Abstract
In this study, antibacterial characteristic of silver/poly (lactic acid) nanocomposite (Ag/PLA-NC) films was investigated, while silver nanoparticles (Ag-NPs) were synthesized into biodegradable PLA via chemical reduction method in diphase solvent. Silver nitrate and sodium borohydride were respectively used as a silver precursor and reducing agent in the PLA, which acted as a polymeric matrix and stabilizer. Meanwhile, the properties of Ag/PLA-NCs were studied as a function of the Ag-NP weight percentages (8, 16, and 32 wt% respectively), in relation to the use of PLA. The morphology of the Ag/PLA-NC films and the distribution of the Ag-NPs were also characterized. The silver ions released from the Ag/PLA-NC films and their antibacterial activities were scrutinized. The antibacterial activities of the Ag/PLA-NC films were examined against Gram-negative bacteria (Escherichia coli and Vibrio parahaemolyticus) and Gram-positive bacteria (Staphylococcus aureus) by diffusion method using Muller–Hinton agar. The results indicated that Ag/PLA-NC films possessed a strong antibacterial activity with the increase in the percentage of Ag-NPs in the PLA. Thus, Ag/PLA-NC films can be used as an antibacterial scaffold for tissue engineering and medical application.
Introduction
Biocompatible and biodegradable polymers became very important and gained a lot of attention from both biomedical and ecological outlooks in the past decade.Citation1 At that time, synthetic biodegradable polymers were given a lot of attention because they could be used in important biomedical applications approved by the US Food and Drug Administration. Among the most popular and important biodegradable polymers are aliphatic polyestersCitation2–Citation5 such as poly (3-hydroxybutyrate), poly (ɛ-caprolactone), poly (glycolic acid), and poly (lactic acid) (PLA), whereby PLA received the most attention due to its renewable resources,Citation6 biodegradation, biocompatibility, as well as excellent thermal and mechanical properties, and superior transparency of the processed materials.Citation7 Moreover, PLA is widely used in various medical applications such as surgical implants, tissue culture, resorbable surgical sutures, wound closure, and controlled release systems.Citation2–Citation5 PLA produced from renewable resources is a linear aliphatic thermoplastic, which is readily biodegradable through hydrolytic and enzymatic pathways.Citation8–Citation10 More importantly, PLA is an immunologically inert synthetic polymer,Citation11,Citation12 and for this reason, it was chosen for designing a composition of tissue engineering scaffold in the present study.Citation13,Citation14
As they possess strong antibacterial properties and low toxicity, silver nanoparticles (Ag-NPs) and their compounds have been studied for many years not only for their low toxicity, but also for their antibacterial activity.Citation15–Citation18 Yeo et al found that fibers containing silver showed excellent antibacterial properties.Citation19 Meanwhile, studies conducted on Ag-NPs using different polymers have been reported; these include the synthesis in poly vinyl pyrrolidone,Citation20 poly (vinyl alcohol),Citation21 hyper branched polyurethaneCitation22 and poly acrylonitrileCitation23 to give discrete Ag-NPs. There are significant variations in the average sizes and shapes of the Ag-NPs when the polymers were used due to the different chemical structures and Ag/polymer interactions. Natural polymers have also attracted great interest, because they are biocompatible and non-toxic in most cases. Among the few stabilizers or matrices investigated for metal nanoparticles are chitosan,Citation24 starch,Citation25 cellulose,Citation26 and natural rubber.Citation27
In this work, Ag/PLA-NC films were synthesized using AgNO3 and sodium borohydride as silver precursor and reduction agent respectively in the PLA as a polymeric matrix in the diphase solvent. The antibacterial activity of the Ag/PLA-NCs was also determined.
Materials and methods
Materials
All reagents in this work were of analytical grades and used in their original form without any purification; in particular, PLA (density: 1.24 g/mL, >98%), which was supplied by Nature Works 4060D (USA). AgNO3 (99.98%) and NaBH4 (98.5%) were obtained from Sigma-Aldrich (USA). N, N-dimethyl formamide (DMF) (99.0%) and dichloromethane (99.0%) were purchased from Merck (Germany). Phosphate buffer saline (PBS) (pH = 7.00) was supplied by JT Baker (Germany). All the aqueous solutions were made using double distilled water (DD-water).
Synthesis of Ag/PLA-NCs by using NaBH4
PLA was dissolved in a mixed solvent of DMF and CH2Cl2 (1/9 v/v) to achieve a concentration of 10 wt%, and certain amounts of AgNO3 (0.8, 1.6, and 3.2 g) were added to the solution. The AgNO3/PLA solution was observed to be colorless when stirred for 48 hours in an ice water bath, and then 10 mL of NaBH4 aqueous solution (molar ratio of AgNO3/NaBH4 was 1:2) was added dropwise into AgNO3/PLA solution under vigorous stirring at the same temperature for 2 hours. The formation of the Ag-NPs started in the aqueous phase, but later the nanoparticles transferred to the organic phase due to the presence of van der Waals interactions between the hydroxyl groups of the PLA and the partial charges surrounded in the surface of the Ag-NPs.Citation28,Citation29 The organic phase containing stable Ag-NP colloid was separated from the aqueous phase and shaken with DD-water twice to remove any silver ions. After drying at room temperature, the Ag/PLA-NC films were dissolved in CH2Cl2 and dried using solution-casting technique after ultrasonication for 15 minutes.
Silver ions release
For determination of silver ions release, the Ag/PLA-NC films were cut into (1.0 cm × 1.0 cm) pieces with approximately 3.0 mg in weight. The in vitro release of silver was carried out in 10 mL of PBS. The samples were incubated at 37°C under water shaker at 70 rev min−1. The concentration of silver in the solution withdrawn from the test medium at the fixed time intervals was determined using atomic absorption spectroscopy.
Evolution of antibacterial activity
In vitro antibacterial activity of the samples was evaluated using disc diffusion method with Muller–Hinton agar and a determination of inhibition zone in millimeters (mm) which conforms to the recommended standards of the National Committee for Clinical Laboratory Standards. The antibacterial activity of Ag/PLA-NC films was scrutinized against Gram-negative bacteria, Escherichia coli ATCC 13706 and Vibrio parahaemolyticus ATCC 17802, and Gram-positive bacteria, Staphylococcus aureus ATCC 12600, at different percentages of Ag-NPs in the polymeric matrix. In order to recover the lyophilized culture, the desired culture contained in the plastic bead was aseptically transferred into a tube containing 5 mL of nutrient broth and maintained in an incubator at 37°C for 24 hours for bacteria and 25°C for 72 hours for mold. The working stock cultures were maintained on the tripton soy agar (TSA) and potato dextrose agar (Oxoid Ltd, England) slants for the bacteria and mold, respectively, at 4°C in a refrigerator. A single bacterial colony for each microorganism was used to inoculate 100 mL sterile tripton soy broth, which was grown aerobically overnight at 37°C in a rotary shaker at 90 rev min−1 for 18 hours and used as the microbial culture. The initial concentration of the cultures was 4.48 × 107 for E. coli, 7.61 × 106 for S. aureus, and 7.24 × 106 for V. parahaemolyticus, and these were determined using the solid agar plate test. Square samples (1.5 × 1.5 cm) of PLA and Ag/PLA-NC films containing different percentages of Ag-NPs were sterilized by dipping them in ethanol alcohol for 15 minutes and placed on the surface of TSA which was seeded by 1.0 mL of microorganism culture. The plates were inoculated at 37°C for 24 hours. The diameters of the inhibition zone around the film specimen were used to determine the antimicrobial activity of each film sample, and the average of 3 replications was recorded.
Characterization methods and instrumental
The synthesis of nano Ag/PLA was characterized using x-ray diffraction (XRD), transmission electron microscopy (TEM), UV-visible (UV-vis) spectroscopy, Fourier transform infrared (FT-IR) spectroscopy, and atomic absorption spectrometer. Meanwhile, the structure of the Ag/PLA produced was studied using the XRD (Philips, X’pert, Cu Ka) and were recorded at a scan speed of 4°/min. The TEM observations were carried out using a Hitachi H-7100 electron microscope, whereas the particle size distributions were determined using the UTHSCSA Image Tool version 3.00 program. The UV-vis spectra were recorded over a range of 300–700 nm with an H.UV.1650PC (SHIMADZU).B UV-vis spectrophotometer. FT-IR spectra were recorded over the range of 400–4000 cm−1 with a Perkin Elmer 1650, FT-IR spectrophotometer. The released Ag+ concentration in the PBS solution was determined using the atomic absorption spectrometer (Thermo Scientific, S. Series).
Results and discussion
X-ray diffraction
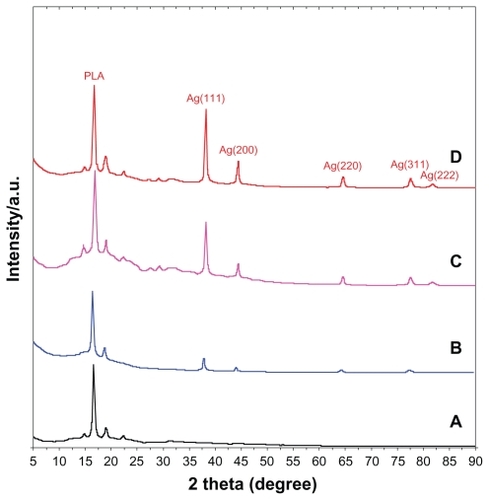
The XRD patterns of PLA and Ag/PLA-NCs are shown in . In addition to the broad diffraction peak, which was centered at 16.25° and could be assigned to PLA, 5 crystalline peaks were observed at 2θ° of 38.18°, 44.3°, 64.55°, 77.54°, and 81.71°. Their intensities were found to be markedly enhanced with the increasing Ag-NP content in the polymeric matrix. In more specific, they were attributed to the 111, 200, 220, 311, and 222 crystallographic planes of face-centered cubic (fcc) silver crystals, respectivelyCitation30 (XRD ref no 01-087-0718). The XRD peaks broadenings of Ag-NPs (8, 16, and 32 wt% respectively) were mostly because of the existing nanosized particles in the nanocomposites. Citation31 Based on the information presented in , five silver crystalline peak intensities were also found to increase with the increase in the percentage of Ag-NPs in PLA. This implies that the Ag-NPs existed in the surface and inside of PLA sheets.
UV-visible spectroscopy
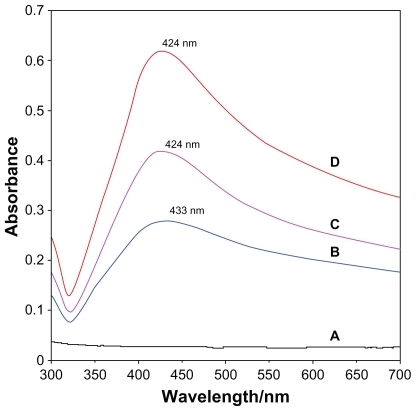
After adding reducing agent, the color of the solution turned light brown, brown, and dark brown. The generation of Ag-NPs could be identified from the UV-vis spectra. The characteristic of the silver SPR bands was detected to be around 400 nm. These absorption bands were presumably corresponding to the Ag-NPs smaller than 10 nm.Citation32,Citation33 Nonetheless, there is no characteristic UV-vis absorption of Ag-NPs before adding NaBH4 into AgNO3/PLA (), while the growth of the Plasmon peak at 433 nm indicates the formation of Ag-NPs (). With the increase in Ag-NP concentration, the corresponding peak intensities in a range of wavelengths from 424–433 nm increases. The absorption peaks for the 16 and 32 wt% of Ag-NPs () are slightly shifted to the lower wavelength (424 nm) indicating that mean diameters of Ag-NPs are different.Citation21,Citation34
Morphology
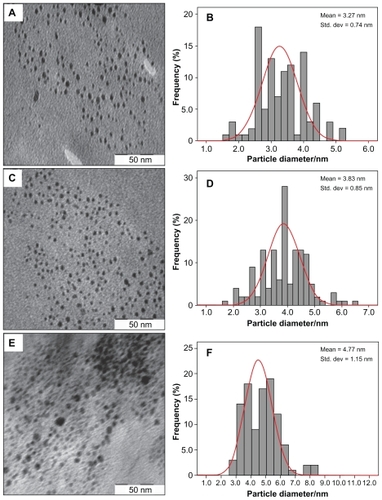
demonstrates TEM and size distribution of Ag/PLA-NC film containing different amounts of Ag-NPs. TEM image and their size distribution show that the mean diameters and standard deviation of Ag-NPs (8, 16, and 32 wt% relative to PLA) are 3.27 ± 0.74, 3.83 ± 0.85, and 4.77 ± 1.15 nm respectively. Furthermore, this confirms the uniform distribution of the Ag-NPs in the PLA matrix, although particles seem to aggregate to some extent. The observation could be attributed to a strong interaction between AgNO3 and PLA molecular chains, which promoted phase separation of polymer, thus preventing Ag-NPs from coagulating in the reduction process. TEM images show that with the increased percentages of Ag-NPs in the polymeric matrix, particle diameters and standard deviation are increased.
FT-IR chemical analysis
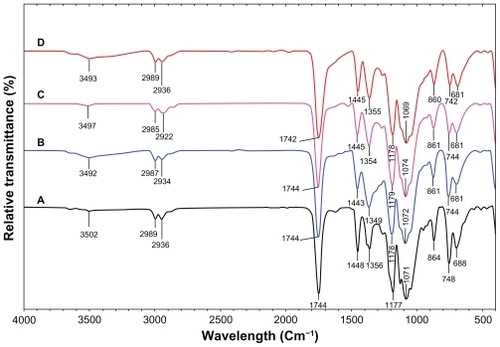
FT-IR spectroscopy was used to characterize the interaction between the Ag-NPs with PLA. shows FT-IR peaks for PLA and Ag/PLA-NCs (8, 16, and 32 wt% relative to PLA). The peaks at 1177 (1071), 2989 (2936), and 3502 cm−1 were assigned to the C–O, C–H (double) and O–H stretching of the –CH (CH3)–OH end group of PLA, respectively. The splitting of the C=O carbonyl stretching at ca. 1744 cm −1 might be due to the presence of –CH–CO–O– group. The peaks at 1448, and 1356 cm−1 were assigned –CH3, and –CH– bending including symmetric and asymmetric bending.Citation35,Citation36 The interactions between PLA chain molecules and Ag-NPs are associated with the peak at 3493 cm−1. Broad peak is due to the presence of van der Waals interactions between the hydroxyl groups of PLA and the partial positive charge on the surface of the Ag-NPs.Citation37
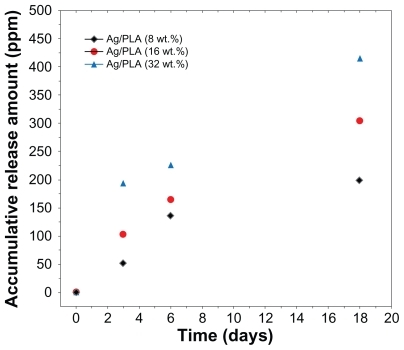
Silver ion release
Silver ions released from the Ag/PLA-NC films were investigated in PBS (pH = 7.00). The released silver was detected using the atomic absorption spectroscopy, and it should be silver cations Ag+. Therefore, the metallic silver in the polymeric matrix was converted to cationic silver during the release process through reaction with water. As shown in , the release of Ag+ for a given silver content is relatively fast at the beginning, but it becomes slower according to incubation time, and the release can last for more than 18 days. The accumulative amount of silver ions released is dependent on the silver content in the polymeric films, whereby more initial silver content leads to a much faster release of Ag+. A steady and prolonged release of silver cations can inhibit the growth of bacteria when their concentrations are above 0.1 ppb. In the present study, the accumulative amount of the silver ions released could reach up to 60 ppm of the polymeric films at the beginning stage of release. This suggests that Ag/PLA-NC films may have antibacterial ability.
Antibacterial results
Based on the results gathered from the agar disc diffusion test, all the Ag/PLA-NC films were found to exhibit a significant inhibition activity against E. coli ATCC 13706, S. aureus ATCC 12600, and V. parahaemolyticus ATCC 17802. The clear zones of the samples are shown in . Meanwhile, the inhibition zones surrounding the film square were formed, and these ranged from 1.43 to 10.33 mm against E. coli, from 4 to 15 mm against V. paraemolyicus, and from 4 to 9.3 against S. aureus, respectively, suggesting an antimicrobial activity of Ag/PLA-NCs. It is important to note that the antibacterial efficacies against Ag/PLA-NC films indicate that Ag-NPs are responsible for the antibacterial activity in the polymer nanocomposites, and this activity is completely strong. The Ag-NPs are gradually released to Ag+, and thus, the antibacterial activity becomes tough. Similarly, the antibacterial efficacy against V. parahaemolyticus is more than the one against E. coli, and this is probably because of the difference in cell walls between the Gram-negative bacteria. These different behaviors are shown between S. aureus (as Gram-positive bacteria) and E. coli (as Gram-negative bacteria). The cell wall of E. coli, containing lipids, proteins, and lipopoly-saccharides (LPS) provides an effective protection against biocides. However, the cell wall of the Gram-positive bacteria, such as S. aureus, does not contain LPS.Citation38 shows typical results of the tests carried out for the purpose of a first qualitative evaluation for V. parahaemolyticus; the antibacterial activity is evidenced by a zone of bacteria-growth inhibition, for the PLA and Ag/PLA-NC (8, 16, and 32 wt%) films. A similar inhibition zone is not present around the PLA film in , and there is bacteria growth also on the top of the sample. As expected, the most notable antibacterial effect is observed for the Ag/PLA-NCs (32 wt%) with the highest percentage (); reduced antibacterial effect are noted in respectively.
Figure 6 Comparison of inhibition zone test for Vibrio parahaemolyticus between PLA (A), Ag/PLA-NC content 8 (B), 16 (C), and 32 (D) wt% respectively.
Abbreviations: PLA, poly (lactic acid); NC, nanocomposite.
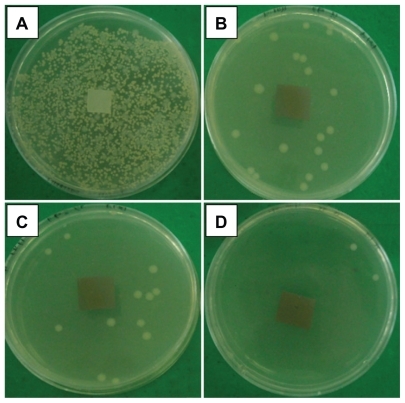
Table 1 Average of inhibition zones for PLA and Ag/PLA-NCs content 8, 16, and 32 wt%
Conclusion
A simple way to prepare uniform size of Ag-NPs in PLA by reacting different percentages of nanoparticles in PLA with sodium borohydride as reducing agent was developed. The average diameters of the Ag-NPs were between 3.27, 3.83, and 4.77 nm with well crystallized structures, and Ag-NP diameters increase with increasing amount of AgNO3 added. XRD analysis confirms that crystallographic planes of the silver crystal are the fcc types. UV-vis absorption spectra show peaks characteristic of the surface plasmon resonance of Ag-NPs. FT-IR shows that interactions exist between molecules of PLA and Ag-NPs. The antibacterial activity of Ag/PLA-NC films was demonstrated and showed a strong antibacterial activity against Gram-negative and Gram-positive bacteria. Further studies investigate the bactericidal effect of Ag/PLA-NC films on the types of bacteria for potentially widening such wound dressings or as anti-adhesion membranes.
Acknowledgments
Thanks are due to Professor Abdolhossein Rustaiyan and Mrs Parvaneh Shabanzadeh for helpful discussions and in giving the authors the idea. The authors are also grateful to the Institute of Bioscience (IBS/UPM) and Mrs A Jusoh in the TEM unit for technical assistance in this project.
Disclosure
The authors have no conflicts of interest to disclose in this work.
References
- IkadaYTsujiHBiodegradable polyesters for medical and ecological applicationsMacromol Rapid Comm20002117132
- JainRAThe manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide) (PLGA) devicesBiomaterials200022475249011055295
- MikosALymanMFreedLWetting of poly (L-lactic acid) and poly (D, L-lactic-co-glycolic acid) foams for tissue cultureBiomaterials19941555588161659
- TaylorMSDanielsAUAndrianoKPSix bioabsorbable polymers: in vitro acute toxicity of accumulated degradation productsJ Appl Biomater19945215115710147175
- ParkTCohenSLangerRPoly (L-lactic acid)/pluronic blends: characterization of phase separation behavior, degradation, morphology and as protein releasing matricesMacromolecules199225116122
- TsujiHIkadaYBlends of aliphatic polyesters. II. hydrolysis of solution-cast blends from poly (L-lactide) and poly (E-caprolactone) in phosphate-buffered solutionJ Appl Polym Sci199867405415
- UrayamaHKanamoriTKimuraYProperties and biodegradability of polymer blends of poly (L-lactide) with different optical purity of the lactate unitsMacromol Mater Eng2002287116121
- IwataTDoiYMorphology and enzymatic degradation of poly (L-lactic acid) single crystalsMacromolecules19983124612467
- SinclairRGThe case for polylactic acid as a commodity packaging plasticJ Macromol Sci199633585597
- SawaiDTakahashiKImamuraTPreparation of oriented β-form poly (L-lactic acid) by solid-state extrusionPolym Sci Poly Phys Ed20024095104
- NovikovaLNNovikovLNKellerthJOBiopolymers and biodegradable smart implants for tissue regeneration after spinal cord injuryCurr Opin Neurol20031671171514624081
- VitoCQuatelaMDJenCMDFacial plastic surgery clinics of North AmericaSynthetic Facial Implants200816110
- ChenYMakAFTWangMPLLA scaffolds with biomimetic apatite coating and biomimetic apatite/collagen composite coating to enhance osteoblastlike cells attachment and activitySurface Coatings Technol2006201575580
- ParkKEKangHKLeeSJBiomimetic nanofibrous scaffolds: preparation and characterization of PGA/Chitin blend nanofibersBiomacromolecules2006763564316471941
- ChenXSchluesenerHJNanosilver: a nanoproduct in medical applicationToxicol Lett200817611218022772
- AltVBechertTSteinrückePAn in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cementBiomaterials2004254383439115046929
- ShanBCaiYZBrooksJDAntibacterial properties of polygonum cuspidatum roots and their major bioactive constituentsFood Chem2008109530537
- LiYLeungPYaoLAntimicrobial effect of surgical masks coated with NanoparticlesJ Hosp Infect200662586316099072
- YeoSYLeeHJJeongSHPreparation of nanocomposite fibers for permanent antibacterial effectJ Materials Sci20033821432147
- ZhengMGuMJinYOptical properties of silver-dispersed PVP thin filmMater Res Bull200136853859
- KhannaPKSinghNCharanSSynthesis and characterization of Ag/PVA nanocomposites by chemical reduction methodMater Chem Phys200593117121
- LuHWLiuSHWangXLSilver nanocrystals by hyperbranched polyurethane assisted photochemical reduction of Ag+Mater Chem Phys200381104107
- ZhangZZhangLWangSA convenient route to polyacrylonitrile/silver nanoparticle composite by simultaneous polymerization–reduction approachPolymer20014283158318
- AdlimMAbu BakarMLiewKWSynthesis of chitosan-stabilized platinum and palladium nanoparticles and their hydrogenation activityJ Mol Catal A: Chem2004212141149
- RaveendranPFuJWallenSLCompletely “green” synthesis and stabilization of metal nanoparticlesJ Am Chem Soc2003125139401394114611213
- KotelnikovaNEWegenerGStollMComparative study of intercalation of zero-valent silver into the cellulose matrix by raster and transmission microscopyChem Polym Mater200376117123
- AppendiniPHotchkissJHReview of antimicrobial food packagingInnov Food Sci Emerg Technol20023113126
- TurkevichJStevensonPCHillierJThe nucleation and growth processes in the synthesis of colloidal goldDisc Faraday Soc1951115575
- RoucouxASchulzJPatinHReduced transition metal colloids: a novel family of reusable catalystsChem Rev20021023757377812371901
- TemgireMKJoshiSSOptical and structural studies of silver nanoparticlesRadiat Phys Chem20047110391044
- PrasadVSouzaCDYadavDSpectroscopic characterization of zinc oxide nanorods synthesized by solid-state reactionSpectrochim Acta A200665173178
- AiharaNTorigoeKEsumiKPreparation and characterization of gold and silver nanoparticles in layered laponite suspensionsLangmuir19981449454949
- LinXZTengXYangHDirect synthesis of narrowly dispersed silver nanoparticles using a single-source precursorLangmuir2003191008110085
- AhmadMBShameliKDarroudiMAntibacterial activity of silver/clay/chitosan bionanocompositesRes J Biol Sci200941111561161
- YounesHCohnDPhase separation in poly (ethylene glycol)/poly (lactic acid) blendsEur Polym J198824765773
- AgarwalMKoellingKWChalmersJJCharacterization of the degradation of poly-lactic acid in a well-controlled composting systemBiotechnol Progr199814517526
- Da SilvaECDa SilvaMGAMeneghettiSMPSynthesis of colloids based on gold nanoparticles dispersed in castor oilJ Nanopart Res200810201208
- SperanzaGGottardiGPederzolliCRole of chemical interactions in bacterial adhesion to polymer surfacesBiomaterials2004252029203714741617