 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Zwitterionic polymers have unique features, such as good compatibility, and show promise in the application of drug delivery. In this study, the zwitterionic copolymers, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide (PCL-ss-PMPC) or poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) or without disulfide (PCL-PMPC) and with different block lengths in PCL-ss-PMPC, were designed. The designed copolymers were obtained by a combination of ring-opening polymerization and atom transferring radical polymerization. The crystallization properties of these polymers were investigated. The micelles were prepared based on the obtained copolymers with zwitterionic phosphorylcholine as the hydrophilic shell and PCL as the hydrophobic core. The size distributions of the blank micelles and the doxorubicin (DOX)-loaded micelles were uniform, and the micelle diameters were <100 nm. In vitro drug release and intracellular drug release results showed that DOX-loaded PCL-ss-PMPC micelles could release drugs faster responding to the reduction condition and the intracellular microenvironment in contrast to PCL-PMPC micelles. Moreover, in vitro cytotoxicity evaluation revealed that the designed copolymers possessed low cell toxicity, and the inhibiting effect of DOX-loaded phosphorylcholine micelles to tumor cells was related to the components of these copolymers. These results reveal that the reduction-responsive phosphorylcholine micelles with a suitable ratio of hydrophilic/hydrophobic units can serve as promising drug carriers.
Introduction
Polymer micelles have a core–shell structure self-assembled from amphiphilic block copolymers and have shown great potential for targeted anticancer drug delivery because they have advantageous physico- and biochemical features similar to other types of nanocarriers such as liposomes and nanoparticles. Polymer micelles have shown enhanced drug-loading capacity with the hydrophobic cores for efficient loading hydrophobic drugs and prolonged circulation time with the hydrophilic shells to warrant good colloidal stability and intrinsic stealth effect.Citation1 Specifically, polymeric micelles undergo dynamic physicochemical changes during drug entrapment and release in terms of molecular assembly and dissociation between block copolymer components. Owing to the abovementioned characteristics and their 20–100 nm size, polymer micelle drug carriers exhibit pharmacological profiles, enhanced drug accumulation in the tumor site via enhanced permeability and retention (EPR) effect, reduced adverse effects and so on.Citation2–Citation4 However, the slow drug release of traditional polymer micellar drug carriers at the tumor site and inside the tumor cells often reduce the therapeutic effect of the drug.Citation3,Citation5,Citation6 To achieve a faster drug release at the tumor site or inside tumor cells, more and more polymeric micellar drug carriers were designed to respond to the intrinsic environment of tumor.Citation7–Citation9 In particular, redox-responsive drug carriers are attractive due to the high reducing potential at the tumor site and inside tumor cells.Citation9 Generally, redox-responsive drug carriers are prepared from reducible polymers.Citation10 One of the most widely used strategies is to introduce the disulfide to serve as the linkage of the hydrophilic block and the hydrophobic block.Citation11–Citation13
Recently, polymer micelles with zwitterionic phosphorylcholine as hydrophilic shells have been used as drug delivery systems.Citation14 The hydrophilic phosphorylcholine groups in these polymers can stabilize their micelles in aqueous environment owing to the strong hydration through electrostatic interactions,Citation15 forming a mimetic cell outer membrane structure.Citation16 Poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) containing phosphorylcholine groups shows better anti-protein adsorption and non-bioadhesive properties than PEG.Citation14,Citation17,Citation18 Therefore, copolymer micelles composed of PMPC have been used to deliver poorly water soluble drugs. The phosphorylcholine micelles exhibit excellent properties, eg, good biocompatibility, high drug-loading ability, excellent prolonged circulation and prominent tumor therapy effect.Citation16,Citation19–Citation24 Zhao et alCitation16 investigated in detail the drug loading, in vitro phagocytic uptake, blood clearance and biodistribution in vivo of random copolymer phosphorylcholine micelles with different compositions. However, investigations on the relation between the component of phosphorylcholine micelles and their properties are far from enough. To our knowledge, studies concerning block copolymer constituent on phosphorylcholine micelle properties have not yet been reported. Furthermore, in the reports about the reductive drug carrier,Citation10 few works dealing with reductive phosphorylcholine micelles are reported.
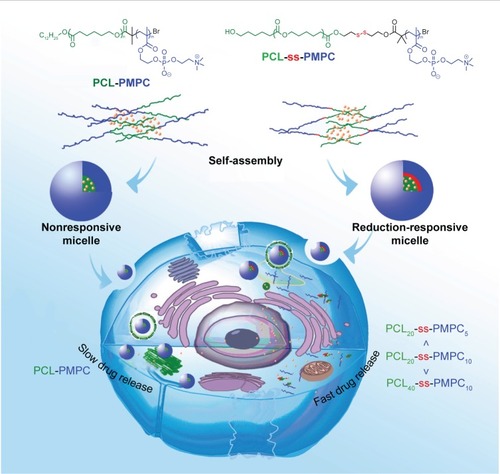
In this study, we investigated the relationship between the polymer component and the properties of zwitterionic phosphorylcholine micelles. As shown in , the polymers forming phosphorylcholine micelles were designed to be with or without disulfide as the linkage of the two blocks, and in reductive phosphorylcholine copolymers, different block polymers were prepared by varying the length of PCL block or PMPC block. The micellization behaviors of designed copolymers, reduction-triggered releases and in vitro antitumor effect of the micelles were evaluated.
Materials and methods
Materials
ε-caprolactone (CL; Sigma-Aldrich, New Jersey, USA) was dried by calcium hydride (CaH2; ChengDu KeLong Chemical Co., Ltd., China) and distilled under reduced pressure. Triethylamine (TEA) (ChengDu KeLong Chemical Co., Ltd.) was dried over CaH2 and distilled. 2-Bromoisobutyryl bromide (98%), stannous octoate (Sn(Oct)2; 95%), copper (I) bromide (CuBr; 99%), pentaerythritol, 2,2′-disulfanediyldiethanol, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), 2,2′-bipyridine (BPy), DL-dithiothreitol (DTT) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA) and used as received. 2-Methacryloyloxyethyl phosphorylcholine (MPC) was supplied by Nanjing Joy-Nature Ltd (Nanjing, China) and used as received. Doxorubicin (DOX)·HCl was neutralized with TEA to remove the hydrochloride to make it become hydrophobic DOX. Toluene and tetrahydrofuran (THF) (ChengDu KeLong Chemical Co., Ltd) were dried by refluxing over sodium and distilled before used. All the other reagents and solvents used were analytically pure and without further purification.
Synthesis of PCL-ss-PMPC
Synthesis of HO-ss-iBuBr and PCL-ss-iBuBr
The initiator 2′-(2″-hydroxyethyl disulfanyl)ethyl-2-bromo-2-methylpropanoate (HO-ss-iBuBr) and the macroinitiator HO-ss-iBuBr modified poly(ε-caprolactone)(PCL-ss-iBuBr) were prepared according to our previous report.Citation25 The yield for HO-ss-iBuBr was 63.5% and the yield for PCL-ss-iBuBr was 87.4%.
Synthesis of PCL-ss-PMPC
PCL-ss-iBuBr (1.00 g, 0.320 mmol), MPC (1.42 g, 4.80 mmol) and Bpy (0.150 g, 0.960 mmol) were dissolved in mixed solvent of THF and methanol (v/v: 2/1). After bubbling the solution with nitrogen for 30 min, CuBr (0.0689, 0.480 mmol) was added under N2 atmosphere. The reaction with the protection of N2 was performed at 25°C for 72 h. The reaction mixture was exposed into the atmosphere to stop the reaction. Then, the reaction mixture was dialyzed against ultra-purified water. White powder product was obtained by the freeze-drying method (yield: 82.8%).
Synthesis of PCL-PMPC
The copolymer was prepared according to the method reported in our previous study.Citation26 PCL was synthesized by ring-opening polymerization (ROP) using laurinol as the initiator and then reacted with 2-bromo-2-methylpropanoyl bromide to generate the macroinitiator, which would initiate the polymerization of MPC through atom transfer radical polymerization to obtain poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide (PCL-PMPC; yield: 89.1%).
Characterization of the PCL-ss-PMPC and PCL blocks
1H·nuclear magnetic resonance (NMR) spectra of the target copolymers and their precursors PCL blocks were recorded on a Unity Inova 400 spectrometer (Varian Inc., California, USA) operating at 400 MHz.
The molecular weight and polydispersity of the precursors were determined by a Waters 1515 gel permeation chromatograph instrument system (GPC, Waters Inc., MA, USA). THF was used as the eluent; the measurements were performed using a flow rate of 1.0 mL/min at 30°C, and a series of narrow polystyrene standards were used for the calibration of the columns.
The spectra of attenuated total reflection Fourier transform infrared (ATR-FTIR) were recorded using a Nicolet Magna 560 FTIR spectrometer (Thermofisher, Massachusetts, USA).
Differential scanning calorimetry (DSC) was performed on a DSC-Q2000 (TA Instruments, New Castle, DE, USA) at a heating rate of 10°C/min in the range of −20°C to 100°C under a steady flow of nitrogen. All samples were first heated at 10°C/min from 40°C to 100°C and held for 5 min to erase the sample thermal history, then cooled at the rate of 10°C/min to −20°C and finally heated at the rate of 10°C/min to 100°C. The crystallization temperature (Tc) and the melting temperature (Tm) of these polymers were obtained at the inflection point of the DSC curve.
Preparation and characterization of blank and DOX-loaded micelles
Ten mg block polymer, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with sulfide (PCL-ss-PMPC) or PCL-PMPC, was dissolved in 4 mL mixed solvent of methanol and dimethyl sulfoxide (DMSO; 1/1, v/v). This copolymer solution was injected into 12 mL phosphate-buffered saline (PBS; pH =7.4, 0.01 M) at a speed of 200 μL/min. After stirring for 8 h, the mixed solution was transferred to a dialysis bag (Spectra/Por MWCO 3500) for dialysis against PBS (pH =7.4, 0.01 M) at 4°C for 48 h. The final volume of the micelle solution was adjusted to 20 mL, and the copolymer micelles were stored at 4°C for further use.
DOX-loaded micelles were prepared as follows: 2 mg DOX·HCl and 2 μL TEA were dissolved in 4 mL mixed solvent of methanol and DMSO (1/1, v/v) followed by 2 h of stirring. The next process was similar to the process of preparing blank micelles.
The critical micelle concentration (CMC) of the copolymers was determined by fluorescence spectroscopy using pyrene as a fluorescence probe. Varied micellar solutions with different concentrations were prepared and were mixed with the pyrene solution (concentration 6×10−7 M) for 6 h with shaking at room temperature. The excitation spectra of pyrene in the micellar solution were recorded with a fluorescence spectrophotometer (F-7000; Hitachi Ltd., Tokyo, Japan). Emission was carried at 395 nm, and excitation spectra ranged from 240 to 360 nm. The CMC was calculated from the plot of fluorescence intensity ratios of I336/I333 at low and high concentration regions. The partition equilibrium constant (Kv) of pyrene in the hydrophobic core of these micelles was estimated based on the method reported by Wilhelm et al:Citation28
where Fmin is the average value of the ratio I336/I333 in the low polymer concentration region, Fmax the average value of the ratio I336/I333 in the very high polymer concentration region, F the intensity ratio I336/I333 in the intermediate polymer concentration region, C the polymer concentration, χPCL the weight fraction of hydrophobic PCL in the block copolymer and ρ the density of the PCL core of micelles, which is assumed to take the same value as bulk PCL (1.146).
The micellar size and distribution were measured by dynamic light scattering (DLS) using a Malvern Zetasizer Nano Series (Malvern Instruments, Malvern, UK) equipped with DTS software and with a He–Ne laser beam at 633 nm (scattering angle: 90°). Analysis was performed at a constant temperature of 25°C.
The drug-loading content (DLC) and drug-loading efficiency (DLE) were determined by ultraviolet spectroscopy. Briefly, a mixed solution of DMSO and methanol (2 mL) was added into the micellar solution (0.2 mL). The concentration of DOX in the solution was analyzed by ultraviolet spectroscopy at an absorbance wavelength of 483 nm. DLC and DLE were calculated by the following equation:
In vitro drug release
The DOX release profiles from DOX-loaded PCL-ss-PMPC or PCL-PMPC micelles were studied in two different media, ie, PBS (pH =7.4, 0.01 M) with 10 mM DTT or PBS (pH =7.4, 0.01 M) without DTT. 1 mL DOX-loaded PCL-ss-PMPC or PCL-PMPC micelle solution (0.2 mg/mL for the polymer concentration) was added into a dialysis tube (MWCO 3500). Then, the tube was sealed and immersed in 10 mL release media at 37°C with shaking. At desired time intervals, 5.0 mL of the release medium was taken out and replenished with an equal volume of fresh medium. The concentration of DOX was determined by a fluorescence spectrophotometer with extinction wavenumber at 480 nm and emission wavenumber at 600 nm.
In vitro cytotoxicity assay
The cytotoxicity of the micelles was investigated by MTT assay. Human epithelial carcinoma cells (HeLa) and mice connective tissue fibroblast cells (L-929) were provided by the Huaxi Medical Research Center of Sichuan University (Chengdu, China). The cells were seeded at a density of 2,000 cells per well in 96-well plates. The culture medium was Roswell Park Memorial Institute (RPMI) 1640 containing 10% fetal bovine serum (FBS; v/v), subjoined with 50 U/mL streptomycin and 50 U/mL penicillin. The cells were cultured at 37°C in 5% CO2 atmosphere for 24 h. Then, different concentrations of blank micelles or drug-loaded micelles were mixed with a fresh culture medium, and these mixtures were used to replace the cell culture medium. In 48, 72 and 96 h, 20 μL of MTT solution (5 mg/mL) was added to each well. After incubation for 4 h at 37°C, the culture medium containing MTT in each well was replaced with 150 μL/well DMSO. The resulting formazan crystals were dissolved in DMSO followed by 10 min shaking. The optical densities (ODs) of each well at 490 nm were determined using a microplate reader (DNMM-9602; Perlong Medical, Beijing, China). The blank control was the cells cultured without adding micelles. The cell viability was obtained according to the following formula:
Here, ODt and ODb are the OD values of surviving cells treated with test group and blank control, respectively. The results given in the work were the average values of five measurements.
Cellular uptake and intracellular drug release of DOX-loaded micelles
Confocal laser scanning microscopy (CLSM) and flow cytometry (FCM) were employed to examine the cellular uptake and intracellular drug release of these DOX-loaded micelles.
HeLa cells were provided by the Huaxi Medical Research Center of Sichuan University. The HeLa cells were seeded in 35-mm-diameter glass dishes at a density of 1×105 cells with RPMI 1640 culture media containing 10% FBS (v/v), subjoined with 50 U/mL penicillin and 50 U/mL streptomycin and cultivated at 37°C in 5% CO2 atmosphere for 24 h. Thereafter, the prepared DOX-loaded micelle solution with the final DOX concentration of 10 μg/mL was added. After incubation at 37°C for 2 or 5 h, the culture media were removed and the dishes were rinsed for three times with PBS of pH 7.4, 0.01 M. After that, the cells were observed by using CLSM (TCP SP5; Leica Microsystems, Wetzlar, Germany).
For quantitative study by FCM, HeLa cells were seeded in six-well plates at a density of 1×105 cells for 24 h. Then, the prepared DOX-loaded micelle solution with the final DOX concentration of 10 μg/mL was added. After incubation at 37°C for 2 or 5 h, the culture media were removed and the dishes were rinsed with PBS (pH =7.4, 0.01 M) for three times. The cells were then harvested by trypsinization and centrifuged at 1,000 rpm for 5 min and resuspended in 0.5 mL PBS medium. The centrifuging and resuspending processes were repeated twice. Finally, the cells were examined by FCM using FACScan instrument (Becton Dickinson, San Jose, CA, USA).
Results and discussion
Synthesis and characterization of the copolymers
The zwitterionic copolymers, PCL-ss-PMPC or PCL-PMPC, were obtained by a combination of ROP and atom transferring radical polymerization. The copolymers with disulfide were synthesized by using an initiator with disulfide with terminal hydroxyl at one end and bromine at the other end. The structure of the target copolymers was confirmed by FTIR and 1H·NMR.
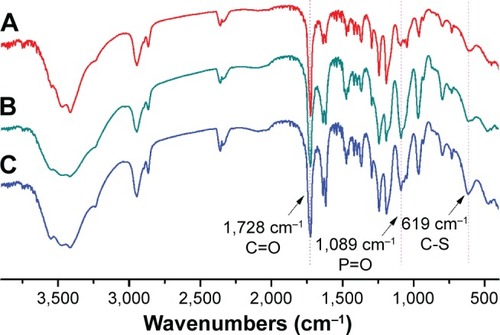
As shown in , the strong peaks ~1,728 cm−1 were attributed to the C=O of the ester group in the PCL block. The absorptions at 1,240, 1,089 (–POCH2–) and 970 cm−1 (–N+(CH3)326) from PMPC were also be observed, indicating the coexistence of PCL and PMPC blocks. Moreover, PCL-ss-PMPC copolymers clearly showed the peaks at ~619 cm−1 assigned to the C-S, while no signal for C-S was found in the spectrum of PCL-PMPC without disulfide.
Figure 1 FTIR spectra of (A) PCL20-ss-PMPC5, (B) PCL20-ss-PMPC10 and (C) PCL40-ss-PMPC10.
Abbreviations: FTIR, Fourier transform infrared; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide.
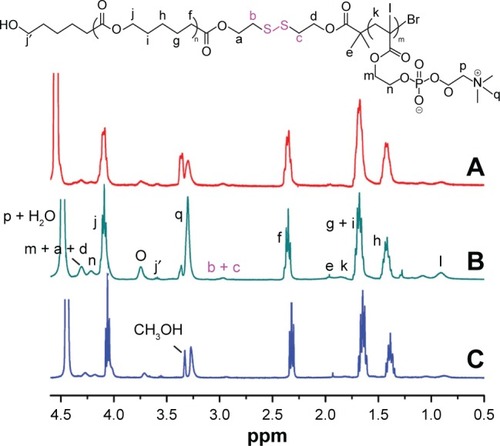
In 1H·NMR spectra of these copolymers as shown in , characteristic peaks attributed to the PCL block arose clearly (COOCH2CH2CH2CH2–, δ1.40; –COOCH2 CH2CH2CH2–, δ1.62; –CH2COO–, δ2.33; –COOCH2–, δ4.06). The characteristic peaks assigned to the PMPC block also appeared (–CH2C(CH3)–, δ0.89; –CH2C(CH3)–, δ1.83; –CH2CH2N+(CH3)3, δ3.29; –CH2CH2 N+(CH3)3, δ3.74; –COOCH2CH2–, δ 4.32; –COOCH2CH2–, δ4.21; –CH2CH2N+(CH3)3, δ4.5). Because of the increase in MPC units, the peaks assigned to the PMPC block in the PCL20-ss-PMPC10 spectrum were stronger than those in the PCL20-ss-PMPC5 spectrum, but weaker than those in the PCL40-ss-PMPC10 spectrum. The peaks assigned to the proton of the methylene next to disulfide were also clear (δ2.90). The degree of polymerization (DP) for the PCL block was calculated by the integral ratio of the tiny peak at δ3.65 that was assigned to hydroxyl methylene protons at the end of PCL chain to the peak at δ2.33 affiliated to the methylene protons among the backbone of PCL. The DP for the PMPC block was determined by the integrated area ratio of the methylene protons (δ2.33) in the PCL block to the methylene protons (δ3.74) in the PMPC block. The composition data of these polymers are summarized in .
Table 1 Synthesis and characteristics of polymers
Figure 2 1H NMR spectra of (A) PCL20-ss-PMPC5, (B) PCL20-ss-PMPC10 and (C) PCL40-ss-PMPC10 in CDCl3 and CD3OD (v/v =2:1).
Abbreviations: NMR, nuclear magnetic resonance; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide.
It is widely reported that the crystallization of the block polymer greatly influenced the properties of their drug carriers, such as DLC, in vitro drug release. In this study, DSC was used to investigate the crystallization properties of the reductive phosphorylcholine copolymers with the varied lengths of PCL block or PMPC block. DSC profiles of these copolymers and their precursors are presented in , and the data for thermal properties are included in . The crystallization peak and melting peak appeared clearly in the DSC curves for PCL20-ss-iBuBr and PCL40-ss-iBuBr. The Tm of the latter was higher than that of the former, but they were both lower than the Tm of bulk PCL. The crystallinity degrees of both precursors were >40%, but were still lower than that of the corresponding bulk PCL. The reasonable explanation for this phenomenon was that the initiator with disulfide might not as stable as the general initiators used during ROP. The polydispersity index (PDI) of PCL20-ss-iBuBr and PCL40-ss-iBuBr was larger than the general value (~1.1) obtained by ROP conventional initiators. Moreover, with the increase in the MPC units, the degree of crystallinity decreased further. With large side groups, the block PMPC was not able to crystallize. Introducing PMPC block into PCL will limit the movement of PCL block. As a result, the capability of crystallization of the PCL block decreased. Furthermore, with more amorphous PMPC in the bulk, there would be more defects, which will further decrease the degree of crystallinity.Citation27
Figure 3 DSC curve of different polymers for heating (A) and cooling (B).
Abbreviations: PCL-ss-iBuBr, poly(ε-caprolactone) with OH-ss-iBuBr opening-ring polymerization; DSC, differential scanning calorimetry; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
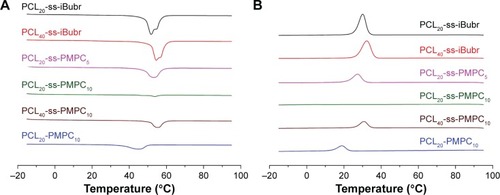
As shown in , the degree of crystallinity of PCL20-ss-PMPC20 was lower than that of PCL20-PMPC20, although both copolymers were with the same hydrophilic/hydrophobic units, since large PDI of PCL20-ss-iBuBr lead to inferior crystallization of the PCL block in PCL20-ss-PMPC20. The crystallinity difference of these copolymers would result in different drug load and release behaviors as we see in the following.
Micellization properties of the polymers
Amphiphilic copolymers can self-assemble into micelles in selective solvents. As reported in our previous studies,Citation24,Citation26 copolymers with hydrophobic PCL and hydrophilic PMPC blocks easily formed micelles in aqueous solution. In this study, the micellization properties of the copolymers were studied by CMC.
CMC is an important parameter for amphiphilic copolymers forming micelles for drug delivery. The CMC curves for the copolymers are shown in . The obtained data are included in . These block polymers possessed low CMC, indicating that their micelles were able to serve as drug carriers.Citation2 The CMC value of the copolymers was related to the ratio of hydrophilic units and hydrophobic units. With the increase in hydrophilic units or the decrease in hydrophobic units, the CMC values increased.Citation28 Hence, the CMC value for PCL20-ss-PMPC10 was the maximum in reductive phosphorylcholine copolymers and was close to the value of PCL20-PMPC10.
Table 2 Preparation and characteristics of blank micelles and DOX-loaded micelles
Figure 4 Measurement of CMC for different block polymers.
Abbreviations: CMC, critical micelle concentration; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide.
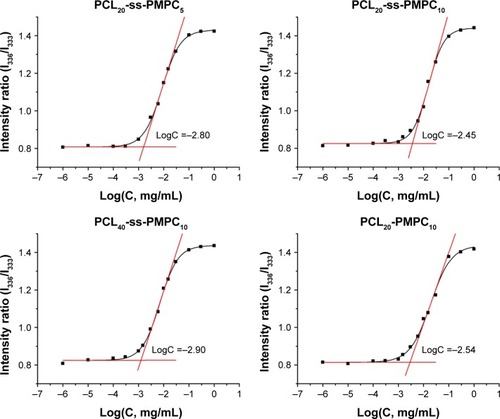
The equilibrium constant Kv for partitioning of pyrene between the micelle core and the exterior solution may be used to estimate the hydrophobicity of micellar core and the stability of micelles.Citation29 It is also a crucial parameter to characterize the thermodynamics of the drug in the micelles determining the drug load. As shown in , the values for all these micelles were similar to other micelles with PCL as the core.Citation27,Citation29 Especially, the values for the PCL20-ss-PMPC10 and PCL20-PMPC10 were almost equal, meaning that the zwitterionic copolymers with/without disulfide might load drugs similarly. Moreover, when the MPC or CL unit changed, the Kv values showed the same trend to CMC, ie, with the increase in the hydrophilic content or the decrease in the hydrophobic content, the Kv value increased.
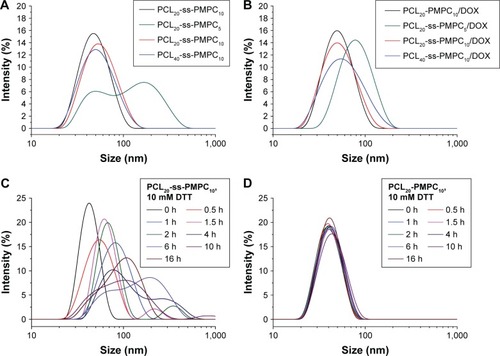
Diameter is another important parameter, because nanoparticles with diameter >200 nm are easy to be cleared by RES during the circulation in blood.Citation2 Blank micelles () and DOX-loaded micelles () showed small diameter of <100 nm with a narrow distribution except blank PCL20-ss-PMPC5 micelles. With the MPC unit number decreasing from 10 to 5 or the CL unit number increasing from 20 to 40, the diameter increased, which led to the increase in the ratio of hydrophobicity to hydrophilicity.Citation2 PCL20-ss-PMPC5 micelles displayed double peak distribution. This might result from too short hydrophilic block leading to the unstability of micelles. Therefore, some micelles had to aggregate into bigger micelles. Thus, PDI of PCL20-ss-PMPC5 micelles was the biggest in all the micelles, as shown in . Furthermore, these phosphorylcholine micelles with disulfide could respond to reductive environment. With <10 mM DTT at 37°C, the diameters and diameter distribution of PCL20-ss-PMPC10 micelles changed obviously over time, which were caused by breaking disulfide linkage between hydrophilic units and hydrophobic units (). On the contrary, PCL20-PMPC10 micelles at the same condition almost kept their diameters and diameter distribution ().
Figure 5 Size distribution of blank micelles (A) and DOX-loaded micelles (B). Diameter change of PCL20-ss-PMPC10 (C) and PCL20-PMPC10 (D) micelles in response to 10 mM DTT under 37°C measured by DLS.
Abbreviations: DOX, doxorubicin; DTT, DL-dithiothreitol; DLS, dynamic light scattering; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
As a hydrophobic drug, DOX is easily loaded into the core of PCL. As shown in , these DOX-loaded micelles showed good drug-loading capability. With the MPC unit number decreasing from 10 to 5 or the CL unit number increasing from 20 to 40, the DLC and the DLE decreased slightly, which may be on account of the increase in the degree of crystallinity.Citation30,Citation31
The abovementioned data of the blank micelles and the DOX-loaded micelles are summarized in .
In vitro drug release
Fast drug release at the site of tumor tissue or inside of tumor cells is a critical property for drug delivery systems. The in vitro drug release behavior of the phosphorylcholine micelles was studied in the presence of 10 mM DTT or without DTT.
As shown in , the drug release of these DOX-loaded micelles was slight and slow in DTT absent condition. The desorption of drug on the shell and diffusion of drug from the core dominated the drug release of these DOX-loaded micelles. However, in the presence of 10 mM DTT, the drug release of these PCL-ss-PMPC/DOX micelles was significantly enhanced, while the drug release of nonsensitive micelles, PCL-PMPC/DOX, appeared almost similar to that without DTT. In reductive phosphorylcholine micelles, as DTT broke the disulfide linkage, PMPC blocks departed rapidly, the micelle shell became thinner or micelles were destroyed, so the drug release was easier. These results indicated that the designed PCL-ss-PMPC micelles with different block lengths could fast release drugs in response to intracellular reductive environment, which may inhibit cancer cell proliferation effectively.
Figure 6 In vitro drug release of PCL-ss-PMPC/DOX micelles (A–C) and PCL-PMPC micelles (D) in PBS (pH =7.4, 0.01 M) with 10 mM DTT or without DTT at 37°C.
Abbreviations: DOX, doxorubicin; PBS, phosphate-buffered saline; DTT, DL-dithiothreitol; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
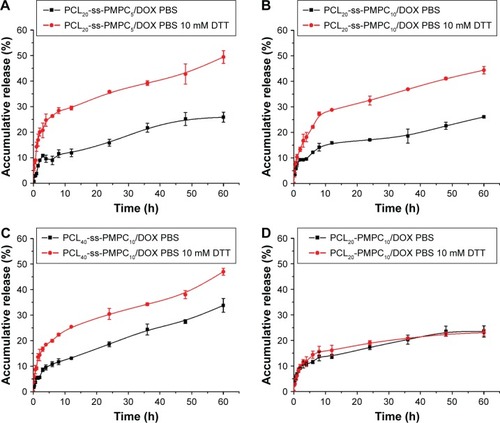
Moreover, the ability of these micelles in responding to DDT was related to their composition. PCL20-ss-PMPC5/DOX showed the greatest accumulative release gap (23.6%) between under reducing environment and nonreducing environment, followed by PCL20-ss-PMPC10/DOX (18.6%) and PCL40-ss-PMPC10/DOX (13.2%) the smallest. It meant that decreasing CL unit number or MPC unit number would increase the responsive ability of these micelles to reduction environment. This may be attributed to higher disulfide content in copolymer molecules by decreasing the CL unit number or MPC unit number.
In vitro cytotoxicity
The cytotoxicity of the blank micelles to HeLa and L929 was evaluated by MTT, and the results are shown in . The in vitro cytotoxicity of these micelles to tumor cells or normal cells was not significant at various concentrations of the blank micelles, which might result from the excellent biocompatibility of the micelles composed by PCL and PMPC.
Figure 7 In vitro cytotoxicity of blank and DOX-loaded micelles.
Notes: Cell viability of HeLa cells (A) and L929 cells (B) incubated with different blank micelles for 72 h at 37°C in 5% CO2 atmosphere. Cell viability of HeLa cells and L929 cells incubated with different DOX-loaded micelles for 48 and 96 h (n=5, SD <0.06; *P<0.05, **P<0.01, ***P<0.001) (C). In vitro cytotoxicity of different DOX-loaded micelles with various DOX concentrations incubated with HeLa cells (D).
Abbreviations: DOX, doxorubicin; IC50, half inhibitory concentration; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
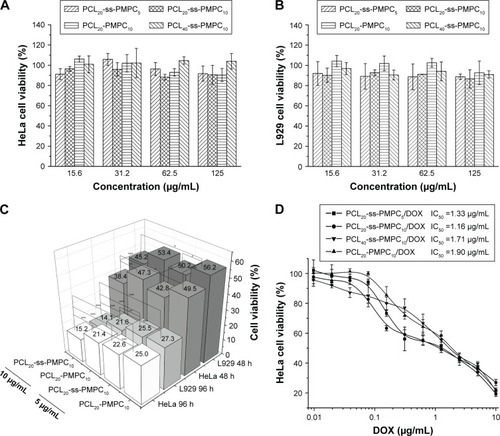
Furthermore, we evaluated the in vitro cytotoxicity of these DOX-loaded micelles to the two cell lines in 48 and 96 h (). The results showed that DOX-loaded PCL20-ss-PMPC10 micelles had obviously stronger inhibition ability to both HeLa and L929 than DOX-loaded PCL20-PMPC10 micelles. Especially, these DOX-loaded micelles at the same time under the concentration toward HeLa cells presented stronger inhibition than toward L929. This might owe to the high concentration of glutathione (GSH) in tumor cells, which could break off disulfide bond and accelerate DOX release of drug-loaded PCL20-ss-PMPC10 micelles. The further assessment inhibition of these phosphorylcholine drug-loaded micelles with different equivalent concentrations of DOX toward HeLa cells in 72 h is shown in . The DOX-loaded PCL20-ss-PMPC10 micelles showed lower half inhibitory concentration (IC50) than that of PCL20-PMPC10/DOX, indicating that the DOX-loaded redox-responsive micelles had higher antitumor activity toward HeLa cells. As proved by the results of investigation on cellular uptake and intracellular drug release by CLSM and FCM (mentioned in the “Cellular uptake and intracellular drug release” section), PCL20-ss-PMPC10/DOX micelles appeared to have faster intracellular drug release. The released DOX could suppress the proliferation of tumor cells and even kill tumor cells. However, the phosphorylcholine drug-loaded micelles with the same composition without the responsive structure possessed slow drug release in the cells, so IC50 of the micelles was high.
In addition, for these PCL-ss-PMPC/DOX micelles, PCL20-ss-PMPC10/DOX micelles showed the lowest IC50, while IC50 of PCL40-ss-PMPC10/DOX was the largest. This might be related to both disulfide ratio and MPC content in the phosphorylcholine micelles, because high disulfide ratio in copolymer molecules could speed up the drug release under reduction condition (); at the same time, the intact shield of PC of PCL20-ss-PMPC10 micelles could make the micelles easily pass through cell membranes and enter into tumor cells.Citation23 Both comprehensive effects lead to that PCL20-ss-PMPC10/DOX micelles showed higher cytotoxicity against HeLa cells than the other PCL-ss-PMPC/DOX micelles.
Cellular uptake and intracellular drug release
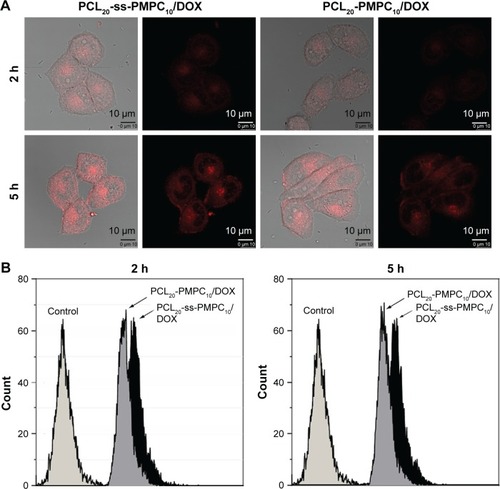
Cellular uptake and intracellular drug release have great influence on tumor treatment. We examined the cellular uptake and intracellular drug release behaviors of PCL20-ss-PMPC10/DOX and PCL20-PMPC10/DOX by CLSM and FCM. As shown in , after incubation with the DOX-loaded micelles for 2 h, red fluorescence could be observed in the cells incubated with PCL20-ss-PMPC10/DOX micelles, while the red fluorescence was weak in the cells incubated with PCL20-PMPC10/DOX micelles. As the incubation time was extended to 5 h, the red fluorescence increased significantly. HeLa cells incubated with PCL20-ss-PMPC10/DOX micelles still exhibited stronger fluorescence than that of HeLa cells incubated with PCL20-PMPC10/DOX micelles. Furthermore, the cells incubated with the DOX-loaded micelles were also quantitatively studied by FCM. The results () indicated that these cells incubated with PCL20-ss-PMPC10/DOX micelles showed stronger red fluorescence than that of cells incubated with PCL20-PMPC10/DOX micelles at both time points.
Figure 8 Cellular uptake and intracellular drug release by CLSM (A) and FCM (B).
Note: HeLa cells were incubated with DOX-loaded micelles with the final DOX concentration of 10 μg/mL at 37°C for 2 or 5 h.
Abbreviations: CLSM, confocal laser scanning microscopy; FCM, flow cytometry; DOX, doxorubicin; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
Scheme 1 Illustration for intracellular drug delivery of reduction-responsive micelles with bioinspired phosphorylcholine as hydrophilic chains.
Abbreviations: PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide.
It is known that DOX fluorescence is self-quenched inside micelles. The measured DOX fluorescence is, therefore, directly related to the amount of released DOX in cells.Citation32 In our previous study and other studies, the micelles or other nanoparticles with PC surface appeared to have effective cell uptake.Citation19,Citation20,Citation24,Citation26,Citation33,Citation34 Moreover, cellular uptake of nanoparticles is usually determined by the size and surficial properties of the nanoparticles.Citation35 For the DOX-loaded micelles of PCL20-PMPC10 or PCL20-ss-PMPC10, they had a similar size of ~50 nm and the same surface was built by PMPC blocks. It might be supposed that they could be internalized by cells effectively at a similar speed. This means the red fluorescence observed by CLSM or recorded by FCM directly reflects the intracellular drug release of these two micelles. From the results, it could be concluded that PCL20-ss-PMPC10/DOX micelles exhibited faster drug release than that of PCL20-PMPC10/DOX micelles. It has been reported there is high concentration of GSH in these tumor cells including HeLa cells.Citation36,Citation37 As the tumor cell internalized the micelles bearing disulfide, the disulfide was cleaved by GSH and the shell of the micelles departed rapidly. As a result, DOX release from reductive phosphorylcholine micelles accelerated, just as the result of in vitro drug release proved.
The results of CLSM and FCM indicated that the reduction-responsive phosphorylcholine micelles could release drug faster than nonreduction-responsive phosphorylcholine micelles in tumor cells. Quick drug release from the reduction-responsive micelles may kill tumor cells or inhibit cancer cell proliferation effectively.
Conclusion
The zwitterionic copolymers with/without disulfide (PCL-ss-PMPC or PCL-PMPC) and with different block lengths in PCL-ss-PMPC were designed and synthesized. The micelles with zwitterionic phosphorylcholine as hydrophilic shell and PCL as hydrophobic core were prepared based on the synthesized copolymers. DLC and DLE of the micelles to hydrophobic drug DOX were related to crystallinity degree of the copolymers; the latter was related to the composition of these copolymers. The desirable redox sensitivity of micelles was verified by in vitro drug release and intracellular drug release results for PCL-ss-PMPC micelles. Moreover, in vitro cytotoxicity evaluation revealed that the copolymer micelles showed low cell toxicity and DOX-loaded reduction-responsive micelles were more effective in inhibiting the growth of tumor cells than PCL-PMPC micelles. The reduction-responsive phosphorylcholine micelles with a suitable ratio of hydrophilic/hydrophobic units can serve as promising drug carriers.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 51273125). We would also like to appreciate our laboratory members for the generous help and gratefully acknowledge the Analytical and Testing Center at Sichuan University for testing.
Disclosure
The authors report no conflicts of interest in this work.
References
- BaeYKataokaKIntelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymersAdv Drug Deliv Rev2009611076878419422866
- KataokaKHaradaANagasakiYBlock copolymer micelles for drug delivery: design, characterization and biological significanceAdv Drug Deliv Rev200147111313111251249
- ParkKControlled drug delivery systems: past forward and future backJ Control Release20141903824794901
- Ebrahim AttiaABOngZYHedrickJLMixed micelles self-assembled from block copolymers for drug deliveryCurr Opin Colloid Interface Sci2011163182194
- RapoportNPhysical stimuli-responsive polymeric micelles for anti-cancer drug deliveryProg Polymer Sci2007328–9962990
- JoglekarMTrewynBGPolymer-based stimuli-responsive nanosystems for biomedical applicationsBiotechnol J20138893194523843342
- ChengRMengFDengCKlokH-AZhongZDual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug deliveryBiomaterials201334143647365723415642
- GaoGHLiYLeeDSEnvironmental pHs-sensitive polymeric micelles for cancer diagnosis and targeted therapyJ Control Release2013169318018423195533
- ChengRFengFMengFDengCFeijenJZhongZGlutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene deliveryJ Control Release2011152121221295087
- DengBMaPXieYReduction-sensitive polymeric nanocarriers in cancer therapy: a comprehensive reviewNanoscale2015730127731279526176593
- HuWChengLChengLRedox and pH-responsive poly (amidoamine) dendrimer-poly (ethylene glycol) conjugates with disulfide linkages for efficient intracellular drug releaseColloids Surf B Biointerfaces201412325426325277289
- YangQTanLHeCRedox-responsive micelles self-assembled from dynamic covalent block copolymers for intracellular drug deliveryActa Biomater20151719320025662913
- CunninghamAKoNROhJKSynthesis and reduction-responsive disassembly of PLA-based mono-cleavable micellesColloids Surf B Biointerfaces201412269370025159511
- JinQChenYWangYJiJZwitterionic drug nanocarriers: a biomimetic strategy for drug deliveryColloids Surf B Biointerfaces2014124808625092584
- ShaoQJiangSMolecular understanding and design of zwitterionic materialsAdv Mater2015271152625367090
- ZhaoJChaiYDZhangJHuangPFNakashimaKGongYKLong circulating micelles of an amphiphilic random copolymer bearing cell outer membrane phosphorylcholine zwitterionsActa Biomater2015169410225637066
- JiangSCaoZUltralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applicationsAdv Mater201022992093220217815
- IshiharaKGotoYTakaiMMatsunoRInoueYKonnoTNovel polymer biomaterials and interfaces inspired from cell membrane functionsBiochim Biophys Acta20111810326827520435095
- MassignaniMLoPrestiCBlanazsAControlling cellular uptake by surface chemistry, size, and surface topology at the nanoscaleSmall20095212424243219634187
- LiuG-YLvL-PChenC-JHuX-FJiJBiocompatible poly(D,L-lactide)-block-poly(2-methacryloyloxyethylphosphorylcholine) micelles for drug deliveryMacromol Chem Phys20112126643651
- SalvageJPRoseSFPhillipsGJNovel biocompatible phosphorylcholine-based self-assembled nanoparticles for drug deliveryJ Control Release2005104225927015907578
- WangHXuFWangYLiuXJinQJiJpH-responsive and biodegradable polymeric micelles based on poly([small beta]-amino ester)-graft-phosphorylcholine for doxorubicin deliveryPolym Chem201341030123019
- MiyataRUedaMJinnoHSelective targeting by preS1 domain of hepatitis B surface antigen conjugated with phosphorylcholine-based amphiphilic block copolymer micelles as a biocompatible, drug delivery carrier for treatment of human hepatocellular carcinoma with paclitaxelInt J Cancer2009124102460246719173297
- HuangLCaiMXieXChenYLuoXUptake enhancement of curcumin encapsulated into phosphatidylcholine-shielding micelles by cancer cellsJ Biomater Sci Polym Ed201425131407142425072747
- CaiMLengMLuASynthesis of amphiphilic copolymers containing zwitterionic sulfobetaine as pH and redox responsive drug carriersColloids Surf B Biointerfaces20151261925531063
- TuSChenY-WQiuY-BZhuKLuoX-LEnhancement of cellular uptake and antitumor efficiencies of micelles with phosphorylcholineMacromol Biosci201111101416142521793214
- LeleBSLerouxJCSynthesis of novel amphiphilic star-shaped poly(ε-caprolactone)-block-poly(N-(2-hydroxypropyl)methacrylamide) by combination of ring-opening and chain transfer polymerizationPolymer2002432155955606
- WilhelmMZhaoCLWangYPoly (styrene-ethylene oxide) block copolymer micelle formation in water: a fluorescence probe studyMacromolecules199124510331040
- CaoJZhaiSLiCNovel pH-sensitive micelles generated by star-shape copolymers containing zwitterionic sulfobetaine for efficient cellular internalizationJ Biomed Nanotechnol20139111847186124059084
- CaoJLuALiCEffect of architecture on the micellar properties of poly (ε-caprolactone) containing sulfobetainesColloids Surf B Biointerfaces2013112354123948152
- ChengFGuanXCaoHCharacteristic of core materials in polymeric micelles effect on their micellar properties studied by experimental and dpd simulation methodsInt J Pharm20154921–215216026196277
- WangWSunHMengFMaSLiuHZhongZPrecise control of intracellular drug release and anti-tumor activity of biodegradable micellar drugs via reduction-sensitive shell-sheddingSoft Matter20128143949
- WangHWangYChenYJinQJiJA biomimic pH-sensitive polymeric prodrug based on polycarbonate for intracellular drug deliveryPolymer Chem201453854861
- PegoraroCCecchinDGraciaLSEnhanced drug delivery to melanoma cells using PMPC-PDPA polymersomesCancer Lett2013334232833723402813
- SahayGAlakhovaDYKabanovAVEndocytosis of nanomedicinesJ Control Release2010145318219520226220
- GanesaratnamKDaburRFraserDThe role of glutathione in cancerCell Biochem Funct20042234335215386533
- HultbergBAnderssonAIsakssonAThiol and redox reactive agents exert different effects on glutathione metabolism in HeLa cell culturesClin Chim Acta1999283213210404728
