir201329216485649423631648
- WolframJSuriKYangYShrinkage of pegylated and non-pegylated liposomes in serumColloids Surf B Biointerfaces201411429430024216620
- HadjidemetriouMAl-AhmadyZMazzaMCollinsRFDawsonKKostarelosKIn vivo biomolecule corona around blood-circulating, clinically used and antibody-targeted lipid bilayer nanoscale vesiclesACS Nano2015988142815626135229
- MiclausTBochenkovVEOgakiRHowardKASutherlandDSSpatial mapping and quantification of soft and hard protein coronas at silver nanocubesNano Lett20141442086209324617413
- GräfeCWeidnerAvd LüheMIntentional formation of a protein corona on nanoparticles-serum concentration affects protein corona mass, surface charge, and nanoparticle-cell interactionInt J Biochem Cell Biol20167519620226556312
- NelAEMädlerLVelegolDUnderstanding biophysicochemical interactions at the nano–bio interfaceNat Mater20098754355719525947
- MahmoudiMLynchIEjtehadiMRMonopoliMPBombelliFBLaurentSProtein-nanoparticle interactions: opportunities and challengesChem Rev201111195610563721688848
- WalkeyCDChanWCUnderstanding and controlling the interaction of nanomaterials with proteins in a physiological environmentChem Soc Rev20124172780279922086677
- TenzerSDocterDRosfaSNanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysisACS Nano2011597155716721866933
- LundqvistMStiglerJEliaGLynchICedervallTDawsonKANanoparticle size and surface properties determine the protein corona with possible implications for biological impactsProc Natl Acad Sci U S A200810538142651427018809927
- YanYGauseKTKamphuisMMDifferential roles of the protein corona in the cellular uptake of nanoporous polymer particles by monocyte and macrophage cell linesACS Nano2013712109601097024256422
- MulikRSMonkkonenJJuvonenROMahadikKRParadkarARTransferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosisInt J Pharm20103981–219020320655375
- RicklinDHajishengallisGYangKLambrisJDComplement: a key system for immune surveillance and homeostasisNat Immunol201011978579720720586
- JohnsonWJMahlbergFHRothblatGHPhillipsMCCholesterol transport between cells and high-density lipoproteinsBiochim Biophys Acta1991108532732981911862
- CaoWMMuraoKImachiHA mutant high-density lipoprotein receptor inhibits proliferation of human breast cancer cellsCancer Res20046441515152114973113
- ActonSRigottiALandschulzKTXuSHobbsHHKriegerMIdentification of scavenger receptor SR-BI as a high density lipoprotein receptorScience199627152485185208560269
- PalchettiSColapicchioniVDigiacomoLThe protein corona of circulating PEGylated liposomesBiochim Biophys Acta20161858218919626607013
- PozziDCaraccioloGDigiacomoLThe biomolecular corona of nanoparticles in circulating biological mediaNanoscale2015733139581396626222625
