Abstract
We investigated the effect of magnetic nanoparticles of Fe3O4 (Fe3O4-MNPs) on the mice immune system. Imprinting control region (ICR) mice were assigned randomly into four groups and treated with normal saline or low, medium, or high doses of Fe3O4-MNPs, respectively. After intravenous administration of Fe3O4-MNPs for 72 hours, the peripheral T cells and the induction of primary immune responses in mice were investigated by flow cytometry and determined using enzyme-linked immunosorbent assay, respectively. The results showed that the ratio of spleen to body weight was not different between the experimental groups and control group (P > 0.05). The lymphocyte transformation rates in the suspension of spleen were higher in low-dose group than those in the control group (P < 0.05), while the proliferation of splenocytes was low in the medium and high groups when compared to the control group (P < 0.05). In peripheral blood, both the proportions of subset CD4+ and CD8+ T lymphocytes in the low-dose group were higher than those in the control group, whereas there was no difference in the number of CD4+ T cells between the medium- and low-dose groups. Interestingly, the Fe3O4-MNPs enhanced the production of interleukin-2 (IL-2), interferon-γ, and IL-10 but did not affect the production of IL-4 in peripheral blood. It is concluded that Fe3O4-MNPs could influence immune functions of normal ICR mice in a dose-dependent manner.
Nanotechnology offers an efficient alternative for cancer diagnostics and tumor target treatment due to the unique properties of nanostructures, such as large surface-to-volume ratio, porous structure, embedded effect, and size effect, which have been recognized as offering potential promising applications in biomedical engineering. Much effort has been extended to the development of novel nanocomposites and biomaterials for DNA detection,Citation1 intracellular labeling,Citation2 drug carrier,Citation3 cancer targeting,Citation4 imaging,Citation5 and so on. Therefore, magnetic nanoparticles of Fe3O4 (Fe3O4-MNPs) as a kind of biocompatible nanomaterial which is feasible to characterize and easily functionalize, may offer an exciting development toward developing an effective drug delivery system while biocompatible superparamagnetic particles like magnetite could be utilized in tissue-specific release of therapeutic agents and magnetic field assisted radionuclide therapy.Citation6–Citation9
As a novel material, though we have already proved that Fe3O4-MNPs have no cytotoxicity, the exact function of Fe3O4-MNPs on immune function has not yet been adequately clarified. In the present paper, we investigated the effects of Fe3O4-MNPs on the immune system in imprinting control region (ICR) mice to elucidate the interactions between Fe3O4-MNPs and immune system and to provide theoretic evidence for the clinical applications.
Materials and methods
Experimental agents
Experimental agents were sourced from the following locations: RPMI1640 (Gibco Chemical Co., Carlsbad, CA, USA); Anti-CD3 (PE-Cy5), Anti-CD4, Anti-CD8 (Pharmingen, San Diego, CA); Calf serum (Gibco Chemical Co); Enzyme-linked immunosorbent assay kit (Gibco, CA, USA); Con A (Sigma Chemical Co., St Louis, MO, USA).
Preparation of Fe3O4-MNPs
Based on our previous studies,Citation10–Citation11 the synthesis of Fe3O4-MNPs was prepared by the electrochemical deposition under oxidizing conditions. Before being applied in the present experiment, the magnetite nanoparticles were well-distributed in RPMI-1640 medium freshly added with 10% heated-inactivated fetal bovine serum (FBS) using ultrasound treatment in order to obtain Fe3O4-MNPs colloidal suspension.
Animals and animal care
Female and male ICR mice, which were age-matched (eight weeks of age) and weight-matched (18–22 g), were purchased from Shanghai National Center for Laboratory Animals. Animals were kept with a 12-hour light/dark cycle and received water and food ad libitumina semi-barrier system. The experiments were performed in adherence to the guidelines for the Care and Use of Laboratory Animals of the National Institute of Health.
Experimental groups and preparation for blood samples
Mice were randomly assigned to one of four groups (n = 10 per group). The doses of 5.14 mg/kg (low dose group), 20.7 mg/kg (medium dose group), and 51.4 mg/kg (high dose group) Fe3O4-MNPs, were dissolved in normal saline and intravenously (iv) injected into mice once. Meanwhile, those injected with 0.2 mL normal saline alone formed the control group. After being monitored for 72 hours, the eyeballs of mice were extirpated for blood collecting at the end of this period and spleens were aseptically removed immediately, blotted, and weighed and then used for various analyses. Blood samples obtained from the mice were centrifuged (1500 rpm) for 5 minutes at 4°C to separate plasma and blood cells. The blood cells were used for analyzing surface markers of lymphocytes and the plasma was stored at −80°C for determination of cytokines.
Lymphocyte proliferation assay
Single-cell suspensions were prepared from the spleens in RPMI-1640 medium. Briefly, a cell suspension was produced by puncturing the spleen with a 20-gauge needle gently flushing the organ with ice-cooled (4°C) culture medium solution. The suspension was freed from debris by centrifugation at 1000 rpm for 20 minutes at 4°C. And the remaining splenocyte suspensions were washed twice and adjusted to 2 × 106 cells/mL with RPMI-1640. The splenocyte cell suspension was placed in a 96-well microtiter plate in 200 μL aliquots, and cultured in the presence or absence of T-cell mitogen (concanavalin A [ConA], Sigma, USA) (5 μg/mL). Meanwhile the wells receiving complete RPMI-1640 were regarded as control. Cells were cultured for 68 hours at 37°C in a 5% humidified CO2 atmosphere, following which 10 μL MTT (0.5 mg/mL) were added to each well at 37°C in the dark for at least 4 hours, the formazan crystals were dissolved in 150 μL dimethyl sulfoxide (Sigma Aldrich) and the reduction of MTT was quantified by absorbance at a wavelength of 570 nm using a microplate reader (Model-550; Bio-Rad Laboratories, Hercules, CA, USA). The results were expressed as a mean differential optical density (ODmitogen-ODcontrol).
The proportions of lymphocyte subset
Phenotypic analyses of blood lymphocytes were performed using FCM. Briefly, the cells were incubated with PE or FITC-conjugated monoclonal antibodies [Anti-CD3 (PE-Cy5), Anti-CD4 (FITC), or Anti-CD8 (PE)] for 10 minutes, washed three times, and then resuspended in FACS permeabilizing solution before determination. At least 10,000 cells were analyzed for each MoAb staining using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Results were expressed as mean fluorescence intensity for a given molecule per cell.
Assessment of cytokines
The levels of interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin- 10 (IL-10), and interferon-γ (INF-γ) in serum were measured in duplicate using enzyme-linked immunosorbent assay kit according to the manufacturer’s instruction. Briefly, 50 μL samples or standard control were added to 50 μL assay diluents for each well, incubated at room temperature for 2 hours; after thorough washing, 100 μL conjugate was added to each well for incubation of 2 hours. Then 100 μL substrate solution was added to each well and incubated for 30 minutes. Finally, a 100 μL stop solution was added to each well and the optical density was measured using ELISA reader (Bio-Rad Laboratories, Hercules, CA, USA) with dual wavelength of 450 nm.
Statistical analysis
Data were analyzed using the Statistical Package for Social Science (version 13.0; SPSS Inc., Chicago, IL, USA). The significant difference between groups was analyzed using one-way ANOVA; P values <0.05 were considered statistically significant.
Results
The characteristic of Fe3O4-MNPs
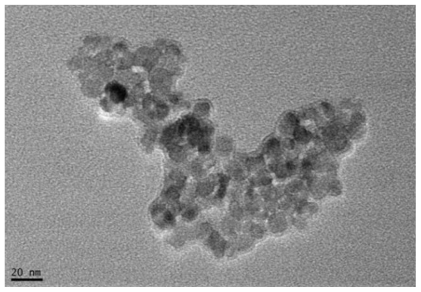
A colloidal suspension of Fe3O4-MNPs was achieved by using ultrasound treatment and the magnetization and the size of Fe3O4-MNPs were found to be 25.6 × 10−3 emu/mg and 20 nm, respectively ().
The changes of spleen weight in Fe3O4-MNPs-treated ICR mice
Mice treated with Fe3O4-MNPs appeared healthy and their body weight gain patterns were similar to controls (data not included). The spleens of Fe3O4-MNPs-treated mice showed same appearance as the controls. Both the spleen weight and the ratio of spleen weight to body weight showed no significant difference between the experimental groups and the controls (). It suggested that Fe3O4-MNPs did not cause splenomegaly, which was due to the deposition of damaged erythrocytes or to recruitment and/or proliferation of splenic cells.
Table 1 Effects of Fe3O4-MNPs on the spleen weight and the ratio of spleen weight to body weight (n = 10, mean ± SD)
Splenocyte proliferation
A significant increase of splenocyte proliferative capacity was noticed after administration of Fe3O4-MNPs in low dose (P < 0.05; low dose versus control). Both administration of medium-dose and high-dose Fe3O4-MNPs affect splenocyte proliferation and reduced the splenocyte proliferative capacity compared with the control group (P < 0.05; control versus medium-dose/high-dose Fe3O4-MNPs) ().Citation12
Table 2 Influence of Fe3O4-MNPs on the rate of lymphocyte transformation in spleen suspension (n = 10, mean ± SD)
The proportions of lymphocyte subsets in peripheral blood
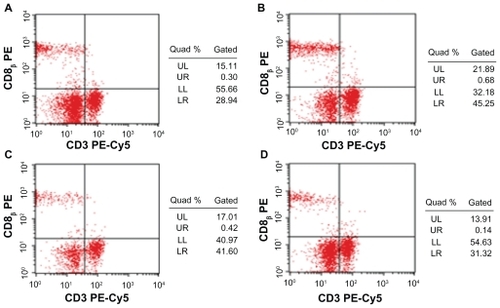
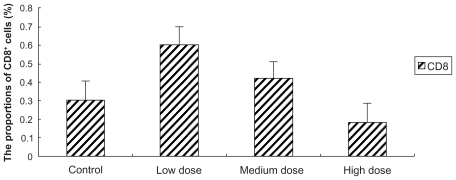
There were no differences between the low-dose group and the medium-dose group in the proportions of CD4+ T-cell subset in peripheral blood of ICR mice, and both had more CD4+ T lymphocytes than the control group. But the highdose group showed no difference compared with control (P > 0.05) ( and ). Furthermore the proportions of CD8+ T lymphocytes subset were slightly lower after the commencement of high dose. Though both low- and medium-dose groups indicated that they have more CD8+ T lymphocytes than those of control group, and the low-dose group showed significantly more when compared to the medium-dose group (P < 0.05) ( and ).
Figure 2 The effects of Fe3O4-MNPs on the proportion of CD4+ T lymphocyte subset in peripheral blood.
Notes: Control: 0.2 mL saline; Low dose: low dose of Fe3O4-MNPs (5.14 mg/kg); Medium dose: medium dose of Fe3O4-MNPs (20.7 mg/kg); High dose: high dose of Fe3O4- MNPs (51.4 mg/kg). CD4+, CD8+ represent CD4, CD8-positive T cells.
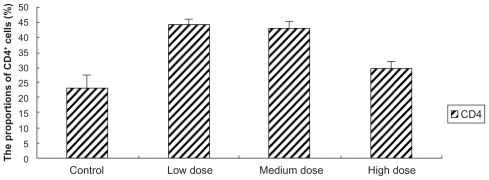
Figure 3 Effect of Fe3O4-MNPs on the proportion of CD4+ T lymphocyte subset in peripheral blood by FCM.
Notes: A) 0.2 mL saline; B) low dose of Fe3O4-MNPs (5.14 mg/kg); C) medium dose of Fe3O4-MNPs (20.7 mg/kg); D) High dose of Fe3O4-MNPs (51.4 mg/kg).
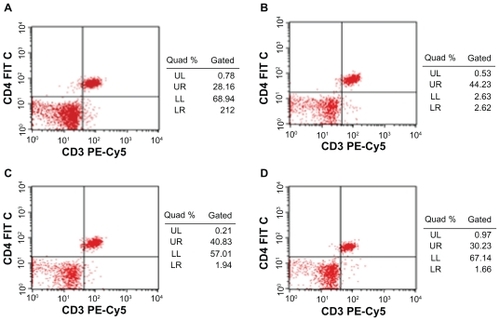
Figure 4 Effect of Fe3O4-MNPs on the proportion of CD8+ T lymphocyte subset in peripheral blood.
Notes: Control: 0.2 mL saline; Low dose: low dose of Fe3O4-MNPs (5.14 mg/kg); Medium dose: medium dose of Fe3O4-MNPs (20.7 mg/kg); High dose: high dose of Fe3O4- MNPs (51.4 mg/kg). CD4+, CD8+ represent CD4, CD8-positive T cells.
Cytokine release
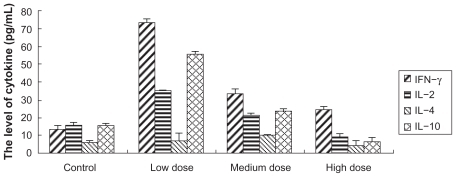
To detect whether Fe3O4-MNPs can alter cytokine production patterns in peripheral blood, enzyme-linked immunosorbent assay was conducted. Fe3O4-MNPs altered the production of IL-2, INF-γ, and IL-10. Interestingly, low dose of Fe3O4-MNPs significantly increased the levels of IFN-γ, IL-2, IL-10 and IL-4 (P < 0.05) when compared to medium or high doses of Fe3O4-MNPs (P < 0.05), and there were no significant differences between medium and high doses of Fe3O4-MNPs (P > 0.05), suggesting that low dose of Fe3O4-MNPs significantly increased the ability of splenocytes to release cytokines. But no significant changes of IL-4 were observed between the experiment groups and the control group ().
Discussion
Close attention has been paid to current nanoparticle techniques. It is well known that nanoparticles could present a versatile nanoscale surface for biomolecular recognition because of the numerous potential benefits in merging biomacromolecules and nanoparticles. Meanwhile, much effort has been explored to the development of new nanocomposites and their application in many research fields such as DNA detection, intracellular labeling, drug carrier, and so on.
The magnetic nanoparticle of Fe3O4 we tested has good biocompatibility and no cytotoxicity.Citation13,Citation14 Our group have proved that Fe3O4-MNPs could increase the intracellular effective concentration of chemotherapeutic drugs in vitro to reverse MDR. The focus of this study was to investigate whether Fe3O4-MNPs have effects on mice immune system, especially the T cell functions after given different dose.
The total body weights of mice were not changed significantly, and the spleen/body weight ratio was unchanged. Actually, quantification of body weight and organ weight forms an integral part of any toxicological study providing an initial assessment of overall animal health status, as well as potential pathology. Descriptions of the tier approach to immunotoxicity evaluation should incorporate measurements of body weight, weights of spleen, thymus, kidney, and liver, as well as organ/body weight ratios in an initial screen.Citation15
Proliferation of lymphocytes following exposure to mitogenic stimuli is an important methodology for the assessment of cell-mediated immunity. This assay has enjoyed frequent use in immunotoxicology studies for its ease of performance and relative reproducibility.Citation16 Therefore, we have investigated the effects of Fe3O4-MNPs on lymphoproliferatior in spleen following exposure to Con-A. The results showed that the proliferation of lymphocytes was significantly increased when mice were injected with 5.14 mg/kg Fe3O4-MNPs compared with the controls. However, the other two groups did not act in a similar way.
To detect the levels of proportion of CD4+ or CD8+ T cells, which are markers for T cell lymphocytes. Fe3O4-MNPs influenced the function of helper T cells or suppresser T cells, when the less the Fe3O4-MNPs were given, the more of an increase in T cell numbers was seen, suggesting that low concentration of Fe3O4-MNPs can regulate T cell functions in ICR mice.
The Th1 cytokines promote cellular immunity by activating macrophages, cytotoxic CD8+ T lymphocytes, and so on, while the Th2 cytokines enhance humoral immunity, including activation and class switching of antibody producing B cells.Citation17 Th1 cells are defined by their ability to secrete the inflammatory cytokines IL-2 and INF-γ and are involved in cellular immunity, some autoimmune disease, and in chronic inflammatory disorders. The Th2-biased cells preferentially produce IL-4, IL-5, IL-10, and IL-13 and participate in humoral response and antibody production. Citation18 In the present study, low dose of Fe3O4-MNPs was found to strongly affect the production of Th1 cytokine, and also affect some Th2 cytokine release, suggesting that Fe3O4-MNPs might be involved in inflammations associated with infections.
Conclusion
In conclusion, our results indicate Fe3O4-MNPs can influence immune functions of mice in a dose-dependent manner. Further study indicated that a high dose of Fe3O4-MNPs has no significant influence on the immune systems of the mice. These data could be useful for improving biomedical applications of Fe3O4-MNPs, but these immunological effects of Fe3O4-MNPs should be further conducted both in vivo and in vitro.
Acknowledgments
This work was supported by 973 National Key Fundamental Research Project of China (No. 2006CB933205), 863 Project of People’s Republic of China (No. 2007AA022007), National Nature Science Foundation of People’s Republic of China (No. 30740062, 30872970), and Special-Purpose Science Research Foundation for High School (No. 20070286042).
Disclosure
The authors confirm no conflicts of interest in this work.
References
- WeizmannYPatolskyFKatzEWillnerIAmplified DNA sensing and immunosensing by the rotation of functional magnetic particlesJ Am Chem Soc2003125123452345412643706
- SongHTChoiJSHuhYMSurface modulation of magnetic nanocrystals in the development of highly efficient magnetic resonance probes for intracellular labelingJ Am Chem Soc2005127289992999316011350
- GaoXHCuiYYLevensonRMChungLWNieSIn vivo cancer targeting and imaging with semiconductor quantum dotsNat Biotechnol200422895996015286644
- ChenYYangLFengCWenLPNano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cellsBiochem Biophys Res Commun20053371526016185655
- ZhangLZhangKPrändlRSchöfflFDetecting DNA-binding of proteins in vivo by UV-crosslinking and immunoprecipitationBiochem Biophys Res Commun2004322370571115336521
- ChoCSChoKYParkIKReceptor-mediated delivery of trans-retinoic acid to hepatocyte using poly (L-lactic acid) nanoparticles coated with galactose-carrying polystyreneJ Control Release20017771511689255
- JainTKMoralesMASahooSKLeslie-PeleckyDLLabhasetwarVIron oxide nanoparticles for sustained delivery of anticancer agentsMol Pharm20052319420515934780
- AlexiouCArnoldWKleinRJLocoregional cancer treatment with magnetic drug targetingCancer Res2000606641664811118047
- TiefenauerLXKuhneGAndresRYAntibody-magnetic nanoparticles: In vitro characterization of potential tumor-specific contrast agent for magnetic resonance imagingBioconjug Chem1993453473528274518
- ChenBASunQWangXMReversal in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/AO2 leukemic cellsInt J Nanomedicine2008327728618686787
- JiangZChenBAXiaGHThe reversal effect of Fe3O4-magnetic nanoparticles loaded with cisplatin on the SKOV3/DDP ovarian carcinoma cellsInt J Nanomedicine200941819421366
- MüllerKSkepperJNPosfaiMEffect of ultrasmall superparamagnetic iron oxide nanoparticles (Ferumoxtran-10) on human monocyte-macrophages in vitroBiomicrofluidics2007144410419693403
- ChengFYSuCHYangYSCharacterization of aqueous dispersions of Fe(3)O(4) nanoparticles and their biomedical applicationsBiomaterials200526772973815350777
- ZhangRWangXWuCSynergistic enhancement effect of magnetic nanoparticles on anticancer drug accumulation in cancer cellsNanotechnology200617143622362619661614
- LusterMIPortierCPaitDGRisk assessment in immunotoxicology Sensitivity and predictability of immune testsFundam Appl Toxicol19921822002101534777
- SnyderCValleCDLymphocyte proliferation assays as potential biomarkers for toxicant exposuresJ Toxicol Environ Health19913411271391890689
- CharltonBLaffertyKJThe Th1/Th2 balance in autoimmunityCurr Opin Immunol1995767937988679122
- Del PreteGFDe CarliMAlmerigognaFHuman IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine productionJ Immunol199315023533608419468