Abstract
We developed a novel method to prepare nanocapsules. A solute often crystallizes when its solubility alters from one solvent to another, and its firstborn crystals are used as templates to prepare nanocapsules for the first time, which is called firstborn microcrystallization method. By using this method, the maximum diameter of the nanocapsules including artesunate is about 76 nm, and wrapping state is well. One important advantage of our method is that the preparation of the nanocapsules operates easily and is a one-time process with no other cumbersome processes necessary, therefore avoiding secondary pollution. The proposed method provides a new route to prepare monodisperse nanocapsules to increase bioavailability of hydrophobic solutes.
Introduction
Drug delivery system (DDS) has been a hot issue in the pharmaceutical field for a long time. To realize this system, some strategies have been explored.Citation1–Citation5 One main strategy has been using submicrometer or nanometer-sized capsules as carriers of a drug. Their characteristics are larger inner volume and much higher interfacial area per unit mass of macro- or nanoparticles, which help carry more drug and having much greater exchange rates.Citation6 Furthermore, microencapsulation of drugs can reduce its toxic side effects and improve its stability. Especially for intravenous injection, the nanocapsules with drugs can pass the smallest capillaries in the body without blockage. Therefore the preparation of the nanocapsules is of both scientific and technological interest.
Artesunate, a water-soluble derivative of artemisinin, which is a traditional Chinese medicine, is a product of artemisinin modified with hydroxyl groups. Its solubility in water is slightly better than that of artemisinin while both do not dissolve in water. It is a white powder, odorless, almost tasteless, and can dissolve easily in ethanol or ethyl acetate. It can play major roles in the asexual body of plasmodium in the erythrocytic stage and can also be used to treat malaria, particularly in multidrug-resistant falciparum malaria, antiprogesterone, antifibrosis, antischistosomiasis, antitoxoplasma, antiarrhythmia, and tumor cell toxicity, and so on. Its structure is shown in . In certain applications, however, a nanocapsule including artesunate is preferred to investigate its behavior, dynamics, and bioavailability. Unfortunately, until very recently, little attention has been paid to microencapsulation of artesunate.
Preparation of microcapsules have different methods such as nanoprecipitation,Citation7,Citation8 emulsion–diffusion,Citation9 double emulsification,Citation10 emulsion–coacervation,Citation11 polymer-coating,Citation12 layer-by-layer,Citation13 and self-templating.Citation14 While the range of size generally obtained is 100–500 nmCitation15 which is not nanoscale in the strict sense of the term, and the preparation is a multistep process including removing emulsifier or templates, loading drug, which means secondary pollution and a long cycle.Citation16 Up till now, research on the nanocapsules is still continuing.
In this work, we report on a novel method, called firstborn microcrystallization method, to prepare drug-containing nanocapsules. Because there is different solubility of drug in different solvents, its firstborn crystals, which arise when the saturated drug in one solvent with larger solubility adds to other with smaller solubility, are used as templates to prepare nanocapsules. To explore preparation conditions, effects of some factors such as organic solvent, formaldehyde dosage, artesunate dissolving and curing temperature, and the mass ratio of gelatin and artesunate on the nanocapsules were investigated.
Materials and methods
Materials
Artesunate powder (98.99%, W/W) was purchased from Wuhan Kanglong Pharmaceutical LTD Company, China. Gelatin (Mmw = 15000–25000; isoelectric point = 4.9) was chemical grade and purchased from Tianjin Tai Lande Chemical Reagent Factory, China. Ethanol alcohol, glacial acetic acid, and formaldehyde were reagent grade (Tianjin Kermel Chemical Reagent LTD Company, China). All were used as received and without any further purification. Well deionized water was used.
Methods
Preparation of nanocapsules by using firstborn microcrystallization method
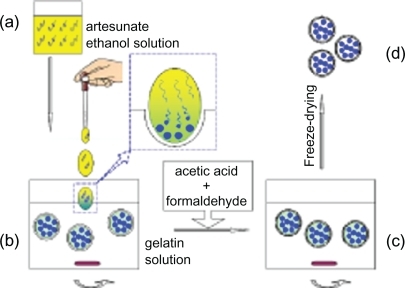
The proposed method included five steps. In the first step, 2 g of gelatin dissolved in 100 mL of water, the mixture was stirred for more than 24 hours at room temperature to form gelatin solution, and the impurities removed by using vacuum distillation. In the second step, artesunate dissolved sufficiently in hydrophilic organic solvent to saturated solution by stirring continuously. And 10% of acetic acid aqueous solution was obtained. In the third step, saturated artesunate solution was dropped into 15 mL of gelatin solution heating at 40°C at low-speed stirring (about 150 rpm). Next, acetic acid solution was added into this mixture to regulate pH 4.9, and then cooled to room temperature. Finally 0.25 mL of formaldehyde, a curing agent, was added slowly at temperature and stirred continuously for 30 min to cure the walls of the capsules. The resulting samples were dialyzed and purified by vacuum distillation and then freeze-dried.
Preliminary determination of experimental results
The aim is to explore preparation conditions of the nanocapsules by using firstborn microcrystallization method. To save time and narrow scopes, Tyndall scattering phenomena and phase separation were used to detect the samples.
Morphological analysis
Morphology is a critical factor to analyze final result. In this work, the morphology of nanocapsules was observed by a TECNAI G2–20 S-Twin-type (Czech Republic) transmission electron microscope (TEM) operating at 100 kV.
Results and discussion
Preparation theory
After the saturated artesunate solution was dropped into water including enough wall materials, gelatin, the hydrophilic organic solvent dissolved immediately in water. Because the solubility parameter of hydrophilic organic solvent is less than that of water, artesunate crystallizes in time. Once its firstborn crystals with nanoscale arose, the wall materials gathered on its surface and wrapped gradually the crystals. Finally the wall materials deposited on the surface to form a continuous membrane, and then form the walls of capsules by adding a curing agent (as shown in ).
Preliminary determination
Tyndall phenomenon is a significant characteristic for colloid solution. Because artesunate cannot dissolve in water, the solution including the products treated by vacuum have Tyndall phenomenon and no phase separation after microencapsulating, which means that the solution is colloid solutions and the capsules including artesunate must belong to the size of colloid. Thus Tyndall phenomenon and phase separation may be used as preliminary determination of the samples.
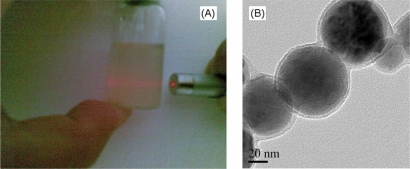
are Tyndall phenomenon and TEM photos, respectively, of the sample when the mass ratio of gelatin and artesunate is 5. Tyndall phenomenon is obvious and there is no phase separation in . From , artesunate was wrapped in higher quality, and the structure of the capsules is very clear, and its maximum size is about 76 nm, which means it is reliable to prepare the nanocapsules by using firstborn microcrystallization method.
Selection of solvents
In the experiments ethanol alcohol and acetone were chosen as solvents. When ethanol alcohol being a solvent, artesunate dissolved well, and there is no precipitating artesunate. While acetone being a solvent, some artesunate precipitated and was not wrapped completely. Because the solubility of artesunate in ethanol alcohol is much higher than that in acetone, and acetone is more volatile than ethanol alcohol resulting in artesunate precipitating easily when the outside temperature is higher. Simultaneously acetone has the toxicity, thus ethanol alcohol was selected as solvent in following experiment. In this study, as wall materials of nanocapsules, gelatin contains mainly protein, therefore much more ethanol alcohol will make gelatin variability and the best artesunate ethanol solution is a saturated solution.
Heat during artesunate dissolving
Because the process of artesunate dissolving in ethanol alcohol is slow, heat was used to promote dissolution. The results displayed that all products by heating at 40°C have precipitation and no Tyndall scattering phenomenon, while the results for others not heating is exciting. The possible reason is that the peroxy bond in artesunate reacts with hydroxyls resulting from ethanol alcohol when heated.
The mass ratio of gelatin and artesunate
Gelatin is a water-soluble protein and has good film-forming characteristics, so it is nontoxic and widely used to prepare microcapsules as wall materials. Overdose of gelatin leads easily to not only the walls of capsules thickening and the monodispersity being not well, but also the nanocapsules crosslinking each other and the final products caking. While its lowdose results in poor encapsulation, some artemisia crystallizating in water, and affected morphology of the capsules. Therefore gelatin dosage is the most influential factor.
In the work, when the mass ratio of gelatin and artesunate is less than 1, the resulting solution has phase separation, which means the experiment fails. When the mass ratio increased from 1, 3 to 5, all products had Tyndall phenomenon, and the phenomenon was more obvious, which mean the package of the nanocapsules is more complete. are the freeze-drying samples and TEM photos of different mass ratio, respectively. shows that the dried product is a small and white powder when the ratio is equal to 1, while the products are coarser and the crosslinking degree increases with increasing the ratio. shows that the diameter becomes larger and the crosslinking degree among the nanocapsules increases when the ratio increases, which was used to explain the scattering phenomenon is more obvious or the crosslinking degree among the nanocapsules with increasing the ratio.
Formaldehyde
Formaldehyde plays the role in curing the walls of the capsules in the study. When gelatin meets formaldehyde at pH 4.9, the curing reaction of the capsules happens. Gelatin, the walls of the capsules, fall off easily when the temperature is too high, so addition of formaldehyde helps to cure the walls. If an excessive amount of formaldehyde cannot remove in time after the curing experiment is finished, it is easy to form the cross-linking gels among the capsules and the gel is not reversible. While formaldehyde is so small that a certain amount of the capsules falls and the formation of the capsules is not complete. In the work, curing time was kept 30 min, and then formaldehyde was removed by using vacuum distillation.
Curing temperature
Control of curing temperature is the key to the success of the experiment. In order to explore a temperature range, higher than 5°C and 0–5°C were chosen because the mixed solution is frozen when the temperature is lower than 0°C. Preliminary results are that there has precipitation in the solution for gelatin itself being off when the temperature is higher than 5°C. When the temperature is from 0–5°C, the results are ideal. Therefore 0–5°C was selected as curing temperature.
Conclusion
In summary, a novel strategy called firstborn microcrystallization method was developed to explore preparation conditions of the nanocapsules. Effects of some factors such as organic solvent, formaldehyde dosage, artesunate dissolving and curing temperature, and the mass ratio of gelatin and artesunate on the nanocapsules were investigated. By using this method, one important advantage is that the preparation is easy to operate and is a one-time process with no other cumbersome processes, therefore avoiding secondary pollution for the final product. The method can be applied to prepare monodisperse nanocapsules to increase bioavailability of hydrophobic solutes, which is highly attractive for targeting drug delivery systems, chemical separations, sensors and so on.
Acknowledgements
This work was financially supported by the Academic Team Foundation of South-Central University for Nationalities (Research and Development of Ethnodrug, XTZ09010).
Disclosure
No conflicts of interest were declared in relation to this paper.
References
- JuXJChuLYLiuLMiPLeeYMA novel thermoresponsive hydrogel with ion-recognition property through supramolecular host-guest complexationJ Phy Chem B200811211121118
- JuXJChuLYMiPSongHLeeYMSynthesis and characterization of a novel thermo-sensitive copolymer of N-isopropylacrylamide and dibenzo-18-crown-6-diacrylamideMacromol Rapid Commun20062720722077
- ZhuYShiJShenWDongXFengJRuanMLiYStimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core-shell structureAngew Chem Int Ed20054450835087
- XiaoXCChuLYChenWMWangSXieRPreparation of submicrometer-sized monodispersed thermoresponsive core-shell hydrogel microspheresLangmuir2004205247525315986659
- XiaoXCChuLYChenWMWangSLiYPositively thermo-sensitive monodisperse core-shell microspheresAdv Funct Mater200313847852
- ChuLYYamaguchiTNakaoSA molecular recognition microcapsule for environmental stimuli responsive controlled releaseAdv Mater200214386389
- Mora-HuertasaCEFessiHElaissariAPolymer-based nanocapsules for drug deliveryInt J Pharm201038511314219825408
- FessiHPuisieuxFDevissaguetJPProcédé de préparation de systèmes colloïdaux dispersibles d’une substance sous forme de nanocapsulesEuropean Patent1988 274961 A1.
- QuintanarDFessiHDoelkerEAllemanEMethod for preparing vesicular nanocapsulesUS Patent2005 6884438
- GrigorievDMillerRMono- and multilayer covered drops as carriersCurr Opin Colloid Interf Sci2009144859
- LertsutthiwongPNoomunKJongaroonngamsangNRojsitthisakPNimmannitUPreparation of alginate nanocapsules containing turmeric oilCarbohydr Polym200874209214
- AntonNBenoitJPSaulnierPDesign and production of nanoparticles formulated from nano-emulsion templates – a reviewJ Control Release200812818519918374443
- ChenYLinXParkHGreeverRStudy of artemisinin nanocapsules as anticancer drug delivery systemsNanomedicine2009531632219523432
- LiuPLiuGFZhangWJiangFCrosslinked polymeric nanocapsules with controllable structure via a ‘self-templating’ approachNanotechnology20102116
- QuintanarDAllémannEFessiHDoelkerEPreparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymersDrug Dev Ind Pharm199824111311289876569
- ZhaLZhangYYangWFuSMonodisperse temperature-sensitive microcontainersAdv Mater20021410901092
![Figure 4 The dried samples (A) and TEM photographs (B) of the nanocapsules of the mass ratio of gelatin and artesunate being 1 [1], 3 [2] and 5 [3].](/cms/asset/a0ec4b6d-d79a-4672-9a72-bf3521f34b05/dijn_a_12184621_f0004_c.jpg)