Abstract
Diabetic neuropathy (DN) is a debilitating disorder occurring in most diabetic patients without a viable treatment yet. The present work examined the protective effect of 25Mg-PMC16 nanoparticle (porphyrin adducts of cyclohexil fullerene-C60) in a rat model of streptozotocin (STZ)-induced DN. 25Mg-PMC16 (0.5 lethal dose50 [LD50]) was administered intravenously in two consecutive days before intraperitoneal injection of STZ (45 mg/kg). 24Mg-PMC16 and MgCl2 were used as controls. Blood 2,3-diphosphoglycerate (2,3-DPG), oxidative stress biomarkers, adenosine triphosphate (ATP) level in dorsal root ganglion (DRG) neurons were determined as biomarkers of DN. Results indicated that 2,3-DPG and ATP decreased whereas oxidative stress increased by induction of DN which all were improved in 25Mg-PMC16-treated animals. No significant changes were observed by administration of 24Mg-PMC16 or MgCl2 in DN rats. It is concluded that in DN, oxidative stress initiates injuries to DRG neurons that finally results in death of neurons whereas administration of 25Mg-PMC16 by release of Mg and increasing ATP acts protectively.
Introduction
The number of people with diabetes is developing due to population growth, aging, and increasing prevalence of obesity and physical inactivity. The total number of people with diabetes is estimated to rise from 171 million in 2000 to 366 million in 2030.Citation1 Diabetic neuropathy (DN) usually develops in about 4%–10% of diabetic patients after five years and in 15% after 20 years.Citation2 It is estimated that at least 50% of all diabetic patients will suffer from neuropathy in their lifetime.Citation3
Hyperglycemia in diabetes causes an overflow of the polyol pathway, advanced glycation, oxidative stress, and deficiencies in neurotrophic factors. On the other hand, hypoinsulinemia results in mitochondrial dysfunction. Unfortunately, to date, a viable treatment for human DN is not available and all current drugs like tricyclic antidepressants, anticonvulsant agents, aldose reductase inhibitors, and drugs such as Y-linolenic acid, zenarestat, mexiletine and midodrine are only used for management.Citation5 Oxidative stress is established to have a fundamental role in development of DN through induction of mitochondrial dysfunction, decline of adenosine triphosphate (ATP), and neural death. 2,3-diphosphoglycerate (2,3-DPG) as one of the factors contributing to tissue hypoxia is decreased in DN. Reduced levels of 2,3-DPG in DN result in a defect in delivery of oxygen to dorsal root ganglion (DRG) neurons leading to hypoxia and neuron death. It is believed that any rise in ATP and 2,3-DPG improve oxygenation.Citation4–Citation6
Recently, nanoparticles of a magnetic isotope of magnesium (25Mg-PMC16) has been developed with low toxicity and membranotropic antioxidant effect. This nanoparticle, which possesses marked cationite properties, is the iron containing porphyrin monoadduct of a classical buckminster fullerene named “Porphylleren-MC16” or, in brief, PMC16. 25Mg isotopes that are released by this nanoparticle activate both substrate and oxidative phosphorylation pathways in acidosis condition and stimulate production of ATP in oxygen-depleted cells.Citation7,Citation8 Positive antiapoptotic and antioxidant effects of Mg, fullerene, and porphyrin that exist within structure of this nanoparticle have been previously known.Citation9–Citation12
Regarding accumulation potential of this nanoparticle in the neuronal mitochondria,Citation7 and the pathophysiology of DN, the hypothesis of this study was that 25MgPMC16 by reduction of oxidative stress and increasing ATP in DRG neurons would protect mitochondrial dysfunction in DN. Therefore, the possible effects of 25Mg-PMC16 in an experimental model of DN were examined in comparison to nonmagnetic 24Mg-PMC16 and the ordinary form of Mg (MgCl2).
Methods
Materials
Adenosine diphosphate (ADP) sodium salt, ATP disodium salt, Tris base, 1,1,3,3-tetraethoxypropane (MDA), 5,5′-dithiobis-2-nitro benzoic acid (DTNB), methanol (high performance liquid chromatography [HPLC]-grade), trichloroacetic acid (TCA), potassium hydroxide, MgCl2, diethyl ether, ketamine, xylazin, tetrabutylammonium hydroxide (TBAHS), n-butanol, 2-thiobarbituric acid (TBA), KH2PO4 (analytical grade), and 2,4,6-tripyridyl-s-triazine (TPTZ) from Merck (Tehran), streptozotocine (STZ) from Pharmacia and Upjohn (USA), 25Mg-PMC16 and 24Mg-PMC16 from Semenov institute (Russian Academy of Sciences), A SUPELCOSIL™ LC-18-T HPLC column from SUPELCO (UK) and 2,3-DPG determination kit from Roche (Germany) were used in this study.
Treatment
Male Wistar rats weighing 200 ± 5 g were randomly divided in five groups with six rats in each group containing the control group that received nothing, DN group which received a single intraperitoneal (ip) injection of STZ (45 mg/kg), the protective group with 25Mg-PMC16 received two intravenous (iv) injections of 25Mg-PMC16 (0.5 lethal dose50 [LD50] = 448 mg/kg) with 48 hours interval before receiving STZ by ip injection, the protective groups with 24Mg-PMC16 and MgCl2 received two iv injections of 24Mg-PMC16 and MgCl2 (0.5 LD50= 448 and 88 mg/kg, respectively) with two days intervals followed by a single ip injection of STZ. Different doses of the nanoparticle according to its LD50 and literature were selected and tested. At first, the work was started with 0.2 LD50 according to previous study (7), but no suitable response was received. Then the dose was increased to 0.5 LD50 and the proper effects were observed in all experiments.
In vivo studies were carried out according to ethical guidelines on the use of animals in research and the protocol was approved by institute review board.
Induction of DN
Experimental diabetes was induced by ip injection of dissolved STZ in normal saline with pH of 7 at the dose of 45 mg/kg. Before an injection of STZ, animals were fasted for 12 hours. Within one week of injection, animals became hyperglycemic, with raised blood glucose between 250 mg/dl and 500 mg/dl that resulted in DN after two months.Citation13
Measurement of blood glucose and weight
Blood glucose and weight of animals at the beginning of study and after two months were measured using a glucometer and a special balance.
Sample preparation
Sample preparation included blood, plasma, and tissues. At first, animals were anesthetized with intramuscular (im) injection of ketamine (4 mg/100 g) and xylazine (1 mg/100 g) mixture, then the abdomen was opened and blood (5 ml) was collected from the heart into a heparinized syringe. Collected blood samples were centrifuged at 1200 g for 10 min at 4°C and plasma frozen at −80°C until further analysis. For tissue preparation, after anesthetizing the animals, the spinal cord and paraspinal tissue from the second cervical to the second lumbar vertebra were removed altogether. Then the cervical spine laminae were removed until the spinal ganglia were exposed. The sixth cervical (C6) DRG on each side was removed and frozen quickly in liquid nitrogen.
Measurement of 2,3-DPG
This measurement was carried out using a kit. Since 2,3-DPG is too labile, the deproteinization procedure had to be performed immediately using perchloric acid (0.6 M) and potassium carbonate solution (2.5 M). 2,3-DPG is stable for at least one day in the neutralized extracts and thus the samples were tested within 24 hours. 2,3-DPG is split by the side activity of phosphoglycerate mutase (PGM) to form phosphoglycerate (PG). Both, 2-PG and 3-PG can be formed. 2-PG is isomerized into 3-PG. 3-PG is converted by phosphoglycerate kinase (PGK), glyceraldehyde-3-phosphate dehydrogenase (GAP-DH), triosephosphate isomerase (TIM) and glycerol-3-phosphate dehydrogenase (GDH). Meanwhile, 2 mole of nicotinamide adenine dinucleotide (NADH) is oxidized per mole of 2,3-DPG. Then absorption of NADH was determined at 340 nm.Citation14
Measurement of total plasma antioxidant capacity
The ability of plasma to reduce Fe3+ to Fe2+ is an indicator of plasma antioxidant capacity. The complex between Fe2+ and 2,4,6-tris(2-pyridyl)-1,3,5-triazine(TPTZ) gives a blue color with absorbance at 593 nm that is read by spectrophotometer.Citation15
Measurement of plasma total thiol groups
Total sulfhydryl level was determined as described previously.Citation16 A plasma volume of 0.2 ml was mixed with 0.6 ml of Tris – EDTA buffer [Tris base (0.25 M), EDTA (20 mM), pH 8.2] in a 10 ml test tube and then mixed with 40 ml of DTNB (10 mM) in methanol. The final volume was made up to 4.0 ml by adding 3.16 ml of methanol. The test tube, after capping, centrifuged at 3000 g for 10 min at ambient temperature. After 15–20 min, the color appeared. The absorbance of the supernatant was measured at 412 nm.Citation16
Measurement of lipid peroxidation (LPO)
LPO of plasma was measured by the reaction of TBA with MDA and other lipid peroxides. Plasma samples were mixed with TCA (20%) and the produced precipitate was dispersed in H2SO4 (0.05 M). After addition of TBA (0.2% in sodium sulfate) the samples were heated for 30 minutes in a boiling water bath. Then LPO adducts were extracted by n-butanol and absorbance was read at 532 nm.Citation17
Measurement of adenosine diphosphate (ADP) and ATP
The frozen DRG was removed on ice and was quickly homogenized (4°C) in 1 mL of ice-cold 6% TCA. The homogenate was centrifuged at 12000 g for 10 min at 4°C. The supernatant was neutralized to a pH of 6.5 with 4 M KOH. Then it was filtered through a millipore filter (pore size 0.45 μm), and the neutralized extract was used to determine the concentrations of ATP and ADP (μg/mL per mg of tissue) using ion pair-high performance liquid chromatography (IP-HPLC). Standard solutions of ATP and ADP were used to get a standard curve and then samples were tested to express energy changes as an ADP/ATP ratio.Citation18
Statistical analysis
All values were expressed as a mean ± standard error (SE). Data was statistically analyzed by analysis of variance (ANOVA) followed by Newman–Keuls using Stat Direct version 2.7.7. P-Values less than 0.05 were considered statistically significant.
Results
Animal’s weight and blood glucose
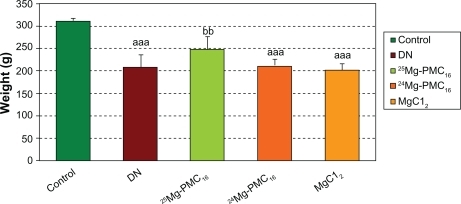
Weights of DN rats significantly reduced as compared with control rats (P < 0.001). Blood glucose in DN rats significantly increased as compared to control rats (P < 0.001). After use of 25MgPMC16, a significant increase in animal weight and decrease in glucose level were observed (P < 0.01 and P < 0.05, respectively) when compared to DN rats. No significant changes were observed by use of 24Mg-PMC16 and MgCl2 ( and ).
Figure 1 Protective effects of various forms of magnesium on blood glucose in DN rats after two months.
Notes: Data are mean ± SE of six animals. Difference between control and other groups is significant at P < 0.001(aaa). Difference between DN and 25Mg-PMC16 is significant at P < 0.05(b).
Abbreviations: DN, diabetic neuropathy; SE, standard error.
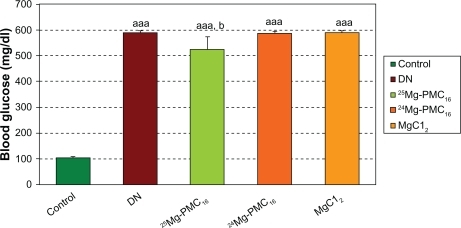
Figure 2 Protective effects of various forms of magnesium on weight of DN rats after two months.
Notes: Data are mean ± SE of six animals. Difference between control and other groups is significant at P < 0.001(aaa). Difference between DN and 25Mg-PMC16 is significant at P < 0.05(bb).
Abbreviations: DN, diabetic neuropathy; SE, standard error.
Blood 2,3-DPG
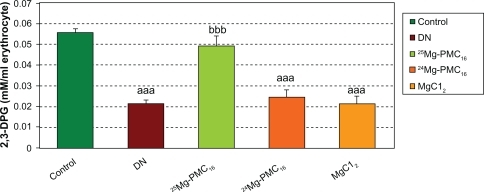
There was a significant decrease in 2,3-DPG concentration in DN group (P < 0.001) as compared to control rats but administration of 25Mg-PMC16 caused a significant increase of this marker (P < 0.001). No significant changes were observed by administration of 24Mg-PMC16 and MgCl2 in DN rats ().
Figure 3 Protective effects of various forms of magnesium on 2,3-DPG concentration in erythrocytes of DN rats after two months.
Notes: Data are mean ± SE of six animals. Difference between control and other groups is significant at P < 0.001(aaa). Difference between DN and 25Mg-PMC16 is significant at P < 0.001(bbb).
Abbreviations: DN, diabetic neuropathy; SE, standard error.
Biomarkers of oxidative stress
A significant decrease in total antioxidant capacity and total thiol molecules (P < 0.01 and P < 0.05, respectively) and an increase of LPO (P < 0.001) in DN group were recorded in comparison to controls. Administration of 25Mg-PMC16 increased total antioxidant capacity and total thiol molecules (P < 0.01 and P < 0.001, respectively) and decreased LPO (P < 0.01) as compared to DN group. No significant changes were detected by administration of 24Mg-PMC16 and MgCl2 ().
Table 1 Protective effects of various forms of magnesium on oxidative stress biomarkers
ATP and ADP in DRG neurons
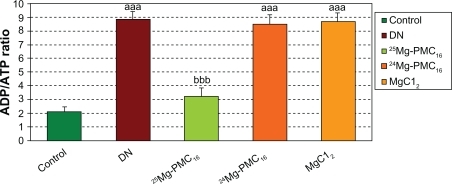
As observed in , a significant increase in ADP/ATP ratio in DN rats is evident in comparison to the control group (P < 0.001). The administration of 25Mg-PMC16 reduced the ADP/ATP ratio in comparison to the DN group (P < 0.001). 24Mg-PMC16 and MgCl2 caused no significant changes.
Figure 4 Protective effects of various forms of magnesium on ADP/ATP level in DN rats after two months.
Notes: Data are mean ± SE of six animals. Difference between control and other groups is significant at P < 0.001(aaa). Difference between DN and 25Mg-PMC16 is significant at P < 0.001(bbb).
Abbreviations: ADP/ATP, adenosine diphosphate/adenosine triphospahte; DN, diabetic neuropathy; SE, standard error.
Discussion
The present findings indicate a significant improvement in biomarkers of DN including blood 2,3-DPG and oxidative stress, DRG neuronal ADP/ATP by use of 25Mg-PMC16. Interestingly no considerable improvement was found in DN when 24Mg-PMC16 or MgCl2 were used.
The unique structure of 25Mg-PMC16 as a nanocationite helps smart release of Mg into DN cells that are in hypoxic acidosis condition and imbalance of oxidative phosphorylation and ATP. Meanwhile, the porphyrin domain of PMC16 provides a tissue selective interaction for porphyrin receptors located in the mitochondrial membrane. It seems that water-soluble C60-fullerene derivatives have the ability to selectively accumulate inside mammalian mitochondria.Citation7 This nanoparticle is safe for use, because the hepatic turnover of PMC16 occurs mainly on the porphyrin domain that forms biologically neutral heme precursor. The hepatic metabolites include alanyl-depleted PMC16 (54.6% of total metabolites amount), deacetylated PMC16 derivatives (27.0%) and cyclohexyl C60-fullerene (16.2%). In hypoxia, PMC16 becomes more resistant to hepatic metabolism.Citation7 Therefore, besides Mg, these metabolites themselves may have useful effects in DN.
As found in the present study, 2,3-DPG is one of the factors contributing to tissue hypoxia significantly decreased in DN. During glycolysis, metabolism can be diverted into the Rapoport–Luebering shunt, generating 2,3-DPG instead of ATP. 2,3-DPG binds to deoxyhemoglobin to modulate oxygen affinity and unloading of oxygen at the capillaries. It is believed that, reduced levels 2,3-DPG in DN result in a defect in oxygen delivery within DRG neurons and followed by hypoxia and neuron death.Citation19,Citation20 As far as we know, 2,3-DPG and ATP decrease the affinity of the hemoglobin for oxygen and a close inverse correlation was found between the oxygen binding and the concentration of these phospho compounds in intact human erythrocytes. ATP and 2,3-DPG have approximately equal effect on hemoglobin oxygenation. However, binding of both phospho compounds decreased at higher hydrogen ion concentrations in DN because of hypoxia and followed acidosis.Citation21 Thus it seems that 25Mg-PMC16 increases 2,3-DPG level via three mechanisms. First of all, the increased ATP by 25Mg-PMC16 in hypoxic condition and reduced 2,3-DPG in DN, bind to hemoglobin compensational in the way to deliver oxygen to DRG neurons. Second of all, in oxygenated erythrocytes that contain a relatively high concentration of Mg, it is estimated that almost all of the Mg is bound to ATP and with somehow lesser affinity to 2,3-DPG.Citation22 Therefore it seems that, deoxygenated erythrocytes in DN, causes complex of Mg and 2,3-DPG to be dissociated easier than that of Mg and ATP due to increased ATP and Mg by 25Mg-PMC16 and high affinity of Mg to ATP. Thus, this leads to increased 2,3-DPG concentration. Finally, in the process of 2,3-DPG production in erythrocytes (Rapoport–Luebering shunt), 1,3-diphosphoglyceric acid is converted to 3-phosphoglyceric acid by phosphoglycerate kinase (PGK) and then to 2,3-DPG by a kinase in the presence of ATP.Citation23 Therefore, 25Mg-PMC16 with improving PGK enzyme activityCitation24 and ATP level proceeds the pathway to increase 2,3-DPG in DN.
The present findings also showed a significant increase in oxidative stress biomarkers in DN that is supported by previous studies. DRG neurons have large mitochondria with high oxidative metabolism making them much susceptible to oxidative injury. The chronic distal sensory deficits that are seen in chronic DN might be more closely related to DRG neurons because the blood-nerve and perineurial barriers are minor at this site.Citation25 Increased ADP/ATP ratio in DRG neurons in DN group is considered as a marker of mitochondrial dysfunction in neurons. Mitochondrial dysfunction itself is a central mediator of neural apoptosis in the peripheral nervous system and a critical modulator of diabetic neuropathy.Citation4 In diabetes, reactive oxygen species (ROS) that are coupled with depolarization of the inner mitochondrial membrane (ΔΨMt) are associated with an increase in the ADP/ATP ratio and an absolute decrease in ATP levels. Also increased metabolic flux in the mitochondria due to high glucose is coupled with reduction of NAD to NADH that results in further formation of ROS. Accumulation of NADH coupled with failure of mitochondrial creatine phosphate pump in regeneration of ATP from ADP disrupts electron transfer chain and therefore results in DRG neuron death.Citation26
Decreased 2,3-DPG and hypoxia and further acidosis in DN conditions activates 25Mg-PMC16 and therefore positive effects of magnetic Mg is guaranteed.Citation7 There is evidence that fullerenes that exist in structure of 25Mg-PMC16 have antioxidant and anti-apoptotic effects due to their potential to react with ROS.Citation9 Other studies have shown that both lipophilic and hydrophilic C60 derivatives have wonderful potential as medicines for treating LPO-related diseases.Citation27–Citation29 Apoptosis is a programmed cell death that is mainly due to the change in growth factor. In this condition, ROS are released, thus, therapies targeted against ROS are greatly useful for minimizing cellular damage in oxidative stress-mediated neurodegeneration and other ROS-dependent disorders.Citation30,Citation31 Fullerene derivatives have also anti-apoptotic activity and can prevent apoptosis by neutralization of tumor growth factor (TGF)-induced ROS, reduction of tumor necrosis factor (TNF) or ultraviolet-triggered activation of caspases. In addition, fullerene derivatives associate with mitochondria more efficiently than conventional antioxidants.Citation9,Citation32 On the other hand, 25Mg-PMC16 by releasing Mg in DN hypoxic conditions induces antioxidant and anti-apoptotic effects. It has been shown that Mg has a dose-dependent neuroprotective effect after a spinal cord injury that is mediated by reduction of neuronal LPO.Citation11 Also it was found that MgSO4 decreases neuron apoptosis after cerebral ischemia-reperfusion injury through suppression of caspase-3 and bax expression.Citation12 Another study showed that MgSO4 significantly inhibits endotoxin-induced up-regulation of inflammatory molecules and NF-κß activation.Citation33 Mg has been also found effective in human poisoning with organophosphates that act through inhibition of cholinesterase and induction of oxidative stress.Citation34 Considering the study indicated a decrease in body Mg concentration in diabetes type 1 patients,Citation35 it is not surprising to conclude that any elevation of intraneuronal Mg is a helpful strategy in the management of DN. Of course it should not be forgotten that we saw positive effects only for 25Mg-PMC16 and not for other forms like 24Mg-PMC16 and MgCl2 that were tested in the present study. Thus, the first idea that comes to mind is the problem that might exist in reaching and accumulating of adequate Mg into neurons. In the plasma with normal pH of 7.4, 25Mg-PMC16 cannot easily release Mg but hypoxic condition in DN when inside the cells will change to acidic pH, then 25Mg-PMC16 will be able to release Mg so easily resulting in a dramatical increase of Mg inside DRG cells. On the other hand since equal doses of these three forms of Mg has been tested, thus one can conclude that magnetic Mg plays the effective role much better than other forms of Mg. Taking collectively, the present finding confirms that 25Mg-PMC16 protects from STZ-induced DN through reduction of oxidative stress and improving ATP by better reaching neuronal cells and release of Mg. Of course positive role of fullerene structure of 25Mg-PMC16 cannot be ignored.
Acknowledgements
This work was supported in part by funds from Tehran University of Medical Sciences. The authors report no conflicts of interest in this work.
References
- WildSRoglicGGreenASicreeRKingHGlobal prevalence of diabetes: estimates for the year 2000 and projections for 2030Diabetes Care20042751047105315111519
- Raymond AzmanAManagement of diabetic neuropathyMalaysian Journal of Medical Science20031022730
- Figueroa-RomeroCSadidiMFeldmanELMechanisms of disease: the oxidative stress theory of diabetic neuropathyRev Endocr Metab Disord20089430131418709457
- FernyhoughPHuangTJVerkhratskyAMechanism of mitochondrial dysfunction in diabetic sensory neuropathyJ Peripher Nerv Syst20038422723514641647
- DouglasWZochodneMDDiabetic NeuropathiesNeuromuscul Disord200022329
- RahimiRNikfarSLarijaniBAbdollahiMA review on the role of antioxidants in the management of diabetes and its complicationsBiomed Pharmacother200559736537316081237
- RezayatSMBoushehriSVSalmanianBThe porphyrin-fullerene nanoparticles to promote the ATP overproduction in myocardium: 25Mg2+-magnetic isotope effectEur J Med Chem20094441554156918782645
- AmirshahiNAlyautdinRNSarkarSPorphyrin-fullerene nanoparticles for treatment of hypoxic cardiopathiesNanotechnol Russ2008310611621
- BosiSDa RosTSpallutoGPratoMFullerene derivatives: an attractive tool for biological applicationsEur J Med Chem20033811–1291392314642323
- WuASKiaeiMAguirreNIron porphyrin treatment extends survival in a transgenic animal model of amyotrophic lateral sclerosisJ Neurochem200385114215012641736
- SüzerTCoskunEIslekelHTahtaKNeuroprotective effect of magnesium on lipid peroxidation and axonal function after experimental spinal cord injurySpinal Cord199937748048410438114
- ZhouHMaYZhouYLiuZWangKChenGEffects of magnesium sulfate on neuron apoptosis and expression of caspase-3, bax and bcl-2 after cerebral ischemia-reperfusion injuryChin Med J (Engl)2003116101532153414570617
- SrinivasanSStevensMWileyJWDiabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunctionDiabetes200049111932193811078462
- CastilhoEMGlassMLMançoJCThe effects of 2,3-diphosphoglycerate, adenosine triphosphate, and glycosylated hemoglobin on the hemoglobin-oxygen affinity of diabetic patientsBraz J Med Biol Res200336673173712792702
- BenziIFStrainSFerric reducing antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentrationMethods Enzymol19992921527
- HuMLDillaredCJPlasma SH and GSH measurementMethods Enzymol19942333853878015474
- RanjbarASolhiHJalali MashayekhiFSusanabdiARezaieAAbdollahiMOxidative stress in acute human poisoning with organo-phosphorus insecticides; a case control studyEnviron Toxicol Pharmacol20052018891
- ChenYXingDWangWDingYDuLDevelopment of an ion-pair HPLC method for investigation of energy charge changes in cerebral ischemia of mice and hypoxia of Neuro-2a cell lineBiomed Chromatogr200721662863417385810
- GoldfrankLewis RFlomenbaumNealHowlandMary AnnHoffmanRobert SLewinNeal AGoldfranksLewis SToxicologic Emergencies8th edNelson-Medical2006238
- NakamuraJKohNSakakibaraFPolyol pathway, 2,3-diphosphoglycerate in erythrocytes and diabetic neuropathy in ratsEur J Pharmacol199529412072148788433
- BunnHFRansilBJChaoAThe interaction between erythrocyte organic phosphates, magnesium ion, and hemoglobinJ Biol Chem197124617527352795094669
- GarbyLGerberGDe VerdierCHBinding of 2,3-diphosphoglycerate and adenosine triphosphate to human haemoglobin AEur J Biochem19691011101155345976
- ShahbaziPMalekniaNGeneral Biochemistry for Students of Medical SciencesTehran, IranTehran University Press20012194
- BuchachenkoALKouznetsovDAOrlovaMAMarkarianAAMagnetic isotope effect of magnesium in phosphoglycerate kinase phosphorylationProc Natl Acad Sci U S A200510231107931079616043694
- SchmeichelAMSchmelzerJDLowPAOxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathyDiabetes200352116517112502508
- RussellJWGolovoyDVincentAMHigh glucose-induced oxidative stress and mitochondrial dysfunction in neuronsFASEB J200216131738174812409316
- WangICTaiLALeeDDC(60) and water-soluble fullerene derivatives as antioxidants against radical-initiated lipid peroxidationJ Med Chem199942224614462010579823
- MotohashiNTakahashiAMifuneMSaitoYInhibitory effects of water-soluble cationic manganese porphyrins on peroxynitrite-induced SOS response in Salmonella typhimurium TA4107/pSK1002Mutat Res20045541–216517415450415
- PessotoFSInadaNMNepomuceno MdeFBiological effects of anionic meso-tetrakis (para-sulfonatophenyl) porphyrins modulated by the metal center. Studies in rat liver mitochondriaChem Biol Interact2009181340040819631199
- AstaneieFAfshariMMojtahediATotal antioxidant capacity and levels of epidermal growth factor and nitric oxide in blood and saliva of insulin-dependent diabetic patientsArch Med Res200536437638115950078
- AfshariMLarijaniBRezaieAIneffectiveness of allopurinol in reduction of oxidative stress in diabetic patients; a randomized, double-blind placebo-controlled clinical trialBiomed Pharmacother2004581054655015589061
- MarkovicZTrajkovicVBiomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60)Biomaterials200829263561357318534675
- LinCYTsaiPSHungYCHuangCJL-type calcium channels are involved in mediating the anti-inflammatory effects of magnesium sulphateBr J Anaesth20101041445119933511
- PajoumandAShadniaSRezaieAAbdiMAbdollahiMBenefits of magnesium sulfate in the management of acute human poisoning by organo-phosphorus insecticidesHum Exp Toxicol2004231256556915688984
- De LeeuwIEngelenWDe BlockCVan GaalLLong term magnesium supplementation influences favourably the natural evolution of neuropathy in Mg-depleted type 1 diabetic patients (T1dm)Magnes Res200417210911415319143