 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A sterically stabilized, mitoxantrone-loaded liposome, tailored to target luteinizing hormone-releasing hormone (LHRH) receptor overexpressing cells, was developed to promote the efficiency of intracellular delivery of mitoxantrone through receptor-mediated endocytosis. Liposomes were prepared by lipid film hydration and an ultrasound dispersion process. Thiolated gonadorelin with affinity for the LHRH receptor was chemically coupled to N-[(3-maleimide-1-oxopropyl) aminopropyl polyethylene glycol-carbamyl] distearoyl-l-phosphatidyl-ethanolamine via a thioether bond and subsequently inserted into polyethylene glycol-grafted liposomes. The liposome was characterized in terms of its size, ligand density, drug loading, and leakage properties. The targeting nature and antitumor effects of the liposomes were evaluated in vitro using cultured MCF-7 breast cancer cells. A protein assay of ligand coupling to the liposomal surface indicated that more than 60% of the LHRH peptides were inserted into the liposome bilayer. Up to 1.0 mg/mL of stable liposomal mitoxantrone loading was achieved, with approximately 98% of this being entrapped within the liposomes. In vitro cell culture studies revealed that the gonadorelin-modified liposomes bound to their target cells had significantly higher affinity and better antitumor efficiency than generic drug-loaded liposomes. These events were presumed to occur through specific interactions of the LHRH with its cognate receptors on the cell surface. It was concluded that the targeting properties of the delivery system would potentially improve the therapeutic benefits of mitoxantrone, as compared with nontargeted liposomes.
Introduction
Chemotherapy is a common procedure for the treatment of advanced or metastatic cancer.Citation1 However, the application of free anticancer drugs has several drawbacks, including lack of selectivity, toxicity to normal cells, rapid elimination from the circulation, and instigation of acquired or intrinsic multidrug resistance of cancer cells.Citation2 Targeted delivery of anticancer drugs to tumors may assist in resolving these issues. Currently, an attractive solution is based on the use of nanocarriers. Encapsulation of anticancer drugs within such carriers could minimize drug degradation, prevent undesirable side effects, and increase the fraction of the drug accumulated in the diseased tissue.
Liposomes represent one of the most efficient and well characterized drug delivery strategies for small molecules.Citation3 These particles are biodegradable, biologically inert, have no antigenic or pyrogenic reactions, and possess limited intrinsic toxicity. Moreover, liposomes are better suited for drug targeting because of the ease of modifying their surface structure when compared with other drug carriers. Rationally designed liposomes, such as polyethylene glycol (PEG)-grafted liposomes (PEGylated liposomes), have the ability to accumulate passively in tumor tissues via “leaky” vasculature through the enhanced permeability and retention effect.Citation4 This passive accumulation can be further enhanced by actively targeting PEGylated liposomes to these tumor areas. Targeting of such liposomes could potentially reduce the extent of nonspecific toxicity associated with many drugs.
Targeting can be achieved by attaching a ligand for specific receptors that are expressed preferentially on tumor cells. Use of ligand-targeted liposomes has been demonstrated to result in increased internalization and more effective nuclear delivery of anticancer drugs, thus overcoming the problem of multiple drug resistance.Citation5 The luteinizing hormone-releasing hormone (LHRH) receptor is characteristically overexpressed in many tumors, such as breast, ovarian, endometrial, prostate, hepatic, colorectal, and pancreatic cancers, renal cell carcinomas, nervous system tumors, oral and laryngeal carcinomas, and melanomas, but is barely expressed in healthy visceral organs.Citation6–Citation8 The elevated expression of LHRH receptors in various cancers makes it possible to use these receptors as target moieties to deliver cytotoxic agents.Citation9 Analogs of LHRH have reportedly been used to deliver cytotoxic molecules to the tumor, and these cytotoxic analogs can be internalized by human ovarian and endometrial cancer cellsCitation10–Citation12 and MCF-7 human breast cancer cells.Citation13,Citation14
Mitoxantrone, a synthetic anthraquinone drug, has high antitumor activity against acute nonlymphocytic leukemia, advanced breast cancer, and non-Hodgkin’s lymphomas. The dose-limiting toxicity associated with mitoxantrone is myelosuppression combined with cardiotoxicity.Citation15,Citation16 Encapsulation of mitoxantrone into PEGylated liposomes not only increases the tumor concentration of the drug via the permeability and retention effect but also decreases its biodistribution and, consequently, the toxicity associated with therapy.Citation17–Citation20
Several attempts have been made to improve the anticancer efficiency of liposomal mitoxantrone further by modifying it with vector molecules specific to receptors typical for cancerous cells. Folate-targeted liposomes loaded with mitoxantrone demonstrated a substantial increase in cytotoxicity toward target cells in vitro.Citation21 Similarly notable results were obtained with mitoxantrone-loaded liposomes composed of anti-epidermal growth factor receptor monoclonal antibody fragments.Citation22 Most of the vector molecules being investigated now are proteins (first of all, monoclonal antibodies) specific to only certain types of tumors. Some small peptide LHRH analogs among these molecules possess an ability to recognize a broad variety of tumors, but not normal cells. Targeting these small peptides has certain advantages, including ease of preparation, lower antigenicity, and increased stability over the use of conventional protein macromolecules. LHRH analog-modified nanocarriers have been used for the selective delivery of diagnostic or therapeutic agents to LHRH receptors overexpressing cancer cells. Its improved efficacy over nontargeted nanoparticles was also clearly evident in previous studies.Citation23–Citation25
We have developed a simple and efficient way to attach gonadorelin to the liposomal membrane. Procedures for the attachment of LHRH analogs to the liposome surface using electrostatic absorption or conventional coupling techniques have been previously reported.Citation26,Citation27 In these two approaches, the ligand was incorporated in the lipid bilayer during their formation which involves several steps and a considerable length of time, and leaves some reactive functional groups on the inner leaflet of the lipid bilayer. In our current work, an alternative design was used, whereby the ligand was first modified with an activated lipid derivative and then incorporated into liposomes by coincubating the micelles of peptide-PEG-DSPE with preformed liposomes. An advantage of this approach is that the preparation of liposomes and the modification of ligand are performed separately, allowing the selection of optimal conditions for each step. The resulting liposomes exhibited strong and selective binding ability to the LHRH receptor on overexpressing tumor cell lines, and subsequent internalization. Furthermore, when compared with generic drug-loaded liposomes, targeting led to a selective increase in cytotoxicity of the mitoxantrone-loaded liposomes toward LHRH-receptor overexpressing tumor cell lines.
Materials and methods
Materials
A peptide analog of LHRH, gonadorelin (a synthetic human luteinizing hormone-releasing hormone), was purchased from ProSpec-Tany TechnoGene Ltd (Rehovot, Israel). The sequence of gonadorelin was Pyr-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2 (molecular weight 1182.3). Mitoxantrone hydrochloride was obtained from Chongqing Kailin Pharmaceutical Co., Ltd (Chongqing, China). Hydrogenated soybean phosphatidylcholine (HSPC) and [N-(carbonyl-methoxypolyethylene glycol 2000)-1, 2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt] (mPEG2000-DSPE) were purchased from Lipoid (Ludwigshafen, Germany). Cholesterol, 2-iminothiolane (Traut’s Reagent), 3-(4,5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide (MTT), and Sephadex G10 were purchased from Sigma Chemical Co (St. Louis, MO). Maleimide-PEG2000-DSPE (Mal-PEG2000-DSPE) was purchased from Avanti Polar Lipids (Alabaster, AL). Phosphate-buffered saline (pH 7.4, 290 mosm) was purchased from Tianjin Haoyang Biologicals Technology Co., Ltd (Tianjin, China). The bicinchoninic acid kit was purchased from Shanghai Shenergy Bicolor Bioscience and Technology Co (Shanhai, China). Ammonium sulfate and all other chemicals used in this study were of analytical grade or of high-performance liquid chromatography grade.
Cell line
Human MCF-7 breast cancer cells (LHRH receptor over-expressing cancer cell line) and human ovarian cancer SK-OV-3 cells (LHRH receptor low-expressing cancer cell line) were purchased from the American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI 1640 medium (Sigma) supplemented with 10% fetal bovine serum (Tianjin Haoyang Biologicals Technology Co., Ltd). Cells were grown at 37°C in a humidified atmosphere of 5% CO2 (vol/vol) in air. All experiments were performed on cells during the exponential growth phase.
Preparation of PEGylated liposomes
Liposomes were prepared by lipid film hydration and ultrasonic dispersion. Briefly, the mixtures of HSPC, cholesterol, and mPEG2000-DSPE (molar ratio 90:10:0.4) were solubilized in chloroform and dried to a thin lipid film at 60°C, followed by incubation overnight under vacuum to remove residual solvent. The dried lipid films were subsequently hydrated with 300 mM ammonium sulfate at 60°C for one hour, and subjected to sonication (VCX-130-PB; Sonics, Newtown, CT) for 10 minutes at 4°C (two minute pulses and two minutes of rest). This procedure formed small unilamellar vesicles. Small unilamellar vesicles were then centrifuged at 1000 g for 10 minutes to remove any titanium particles and larger aggregates. The particle size, polydispersity index, and zeta potential of the liposomes were determined by dynamic light scattering techniques (Zeta sizer Nano ZS; Malvern Instruments Ltd., UK) at 25°C and at a scattering angle of 90°. The obtained particle suspension was diluted appropriately in phosphate-buffered saline before measurement.
Preparation of thiolated gonadorelin and coupling procedure
Gonadorelin and Traut’s reagent were both dissolved in HEPES buffer (25 mM HEPES, 140 mM NaCl, pH 8.0) and mixed at a 1:4 molar ratio. This mixture was left to react for one hour, at room temperature, under dark conditions and under an inert N2 atmosphere. Unreacted Traut’s reagent was removed by size exclusion chromatography using a Sephadex G10 column with degassed HEPES buffer (pH 8.0). Separately, the dried lipid films of Mal-PEG2000-DSPE were prehydrated at a concentration of 4 mg/mL in HEPES buffer (pH 8.0) at 60°C to form micelles. The thiolated-gonadorelin was then added to freshly prepared Mal-PEG2000-DSPE micelles at a 2:1 molar ratio. The resulting suspension was left to react overnight in the dark at room temperature with magnetic stirring under an inert N2 atmosphere. Free maleimide groups were quenched with an excess of histidine for 30 minutes at room temperature. Gonadorelin-coupled PEG-DSPE micelles were then concentrated in a Centriprep® Centrifugal filter, using a size cutoff of 3000 Da (Millipore, Billerica, MA). The amounts of conjugated and free gonadorelin were indirectly measured with a bicinchonic acid protein assay using pure bovine serum albumin as standard.Citation30 The coupling efficiency of gonadorelin to Mal-PEG-DSPE was calculated as μg gonadorelin/μmol lipid.
Preparation of gonadorelin-modified liposomes
Gonadorelin-PEG-DSPE was then transferred into preformed PEGylated liposomes based on the procedure described previously.Citation25,Citation26 Briefly, the gonadorelin-PEG-DSPE solution was added to the preformed PEGylated liposome solution at 1:1000 gonadorelin-PEG-DSPE:phospholipid molar ratios and incubated at 60°C for one hour. The control PEGylated liposomes were prepared similarly by substituting gonadorelin-PEG-DSPE with mPEG2000-DSPE.
Transmembrane ammonium sulfate gradient loading of mitoxantrone
Mitoxantrone was encapsulated into the gonadorelin-modified or control PEGylated liposome formulations using transmembrane ammonium sulfate gradient-driven loading procedures. The procedure consisted of initially exchanging the buffer by dialysis for four hours against sucrose (300 mM)-histidine (10 mM) buffer (pH 7.5), using dialysis tubing with a 10–12 kDa molecular weight cutoff (Pierce Chemical Co., Rockford, IL). Liposomes were incubated at 60°C for 10 minutes before addition of mitoxantrone, to achieve a final lipid to drug weight ratio of 10:1. The resulting mixture was incubated at 60°C for an additional 30 minutes, followed by dialysis against phosphate-buffered saline to remove the free mitoxantrone for determination of entrapment efficiency. The concentration of mitoxantrone was determined by measuring absorbance at 655 nm in a Varioskan Flash spectral scanning multimode reader (Thermo Scientific, Waltham, MA) after lysis of liposomes with 3% Triton-X 100.
In vitro drug release
Drug release experiments were performed in two different release buffers, (release buffer 1: phosphate-buffered saline at pH 7.4 and release buffer 2: 90% isotonic glucose solution buffered with 10 mM histidine to pH 7.5 and 10% human plasma). The liposomes were first diluted using the release media, to a HSPC concentration of 20 mM, and then 2 mL of diluted liposomes were put into dialysis tubing with a molecular weight cutoff of 10–12 kDa and dialyzed against 100 mL release buffers at 37°C. Each buffer contained 100 IU/mL penicillin and 100 μg/mL streptomycin.
At predetermined time intervals, samples were withdrawn from the release medium and assayed for mitoxantrone by high-performance liquid chromatography. Before analysis, samples containing plasma were extracted by protein precipitation,Citation31 and samples without plasma were analyzed directly. Mitoxantrone was separated on a Zorbax SB C18 column (150 mm × 4.6 mm, 5 μm, Agilent Technologies, Inc. Santa Clara, CA) at 35°C, and quantified by ultraviolet absorbance at 655 nm. The mobile phase consisted of acetonitrile and solutions containing 30 mM of sodium 1-heptanesulfonate and 9.0 ml/L of glacial acetic acid (37:63, v/v) at a flow rate of 1 mL/min.
In vitro intracellular uptake of liposomal mitoxantrone
LHRH receptor overexpressing MCF-7 cells or LHRH receptor low-expressing SK-OV-3 cells were seeded at densities of 2 × 105 cm−2 in 35 mm diameter culture dishes and cultured in RPMI 1640 medium. After 24 hours of incubation, 50% confluency was reached and the medium was changed with 1 mL of fresh culture medium containing either gonadorelin-modified mitoxantrone-loaded liposomes (LHRH-MTX-SL) or nontarget mitoxantrone-loaded liposomes (MTX-SL) at a dose of 0.2 mg total lipids (10 μg/mL as mitoxantrone) per dish. The cells were treated identically, except for the omission of reacting with mitoxantrone formulations was used as a negative control. After four hours of incubation at 37°C, the cells were washed three times with cold phosphate-buffered saline (pH 7.4) to remove unbound liposomes. The internalized liposomes were visualized using a confocal laser scanning microscope (Zeiss LSM 510, Germany), with excitation and emission wavelengths for mitoxantrone of 514 nm and 685 nm, respectively.
In vitro antitumor effect
A comparison of the cytotoxicity of free drug and various liposomal formulations was performed on human MCF-7 cells by means of an in vitro proliferation assay using MTT. Briefly, 2 × 105 cells were seeded in 96-well plates and incubated overnight. Culture media with various concentrations of free mitoxantrone or its liposomal formulations were then added in sextuplet, and incubation continued for an additional 24–120 hours. At designated time points, the medium was discarded and fresh medium containing MTT was added to each well. After four hours, the medium was removed, replaced with 100 μL methyl sulfoxide, and mixed on a vortex mixer for 10 minutes. Absorbance was recorded at 570 nm using the Varioskan Flash spectral scanning multimode reader. Cell viability was calculated as follows:
IC50 was defined as the concentration of drug that reduced the optical density measured at 570 nm by 50% of the control value.
Statistical analyses
Statistical comparisons were carried out using one-way analysis of variance with SPSS 10.0 software (SPSS Inc., Chicago, IL). P values less than 0.05 were considered significant.
Results
Preparation and characterization of liposomal formulations
Gonadorelin-modified PEGylated liposomes were produced by transferring the conjugate of DSPE-PEG and gonadorelin from their loose micelles onto the liposome surface. Liposomes consisted of small unilamellar vesicles containing HSPC, cholesterol, mPEG2000-DSPE, and Mal-PEG-DSPE for ligand conjugation. As controls, nontargeted PEGylated liposomes were prepared identically, except for the substitution of mPEG2000-DSPE for gonadorelin-PEG-DSPE. Liposomes were then loaded with mitoxantrone using the transmembrane ammonium sulfate gradient loading method (). Up to 1.0 mg/mL of stable liposomal mitoxantrone loading was achieved, with approximately 98% of this being entrapped within the liposomes. The physical properties of the liposomes before and after coupling of gonadorelin are presented in . The size of the liposomes was in the range of 120–150 nm. The size of the gonadorelin-modified liposomes was found to be 15–20 nm larger than the original liposome. The zeta potential was slightly lower after the ligand conjugation event due to the presence of peptides attached to the liposomal membrane via a longer PEG-linker. Regardless, the encapsulation of mitoxantrone did not significantly affect the particle size of the liposome. The amount of gonadorelin in the liposome (assessed by bicinchoninic acid assay) in comparison with the amount of total phospholipidsCitation30 are also presented in .
Figure 1 Synthesis of LHRH-PEG-DSPE and preparation of LHRH-MTX-SL.
Abbreviations: LHRH, luteinizing hormone-releasing hormone; PEG, polyethylene glycol; MTX, mitoxanthrone; SL, loaded liposomes.
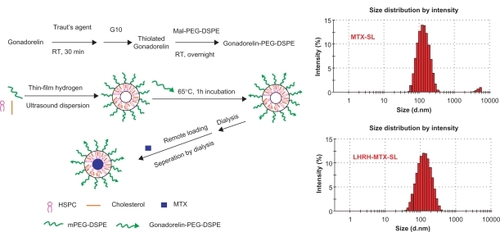
Table 1 Particle size, polydispersity index, zeta potential and gonadorelin:lipid ratio of liposomal formulations with or without mitoxantrone. The dynamic light scattering analysis was performed at 25°C and at a scattering angle of 90°. The obtained particle suspension was diluted appropriately in phosphate-buffered saline before measurement
In vitro release of liposomal mitoxantrone
The in vitro release experiments were performed for quantitative comparison of the drug release characteristics of mitoxantrone from different mitoxantrone-loaded liposomal formulations. For all the formulations, the mitoxantrone/HSPC molar ratio and the entrapment ratio were similar. The drug release experiment was first performed in phosphate-buffered saline using a dialysis method. Under these conditions, the release should be attributed to “dilution effect”. As shown in , both LHRH-MTX-SL and MTX-SL exhibited almost no release during the overall experimental period. The results clearly showed that dilution has little influence on the release of mitoxantrone from the liposomes.
Figure 2 Release profiles of mitoxantrone from MTX-SL and LHRH-MTX-SL in phosphate-buffered saline A or isotonic glucose containing 10% human plasma B Release of mitoxantrone from different liposome preparations was evaluated by a dialysis method, followed by high-performance liquid chromatography analysis for determination of mitoxantrone. Results are expressed as means ± standard error of measurement of three independent measurements.
Abbreviations: LHRH, luteinizing hormone-releasing hormone; MTX, mitoxanthrone; SL, loaded liposomes.
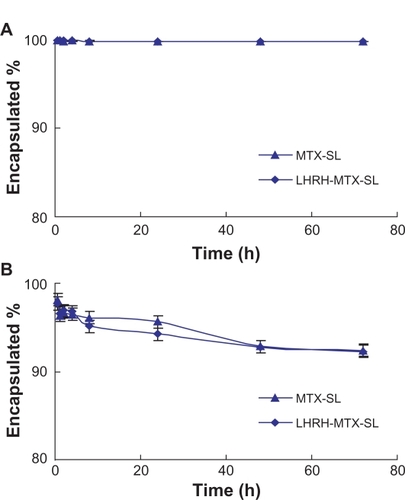
The plasma-containing release buffer was also used to investigate the effect of plasma protein on mitoxantrone retention by liposomes. Because mitoxantrone has a strong affinity to some plasma proteins, protein binding might drive release of mitoxantrone from the vesicles. As shown in , increased drug release of the two formulations was observed. After about 72 hours of incubation, more than 8% mitoxantrone was released from both LHRH-MTX-SL and MTX-SL. Overall, the release experiments in different release media clearly showed the modification of liposomes with gonadorelin had no influence on the mitoxantrone retention.
Intracellular uptake of mitoxantrone formulations
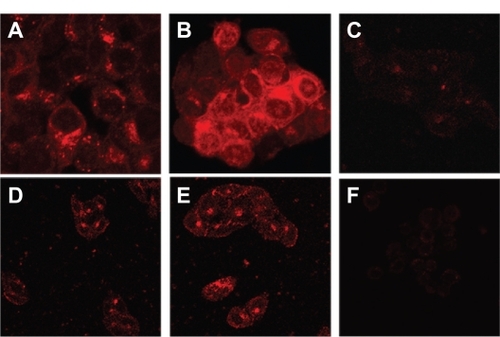
To confirm LHRH receptor-mediated internalization of the gonadorelin-modified liposomes, the extent of cellular uptake of different liposomal formulations was evaluated by confocal laser scanning fluorescence microscopy in LHRH receptor overexpressing or low-expressing cell lines. The cells were incubated with either the MTX-SL or LHRH-MTX-SL (10 μg/mL as mitoxantrone) at 37°C for four hours. Cells treated with drug-free medium were used as controls. In LHRH receptor overexpressing MCF-7 cells (), incubation with LHRH-MTX-SL () resulted in more extensive internalization and cytoplasmic accumulation than MTX-SL (). In contrast, LHRH-MTX-SL () did not show improved uptake over MTX-SL () in the LHRH low-expressing SK-OV-3 cell type ().
Figure 3 Uptake of different liposomes encapsulating mitoxantrone in various cell lines by confocal laser scanning fluorescence microscopy. Various cell lines were treated with either LHRH-MTX-SL or MTX-SL for four hours at 37°C. A LHRH-MTX-SL in LHRH receptor high-expressing MCF-7 cells; B MTX-SL in LHRH receptor high-expressing MCF-7 cells; C MCF-7 cells treated with drug-free medium used as a control; D LHRH-MTX-SL in LHRH receptor low-expressing SK-OV-3 cells; E MTX-SL in LHRH receptor low-expressing SK-OV-3 cells; F SK-OV-3 cells treated with drug-free medium used as a control.
Abbreviations: LHRH, luteinizing hormone-releasing hormone; MTX, mitoxanthrone; SL, loaded liposomes.
In vitro antitumor effect
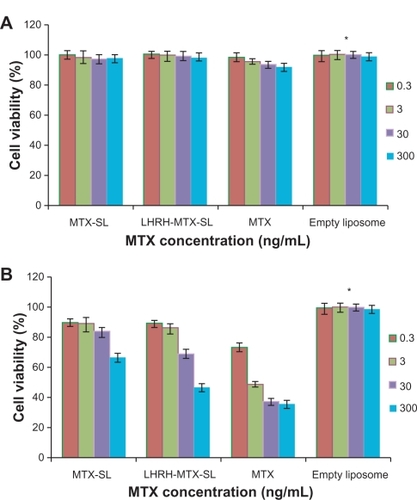
Comparison of the in vitro cytotoxicity of free mitoxantrone and mitoxantrone-loaded liposomal formulations was performed using MCF-7 cells, which are known to overexpress the LHRH receptor. Cells were incubated with either free mitoxantrone or its liposomal formulations ranging from 3 × 10−1 to 3 × 102 ng/mL for 24–120 hours. As shown in , the empty liposomes exerted no effect on cell growth. Increasing the exposure time or drug concentration of either mitoxantrone or the mitoxantrone-loaded liposomal formulation led to improved efficacy, with the effects being more significant with the gonadorelin-modified liposome compared with the control nontargeted liposome.
Figure 4 Viability of MCF-7 cells after treatment with mitoxantrone, mitoxantrone-containing liposomes, and empty liposomes for 24 hours A and 120 hours B The cells were exposed to serial concentrations of free mitoxantrone, MTX-SL, LHRH-MTX-SL at 37°C for 24 hours or 120 hours. The drug concentration of free mitoxantrone and MTX-SL used in this assay was 0.3, 3.0, 30, 300 ng/mL. Empty liposomes were used as a control. The antitumor effect was evaluated usings the MTT method. MCF-7 cell viability (as a percentage of control cells) was calculated as follows: Viability(%) = Atreated/Acontrol × 100%. Results are expressed as means ± standard error of measurement from six independent measurements.
The average in vitro IC50 values for free mitoxantrone, MTX-SL, and MTX-LHRH-SL from three parallel cytotoxicity assays were 0.0445, 11.7, and 0.578 μg/mL, respectively (). Free mitoxantrone was significantly more cytotoxic than either MTX-SL or MTX-LHRH-SL after 120 hours of incubation (P < 0.05). Targeted formulations were significantly more cytotoxic than nontargeted formulations (P < 0.05).
Table 2 Cytotoxicity data (IC50) for free mitoxantrone and mitoxantrone liposomal formulations (with or without LHRH) determined with MCF-7 cells (n = 3). Data are represented as percentage of control. Each assay was done in triplicate (mean ± standard error of measurement). The cells were exposed to serial concentrations of free mitoxantrone, MTX-SL, and LHRH-MTX-SL at 37°C for 120 hours. The cytotoxicity was evaluated using the MTT method. MCF-7 cell viability (as a percentage of control cells) was calculated as follows: Viability (%) = Atreated/Acontrol × 100%. IC50 was defined as the concentration of drug where cell growth and/or viability was 50% of that observed in control (drug-free) cultures in the MTT assay
Discussion
In this study, we developed a simple yet highly efficient way to attach gonadorelin to liposomal membranes without compromising its specific activity or significantly affecting the amount or stability of the encapsulated drug. The LHRH analog, gonadorelin, was coupled to end-functionalized groups in PEG-DSPE micelles, and then the gonadorelin-PEG-DSPE conjugates were transferred in a simple incubation step from the micelles into the outer monolayer of preformed liposomes. This method represents a combinatorial approach to the design of targeted liposomes that minimizes manufacturing complexities and allows a variety of ligands to be inserted into a variety of preformed liposomes containing a large range of drugs.Citation32
The covalent coupling of thiolated gonadorelin to the maleimide terminus of DSPE-PEG was specifically explored. This methodology was chosen because the reaction between thiol and maleimide groups has been reported to be one of the most efficient reactions in bioconjugate chemistry, resulting in a stable thioether linkage.Citation33
Moreira et alCitation29 observed that insertion of peptide ligands into PEGylated liposomes may cause some membrane perturbation, resulting in drug leakage from liposomes. Therefore, the loading of mitoxantrone was performed after insertion of the gonadorelin-PEG-DSPE conjugates. In these preparations, over 98% of drugs were entrapped within the liposomes. Subsequently, mitoxantrone release was evaluated in different media (). After 72 hours at 37°C, regardless of the incubation media, the drug release was approximately the same as that observed for control nontargeted liposomes, demonstrating that modification with gonadorelin had no influence on the membrane integrity of the liposomes.
Nonetheless, the liposomal formulations evaluated here exhibited sustained drug release characteristics. This finding is in agreement with a previous report that indicated liposomal mitoxantrone was difficult to release from vesicles.Citation34 This behavior is consistent with the presence of drug precipitates in the liposomal interior. After ammonium sulfate gradient-mediated uptake, mitoxantrone forms insoluble precipitates within the liposome. The permeability characteristics of the drug in a precipitated form may be less dependent on membrane characteristics or the collapse of the transmembrane ammonium gradient. The efflux rate of drugs is proportional to the concentration of the soluble form of the drug, which will remain constant until the precipitated form is dissolved. Thus, the time required for drug release should be proportional to the amount of drug present in the precipitated form.Citation35,Citation36
In the cell uptake test, gonadorelin-modified liposomes showed significantly higher uptake by their respective target cells in vitro, as compared with the control nontargeted liposomes, implying that the gonadorelin-modified liposome binds specifically to the LHRH receptor and is efficiently internalized by LHRH receptor-mediated endocytosis.Citation37 Endocytosis of the drug-liposome package into the cell interior would effectively limit the diffusion potential of the drug away from the target cell, which would in turn increase the amount of drug delivered to the target cell interior.
Using mitoxantrone-loaded, gonadorelin-modified liposomes, we showed that uptake by LHRH receptor overexpressing cells translates into increased cytotoxicity, as compared with the drug-loaded control without the gonadorelin-loaded liposome, probably due to distinct mechanisms of action. The cytotoxicity of MTX-SL is mainly owing to the gradual release of the encapsulated drug from liposomes close to the cell and uptake of the free drug into the target cell. The cytotoxicity of LHRH-MTX-SL may be attributed to receptor-mediated endocytosis of the liposomes, followed by slow breakdown of the liposomes in the cytoplasm and subsequent intracellular trafficking of the released drug. The empty gonadorelin-modified liposomes were nontoxic, indicating that cytotoxicity was not mediated by the LHRH peptide displayed on the liposome surface.
Cytotoxicity of the LHRH-MTX-SL formulation used in this study did not reach that of the free drug. This is in accordance with previous studies for anti-epidermal growth factor receptor immunoliposomal formulations of mitoxantrone.Citation22 Several factors may account for this observed discrepancy. For example, subcellular localization of endocytosed immunoliposomes and the release rate of encapsulated drugs may all have profound influences on the efficiency of liposomal drugs.Citation38 Endocytosed immunoliposomes are often routed through the endosomal and lysosomal pathways.Citation39 For encapsulated mitoxantrone, release from liposomes and further escape from endosomes or lysosomes are required for translocation to the nucleus to effect disruption of DNA metabolism. In this study, the designed immunoliposomes exhibited sustained drug release characteristics. Moreover, the release rate of free drugs from the endosomes or lysosomes was also low, which depressed the action of mitoxantrone on tumor cells. Further development of pH-sensitive liposome formulations may facilitate the collapse of liposomes, allowing more efficient release of encapsulated drug.Citation40
Conclusions
The present study provides clear evidence that a simple immunomodification of mitoxantrone-loaded PEGylated liposomes with the LHRH analogs, Gonadorelin, against cancer-specific LHRH receptors results in successful development of a rather universal tumor-targeted mitoxantrone delivery system. LHRH-MTX-SL significantly facilitated specific delivery of mitoxantrone to LHRH receptor over-expressing tumor cells and efficiently inhibited tumor growth. This observation leads us to believe that LHRH-MTX-SL has potential value for drug delivery therapy in future nanomedicine to treat human cancer and other diseases.
Acknowledgements
The authors are grateful to the Natural Science Foundation of China (No 50873114) and to the Central Level Nonprofit, Scientific Research Institutes’ Basic R and D Operations Special Fund (Contract No 0609) for funding this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- BiganzoliLMinisiniAAaproMDi LeoAChemotherapy for metastatic breast cancerCurr Opin Obstet Gynecol2004161374115128006
- MezoGManeaMReceptor-mediated tumor targeting based on peptide hormonesExpert Opin Drug Deliv201071799619947889
- AllenTMCullisPRDrug delivery systems: Entering the mainstreamScience200430356651818182215031496
- DrummondDCMeyerOHongKKirpotinDBPapahadjopoulosDOptimizing liposomes for delivery of chemotherapeutic agents to solid tumorsPharmacol Rev19995169174310581328
- ElbayoumiTATorchilinVPTumor-specific anti-nucleosome antibody improves therapeutic efficacy of doxorubicin-loaded long-circulating liposomes against primary and metastatic tumor in miceMol Pharm20096124625419049322
- KakarSSJennesLExpression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non reproductive human tissuesCancer Lett199598157628529206
- SchallyAVSzepeshaziKNagyAComaru-SchallyAMHalmosGNew approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogueCell Mol Life Sci20046191042106815112052
- SchallyAVComaru-SchallyAMNagyAHypothalamic hormones and cancerFront Neuroendocrinol200122424829111587553
- NagyASchallyAVTargeting of cytotoxic luteinizing hormone-releasing hormone (LH-RH) analogs to breast, ovarian, endometrial and prostate cancersBiol Reprod200573585185916033997
- DharapSSQiuBWilliamsGCSinkoPSteinSMinkoTMolecular targeting of drug delivery systems to ovarian cancer by BH3 and LHRH peptidesJ Control Release2003911–2617312932638
- NagyASchallyAVCytotoxic analogues of luteinizing hormone-releasing hormone (LH-RH): A new approach to targeted chemotherapyDrugs Fut200227359370
- WestphalenSKotullaGKaiserFReceptor mediated anti-proliferative effects of the cytotoxic LHRH agonist AN-152 in human ovarian and endometrial cancer cell linesInt J Oncol20001751063106911029513
- KrebsLJWangXPudavarHERegulation of targeted chemotherapy with cytotoxic luteinizing hormone-releasing hormone analogue by epidermal growth factorCancer Res200060154194419910945629
- KakarSSJinHHongBEatonJWKangKALHRH receptor targeted therapy for breast cancerAdv Exp Med Biol200861428529618290339
- FauldsDBalfourJAChrispPLangtryHDMitoxantrone: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in the chemotherapy of cancerDrugs19914134004491711446
- DunnCJGoaKLMitoxantrone: A review of its pharmacological properties and use in acute nonlymphoblastic leukaemiaDrugs Aging1996921221478820798
- UgwuSZhangAParmarMPreparation, characterization, and stability of liposome-based formulations of mitoxantroneDrug Dev Ind Pharm200531222322915773289
- LiCCuiJLiYCopper ion-mediated liposomal encapsulation of mitoxantrone: The role of anions in drug loading, retention and releaseEur J Pharm Sci2008344–533334418573336
- CuiJLiCWangLNi2+-mediated mitoxantrone encapsulation: Improved efficacy of fast release formulationInt J Pharm20093681–2243018973800
- LiCCuiJWangCLipid composition and grafted PEG affect in vivo activity of liposomal mitoxantroneInt J Pharm20083621–2606618598745
- KawanoKOnoseEHattoriYMaitaniYHigher liposomal membrane fluidity enhances the in vitro antitumor activity of folate-targeted liposomal mitoxantroneMol Pharm2009619810419072653
- BeuttlerJRothdienerMMüllerDFrejdFYKontermannRETargeting of epidermal growth factor receptor (EGFR)-expressing tumor cells with sterically stabilized affibody liposomes (SAL)Bioconjug Chem20092061201120819435362
- DharapSSWangYChandnaPTumor-specific targeting of an anticancer drug delivery system by LHRH peptideProc Natl Acad Sci U S A200510236129621296716123131
- LeuschnerCKumarCSHanselWSoboyejoWZhouJHormesJLHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastasesBreast Cancer Res Treat200699216317616752077
- SundaramSDurairajCKadamRKompellaUBLuteinizing hormone-releasing hormone receptor-targeted deslorelin-docetaxel conjugate enhances efficacy of docetaxel in prostate cancer therapyMol Cancer Ther2009861655166519509261
- SaadMGarbuzenkoOBBerEReceptor targeted polymers, dendrimers, liposomes: Which nanocarrier is the most efficient for tumor-specific treatment and imaging?J Control Release200813010711418582982
- QinYSongQGZhangZROvarian tumor targeting of docetaxel-loaded liposomes mediated by luteinizing hormone-releasing hormone analogues. In vivo distribution in nude miceArzneimittelforschung2008581052953419025064
- AllenTMSapraPMoaseEUse of the post-insertion method for the formation of ligand-coupled liposomesCell Mol Biol Lett20027388989412378272
- MoreiraJNIshidaTGasparRAllenTMUse of the post-insertion technique to insert peptide ligands into pre-formed stealth liposomes with retention of binding activity and cytotoxicityPharm Res200219326526911934232
- DeryckeASDe WittePATransferrin-mediated targeting of hypericin embedded in sterically stabilized PEG-liposomesInt J Oncol200220118118711743662
- JohnsonJLAhmadAKhanSImproved liquid chromatographic method for mitoxantrone quantification in mouse plasma and tissues to study the pharmacokinetics of a liposome entrapped mitoxantrone formulationJ Chromatogr B Analyt Technol Biomed Life Sci20047991149155
- ElbayoumiTATorchilinVPTumor-targeted immunoliposomes for delivery of chemotherapeutics and diagnosticsJ Pharm Innov200835158
- AllenTMBrandeisEHansenCBKaoGYZalipskySA new strategy for attachment of antibodies to sterically stabilized liposomes resulting in efficient targeting to cancer cellsBiochim Biophys Acta199512372991087632714
- RegevRYeheskely-HayonDKatzirHEytanGDTransport of anthracyclines and mitoxantrone across membranes by a flip-flop mechanismBiochem Pharmacol200570116116915919056
- LiCCuiJWangCEncapsulation of mitoxantrone into pegylated SUVs enhances its antineoplastic efficacyEur J Pharm Biopharm200870265766518582570
- YamauchiMTsutsumiKAbeMUosakiYNakakuraMAokiNRelease of drugs from liposomes varies with particle sizeBiol Pharm Bull200730596396617473443
- NagyASchallyAVArmatisPCytotoxic analogues of luteinizing hormone-releasing hormone containing doxorubicin or 2-pyrrolino-doxorubicin, a derivative 500–1000 times more potentProc Natl Acad Sci U S A19969314726972738692981
- RothADrummondDCConradFAnti-CD166 single chain antibody-mediated intracellular delivery of liposomal drugs to prostate cancer cellsMol Cancer Ther20076102737274617938267
- CollinsDMaxfieldFHuangLImmunoliposomes with different acid sensitivities as probes for the cellular endocytic pathwayBiochim Biophys Acta1989987147552597686
- KimMJLeeHJLeeIAPreparation of pH-sensitive, long-circulating and EGFR-targeted immunoliposomesArch Pharm Res200831453954618449514