Abstract
This study concerns the supercritical antisolvent process which allows single-step production of 5-fluorouracil (5-FU) nanoparticles. This process enhances the physical characteristics of 5-FU in order to deliver it directly to the respiratory tract. Several mixtures of methanol with dichloromethane, acetone, or ethanol were used for particle preparation, and their effects on the physical characteristics of the final products were studied. The conditions of the experiment included pressures of 100 and 150 bar, temperature of 40°C, and a flow rate of 1 mL/min. The particles were characterized physicochemically before and after the process for their morphology and crystallinity. In spite of differences in size, the particles were not very different regarding their morphology. The resulting particles were of a regular shape, partly spherical, and appeared to have a smooth surface, whereas the mechanically milled particles showed less uniformity, had surface irregularities and a high particle size distribution, and seemed aggregated. Particles of 5-FU precipitated from methanol-dichloromethane 50:50 had a mean particle size of 248 nm. In order to evaluate the aerodynamic behavior of the nanoparticles, six 5-FU dry powder formulations containing mixtures of coarse and fine lactose of different percentages were prepared. Deposition of 5-FU was measured using a twin-stage liquid impinger and analyzed using a validated high pressure liquid chromatography method. Addition of fine lactose improved the aerodynamic performance of the drug, as determined by the fine particle fraction.
Introduction
Lung cancer is one of the most common cancers worldwide, and is associated with significant morbidity and mortality. In fact, lung cancer accounts for approximately 15% of all new cases of cancers, and is the most important cause of cancer-related deaths worldwide, with a total death rate exceeding that from colon, breast, and prostate cancers.Citation1 Regardless of the efforts in the conventional treatment of lung cancer by surgery, radiotherapy, and chemotherapy, the outcome of treatment for lung cancer has not changed dramatically, and the cure rate remains one of the lowest among all malignancies.Citation2–Citation4 In chemotherapy, the delivery of an insufficient drug concentration to the tumor site after intravenous or oral administration is the probable reason for limited efficacy of anticancer agents.Citation5
Targeting chemotherapeutic drugs to the lung via inhalation offers several potential advantages over the oral and parenteral routes, ie, higher local drug concentration, avoidance of first-pass metabolic degradation in the liver and/or intestines, decreased systemic side effects, and the noninvasive nature of administration.Citation5,Citation6 While preclinical studies have shown significant antitumor activity of aerosolized drugs, both in primary or metastatic lung cancer as therapeutic or preventive agents, inhalation remains minimally explored as a model of drug administration for lung cancer treatment.Citation7
5-Fluorouracil (5-FU) is one of the most extensively used chemotherapeutic drugs and radiosensitizers, but its use has been restricted by its systemic toxicities, ie, severe gastrointestinal toxicities, hematologic side effects, and severe bone marrow disturbances.Citation8,Citation9 Intravenous administration of 5-FU results in a large systemic distribution with only a small fraction of the dose reaching the site of action, while oral delivery is not a realistic option due to low and variable bioavailability. There would be an immediate benefit if the drug could be localized near the cancerous tissue within the lungs, as has been confirmed for other drugs, such as doxorubicin, melphalan, and floxuridine.Citation10 The direct delivery of 5-FU to the lung as an aerosol could maximize the local concentration, while minimizing the concentration of 5-FU in the rest of the body.Citation11–Citation14
For aerosol delivery of pharmaceuticals to the lung, a narrow size distribution of fine particles is necessary.Citation15 Several conventional methods are currently being used to produce fine powders, including milling, spray-drying, recrystallization using solvent evaporation, and liquid antisolvents. However, all these techniques have their own specific disadvantages. Production of contaminant-free micro- and nanoparticles with controlled particle size and desired product qualities in an environmentally responsible manner will continue to be a major challenge.Citation16
Supercritical technology is increasingly anticipated to become an alternative to many conventional processes. In the last few years, there has been a rapid increase in use of supercritical fluids (SCFs) for particle engineering. The use of SCFs has provided a more controlled and tunable method to produce a variety of drug delivery systems, whereby many of the problems associated with traditional techniques are avoided. The advantages conferred by SCFs come from both their property and process characteristics, a comparatively mild operating temperature, and the dryness and purity of the produced particles, making the supercritical approach particularly attractive for pharmaceutical applications.Citation17,Citation18
The principal aim of this study was to improve the physical characteristics of 5-FU via formation of SCF-processed nanostructures. In this process, supercritical carbon dioxide (SC-CO2) was used both for its chemical properties and as a “spray enhancer” with a mechanical effect, whereby a nozzle with two coaxial passages allowed introduction of SC-CO2 and a solution of 5-FU into the particle formation vessel where pressure and temperature were controlled.
To the best of our knowledge, the preparation of 5-FU as an anticancer drug in the form of nanoparticles by use of supercritical antisolvents and preparation of dry powder inhalation of the drug has not been reported. In this study, the effect of the solvents on the production of the polar antitumor drug, 5-FU, was studied in different conditions. Dry powder inhalers were then produced from nanoparticles, and the aerodynamic behavior of the nanoparticles was evaluated.
Materials and methods
Materials
The 5-FU (USP) was purchased from Alpha Aesar (Germany). The CO2 (99.99%) was obtained from Sabalan Co. (Iran). Alpha-lactose monohydrate (Pharmatose® 80) was supplied by DMV International (The Netherlands). Solvents including methanol, ethanol, acetone, and dichloromethane, all of high-pressure liquid chromatography grade, were obtained from Merck Chemical Co. (Germany). All aqueous solutions were prepared using distilled water.
Methods
Preparation of 5-fluorouracil nanoparticles using supercritical antisolvents
The technical details of the apparatus used in this study have already been described elsewhere.Citation19,Citation20 The experiments began by delivering CO2 after passing it through a cooling device to the precipitator (a vessel with an 0.4 L internal volume) by a syringe pump until the desired pressure was achieved. The precipitator was placed in an equipped oven for exact temperature regulation. The gas entered the precipitation vessel through a cocentric nozzle placed in the upper side of the precipitator until the pressure reached 100 bar.
Firstly, 5-FU was micronized using a ball mill apparatus (F. Kurt Retch MM2000, Germany). Different mixtures of organic solvents were selected for preparation of the 5-FU solutions (). Organic solution of drug at a concentration of 5 mg/mL was delivered through the nozzle at flows of 1 mL/min and CO2 flow of 20 mL/min. During this process, particles were precipitated on a filter located at the bottom and on the walls of the chamber. This stage took about one hour or so to allow the collection of sufficient solid particles. These experiments were completed simultaneously with delivery of the drug solution to the chamber. However, SC-CO2 was allowed to continue to flow for 20 minutes for washing the residual solvents from the chamber. When the washing process was completed, the CO2 flow was stopped and the chamber was depressurized down to atmospheric pressure.
Table 1 Summary of operational parameters and particle size of processed 5-FU by supercritical antisolvent
Physical characterization of nanoparticles
The hydrodynamic diameters of the nanoparticles were determined at 25°C by quasi-elastic light scattering using a Nanosizer® N4 PLUS (Malvern, England). Samples were suspended in chloroform. The results were expressed as the mean hydrodynamic diameter. Results corresponded to the average of three determinations and polydispersity index.
The morphology of unprocessed and processed particles was qualitatively assessed using a CamScan MV 2300 (Cambridge, England) scanning electron microscope. Particles of representative samples were coated with gold-palladium at room temperature before examination. The accelerator voltage for scanning was 25.0–30.0 kV.
Differential scanning calorimetry was accomplished using a DSC-60 (Shimadzu, Japan). Approximately 10 mg of the material was placed in an aluminum pan and analyzed under dry nitrogen purge. The temperature ranged between 30°C and 320°C.
Structural analysis of the samples was performed using an X-ray diffractometer, Xpert-Pro (Philips, The Netherlands), with a Cu Kα source operating at a tube load of each sample assessed between 5° and 80° (2θ) with a step size of 0.02°.
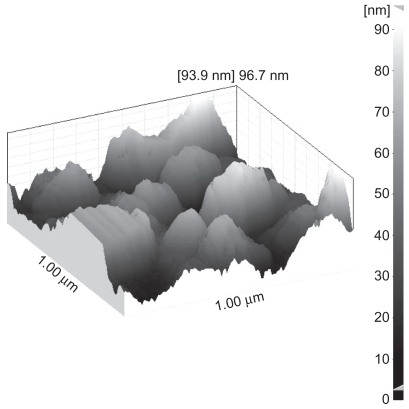
Atomic force microscopy topographic measurements were performed using a DME DualScope/Rasterscope® scanning probe microscope (DME Co., Denmark). The atomic force microscopy studies were performed in AC mode, with a spring constant of 2.8 N/m and a resonance frequency of 100 KHz, a speed of 30–40 μm/s, and a force constant of 42 N. The processing of topographic or phase images was carried out using DME scanning probe microscopy software version (DME Co., Denmark). The particles were dispersed over an adhesive tape onto a glass slide. Groups of particles were located and scanned for their topographic images. The scans were done at room temperature.
Preparation of 5-fluorouracil dry powder formulations
It has been recognized in several earlier studies that the efficiency of a dry powder formulation is highly dependent on the size distribution of the lactose carrier usedCitation21,Citation22 and the content of fine lactose.Citation23 Two binary and four ternary 5-FU dry powder inhaler formulations with different fine lactose ratios were prepared (). The 5-FU:carrier ratio was 1:67.5 (w/w) in all formulations. Alpha-lactose monohydrate was sieved manually to produce 63–90 μm fraction carriers. Fine lactose was prepared by micronization of alpha-lactose monohydrate using an air-jet micronizer (Esco Labor, Switzerland) operating at injecting air pressures of 7 bar, and was collected after five passages through the instrument. Fine lactose was preblended with coarse lactose for 15 minutes before addition of 5-FU to optimize the efficiency of the mixing process. The formulations were prepared in a Turbula mixer (Dorsa, Iran) at 90 rpm for 30 or 90 minutes. Each formulation was manually loaded into size 3 gelatin capsules. Fill weight was 15 ± 1 mg, giving a nominal dose of 220 μg 5-FU per capsule. Following filling, capsules were stored in a sealed desiccator over silica gel prior to the experiments. The uniformity of the blends was examined by analyzing the quantity of 5-FU in 10 capsules, and drug concentration assessed using high-pressure liquid chromatography, based on the USP 29 monograph for 5-FU. The quantity of drug in each capsule was calculated and the content uniformity expressed as the relative standard deviation.
Table 2 Formulations of 5-fluorouracil dry powder inhalers
In vitro deposition profiles of nanoparticles by twin-stage liquid impinger
According to the British Pharmacopeia, the twin-stage liquid impinger is a simple, widely used device for evaluation of the lung disposition of inhaled particles.Citation24 Four capsules were individually installed in a Cyclohaler® device to analyze each formulation. The Cyclohaler was attached to the impinger which contained 7 and 30 mL of distilled water in stages 1 and 2, respectively. The capsules were placed inside the inhalator and their contents were released by twisting the Cyclohaler, and the system was vacuumed to produce air streams of 60 L/min for five seconds. After inspiration, the twin-stage liquid impinger apparatus was disassembled. Thereafter, the thorax, emptied capsules, and each stage were washed with appropriate volumes of distilled water then collected into separate volumetric flasks. The total amount (μg) of 5-FU recovered from the inhaler, the capsule shells, and the upper and lower stages of the twin-stage liquid impinger was calculated per four capsules and defined as the recovered dose. The fine particle dose was defined as the amount of drug recovered per four capsules from the lower stage of the twin-stage liquid impinger. The fine particle fraction was calculated as the ratio of the fine particle dose to recovered dose and expressed as a percentage. The emitted dose was defined as the total quantity of drug recovered from upper and lower stages of the twin- stage liquid impinger per four capsules. The 5-FU concentration was assayed by high-pressure liquid chromatography according to the USP 29 monograph.
Results and discussion
Preparation of 5-FU nanoparticles using supercritical antisolvents
5-FU was recrystallized using supercritical antisolvents and the effect of the solvents on improvement of the physical characteristics of the particles was studied. 5-FU is a polar compound with low solubility in SC-CO2. Among the organic solvents, methanol is the main solvent for 5-FU. Therefore, methanol was used in combination with dichloromethane, acetone, or ethanol to solve 5-FU. The drug was dissolved in the mixture of solvents and then the solution was continuously sprayed through a nozzle into the SC-CO2.
The dispersion of solution in the SCF leads to an expansion of the droplets and, at the same time, an extraction of the liquid into the fluid occurs. The solvent power of the organic solvent decreases dramatically and supersaturation leads to the precipitation of particles.Citation25 If a solvent has high solubility in CO2, the dissolution of the SCF into the organic solvent will be accompanied by a large volume expansion and, as a result, a reduction of the liquid density. This will lead to the reduction of its solvent power, and therefore, a sharp rise in the supersaturation within the liquid mixture. Due to the high and uniform degree of supersaturation, small particles with a narrow particle size distribution are expected.Citation26
In this study, acetone and dichloromethane were the solvents with the highest solubility in SC-CO2 compared with methanol and ethanol.Citation27 This difference in the solvent properties could be the reason for the marked changes in the physical characteristics of the resulting particles.
The level of supersaturation in the mixture of methanol-ethanol was less than in methanol-acetone, and methanol-dichloromethane. As a result, particles with bigger sizes were observed. In the case of particles precipitated from the mixture of methanol-acetone and methanol-dichloromethane, it was noticeable that those from methanol-acetone had not only a bigger size in comparison with the particles produced by methanol-dichloromethane, but also showed more aggregation due to interparticulate bridges (). Also, the higher proportion of dichloromethane in the combinations of methanol and dichloromethane showed marked effects on the particle size of 5-FU nanoparticles. This could be related to the physical properties of dichloromethane as a solvent with high solubility in CO2.
Figure 1 SEM photographs of 5-FU Particles before and after processing by supercritical anti-solvent. Ball milled A, M2 B, MD1 C, MD2 D, MA1 E, and ME1 F.
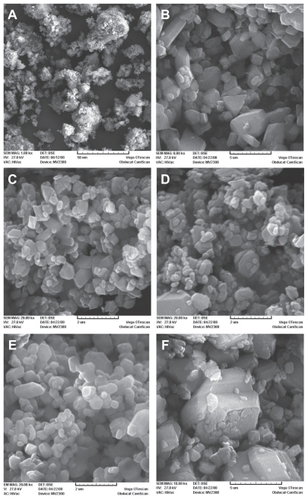
represents the process conditions based on solvent types and proportions. The scanning electron microscopic photographs of the processed 5-FU particles exhibited similar morphology, regardless of the process conditions used for precipitation (). The 5-FU crystals formed were of regular shape, partly spherical, and appeared to have a smooth particle surface, whereas the scanning electron microscopic photograph of the milled 5-FU showed a less uniform particle shape with surface irregularities. Furthermore, it can be noted that the precipitates obtained at different conditions were of different sizes; the particles of 5-FU precipitated from methanol- dichloromethane 50:50 (MD1) and methanol- acetone 50:50 (MA1) exhibited a mean particle size of 248 nm and 732 nm, respectively, whereas the particles from methanol-ethanol 50:50 (ME1) showed two peaks with mean particle sizes of 1987 nm and 5560 nm, respectively.
The relatively smooth surface and nonporous texture of nanostructured 5-FU particles in the scanning electron microscopic photomicrographs correlates appropriately with the corresponding high-resolution atomic force microscopic image of a small sectional area of a nanosized particle surface. shows a representative surface topographic atomic force microscopic image of a supercritically processed particle of MD1.
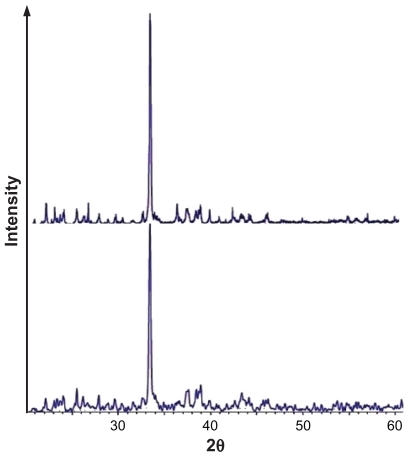
Supercritical processing of pharmaceuticals generally results in the formation of crystalline structures.Citation28 represents the x-ray diffractometry patterns of the 5-FU before and after the process. It illustrates the crystallinity of unprocessed and processed drug, which are qualitatively assessed on the basis of the sharpness of the major diffraction peaks. It also indicates that the same pattern of 5-FU has been produced by the SCF process.
Figure 3 XRD pattern of 5-FU particles before (top) and after (bottom) processing by supercritical anti-solvent (MD1).
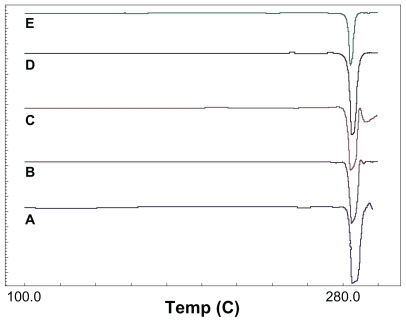
Differential scanning calorimetry thermographs of processed and unprocessed 5-FU particles have confirmed the x-ray diffractometry findings. The melting point of unprocessed 5-FU was 285.3°C, whereas after processing it was 284.3°C–285.0°C. There were no significant changes in melting point, suggesting that the solid states of particle crystals did not change after the supercritical antisolvent process. shows the heat flow with temperature plot of 5-FU particles.
The size and surface properties of solid-state particles have been shown to play a critical role in the behavior of active ingredients in a variety of pharmaceutical solid dosage forms. Considering the results from analyzing the particle size by scanning electron microscopy, atomic force microscopy, x-ray diffractometry, and differential scanning calorimetry, it appears that this supercritical processing method was influential in the preparation of 5-FU nanoparticles. With the results obtained, it is possible to conclude that, for this system, the improved operating conditions were for the MD1 formulation, which resulted in the formation of regular shapes of the drug crystal nanoparticles with smooth surfaces.
Preparation of 5-fluorouracil dry powder formulations
5-FU inhalation is a potential route for the treatment and prevention of lung cancer.Citation29,Citation30 In a study published by Tatsumura et al aerosol administration (via nebulization) of 5-FU to a small group of patients with lung cancer showed an antitumor response in 60% of the participants and no adverse effects were detected.Citation30 Wattenberg and his colleagues also initiated a primary study using 5-FU in the Syrian golden hamster. The progression of squamous cell carcinoma in the trachea, larynx, and oropharynx was reduced by inhalation of nebulized 5-FU.Citation29
Given the treatment potential of lung cancer by aerosol therapy, 5-FU dry powder inhaler formulations have been prepared (). Six dry powder inhaler formulations with different fine lactose ratios were prepared using the MD1 product. Only coarse lactose was used as the carrier in formulations F1 and F2. In formulations F3 and F4, 5% fine lactose was the third component used and, in formulations F5 and F6, 10% fine lactose was used. Two mixing times (30 and 90 minutes) were selected for preparation of the formulations.
After blending, the content uniformity of the different formulations was assessed. The average recovery of 5-FU of all formulations in this test was approximately 85%–105%, suggesting that the overall mixing, sampling, and analysis were satisfactorily accurate. The coefficient of variation (CV) in 5-FU content within each formulation was a function of the components present and the mixing time. Only formulations F4 and F6 showed a mean content of 5-FU between 95.5% and 104.5% and a satisfactory mixing uniformity of the drug with a CV less than 5%. The mixture prepared by mixing coarse lactose with the drug was shown to be the least uniform in 5-FU content, with a CV of over 20% for F1and 18.7% for F2.
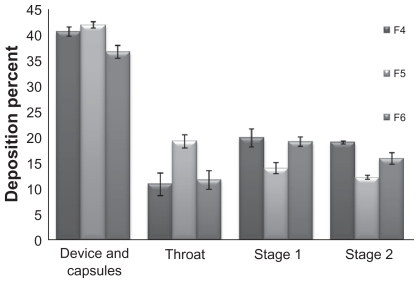
The in vitro deposition profile of the formulations was estimated with the twin-stage liquid impinger. The recovered dose was 85%–90% for all runs. The emitted doses obtained were between 17% (F2) and 43% (F4). The fine particle fractions obtained were between 7% for F2 and 21% for F4. However, formulations F4 and F6, with a fine particle fraction of 21% and 19%, respectively, showed better aerosolization behavior compared with formulation F2. In this study the superior formulation was F4 with 95% coarse lactose, 5% fine lactose, and a 90-minute mixing time, which showed a uniform mixing of the particles and carrier (), and a fine particle fraction of 21%. presents the deposition percentage of drug in different stages of the twin-stage liquid impinger.
Figure 5 SEM micrograph of DPI formulation of 5-FU. A) magnification 1.00, B) magnification 6.00, C) magnification 20.00.

Depending on the particle size, shape, and ventilation parameters, deposition occurs in all regions of the lung, ie, the airways and the alveoli.Citation31 There are three principal mechanisms that lead to pulmonary deposition, ie, inertial impaction for 5–9 μm particles in large airways, gravitational sedimentation for 1–5 μm particles in smaller airways and for 1–3 μm particles in respiratory bronchioles, and Brownian diffusion in alveoli for ≤0.5μm particles.Citation32–Citation34 With decreasing particle diameter below about 500 nm, the deposition increases in all regions of the lung because of the increasing diffusional mobility.Citation32,Citation35
Conclusion
In the present study, the effects of using several solvents in the supercritical processing of 5-FU were evaluated. The results showed that marked changes in the physicochemical properties of the produced particles were possible by this procedure. The sequence of the process was as follows. Firstly, the solvents succeeded each other from low solubility to high solubility in SC-CO2, which led to reduction of the particle size, in addition to a change in the morphologic properties of the particles. When a solvent has higher solubility in SC-CO2, its solvent power reduces faster in the presence of SC-CO2. Therefore, supersaturation happens sooner and smaller particles are formed. The particles, subsequently were produced, followed by recrystallization of 5-FU from methanol-dichloromethane treated nanoparticles characterized by relatively smooth surface and narrow particle size distribution. In vitro evaluation of the aerodynamic behavior of the nanoparticles showed that the respiratory fraction could increase up to 21% using the mixture of coarse lactose and fine lactose as a carrier. Our results could be used as the basis for developing 5-FU as an anticancer agent in the form of dry powder inhalation through the respiratory route. More experimental studies will be carried out to optimize dry powder inhaler formulations to obtain more efficient 5-FU delivery system to the lung.
Acknowledgment
The authors gratefully acknowledge the financial support of the Tehran University of Medical Sciences.
Disclosure
The authors report no conflict of interest in this work.
References
- American Cancer SocietyCancer Facts And Figures, 2008OaklandAmerican Society2008216
- MailletACongy-JolivetNLe GuellecSAerodynamical, immunological and pharmacological properties of the anticancer antibody cetuximab following nebulizationPharm Res2008251318132618030605
- MyrdalGLambergKLambeMRegional differences in treatment and outcome in non-small cell lung cancer: A population-based studyLung Cancer200963162218571760
- ProvencioMCampsCAlberolaVLung cancer and treatment in elderly patients: The Achilles StudyLung Cancer20096610310619193470
- GagnadouxFPapeALLemarieEAerosol delivery of chemotherapy in an orthotopic model of lung cancerEur Respir J20052665766116204597
- HersheyAEKurzmanIDForrestLJInhalation chemotherapy for macroscopic primary or metastatic lung tumors: Proof of principle using dogs with spontaneously occurring tumors as a modelClin Cancer Res199952653265910499645
- LipworthBJPharmacokinetics of inhaled drugsBr J Pharmacol199642697706
- HeYCChenJWCaoJToxicities and therapeutic effect of 5-fluorouracil controlled release implant on tumor-bearing ratsWorld J Gastroenterol200391795179812918123
- YanoTYamazakiKMaruyamaRFeasibility study of postoperative adjuvant chemotherapy with S-1 (tegaful, gimeracil, oteracil potassium) for non-small cell lung cancer – LOGIK 0601 studyLung Cancer20106718418719409643
- SharmaSWhiteDImondiARDevelopment of inhalational agents for oncologic useJ Clin Oncol2001191839184711251016
- HitzmanCJElmquistWFWattenbergLWDevelopment of a respirable, sustained release nicrocarrier for 5-fluorouracil I: In-vitro assessment of liposomes, microspheres, and lipid coated nanoparticlesJ Pharm Sci2006951114112616570302
- HitzmanCJWattenbergLWWiedmannTSPharmacokinetics of 5-fluorouracil in the hamster following inhalation delivery of lipid-coated nanoparticlesJ Pharm Sci2006951196121116639722
- HitzmanCJElmquistWFWiedmannTSDevelopment of a respirable, sustained release microcarrier for 5-fluorouracil II: In-vitro and in vivo optimization of lipid coated nanoparticlesJ Pharm Sci2006951127114316570303
- BaileyMMBerklandCJNanoparticle formulations in pulmonary drug deliveryMed Res Rev20092919621218958847
- DhumalRSBiradarSVParadkarARParticle engineering using sonocrystallization: Salbutamol sulphate for pulmonary deliveryInt J Pharm200938612913718996462
- ShariatiAPetersCJRecent developments in particle design using supercritical fluidsCurr Opin Solid State Mater Sci20037371383
- GintyJWhitakerMJShakesheffKMDrug delivery goes supercriticalMaterials Today200584248
- KimMJinSKimJPreparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical anti-solvent (SAS) processEur J Pharm Pharmacol200869454465
- NajafabadiARVatanaraAGilaniKFormation of salbutamol sulphate microparticles using solution enhanced dispersion by supercritical carbon dioxideDARU20051316
- VatanaraANajafabadiARGilaniKADesign for screening of the operational variables in the formation of salbutamol sulphate particles by supercritical anti-solventJ Supercritical Fluids200740111116
- ZengXMTeeSKMartinGPEffect of mixing procedure and particle size distribution of the carrier particle on the deposition of salbutamol sulphate from dry powder inhaler formulationsProceedings of Drug Delivery to the Lungs VIILondonThe Aerosol Society1996
- GiryKPeanJMGiraudLDrug/lactose co-micronization by jet milling to improve aerosolization properties of a powder for inhalationInt J Pharm200632116216616797150
- LucasPClarkeMJAndersonKTobynMJStaniforthJNDalbyRNByronPRFarrSTThe role of fine particle excipients in pharmaceutical dry powder aerosolsRespiratory Drug DeliveryBuffalo Grove, ILInterpharm Press Inc1998
- YoungSTrainiDJonesMDThe influence of dose on the performance of dry powder inhalation systemsInt J Pharm2005296263315885452
- GallagherPMCoffeyMPKrukonisVJJohnstonKPenningerJGas antisolvent recrystallization: New process to recrystallize compounds insoluble in supercritical fluidsACS Symposium Series 406. Supercritical Fluid Science and TechnologyWashington, DCAmerican Chemical Society1989
- DukhinSSShenYDaveRDroplet mass transfer, intradroplet nucleation and submicron particle production in two-phase flow of solvent-supercritical antisolvent emulsionColl Surf A2005261163176
- StievanoMElvassoreNHigh-pressure density and vapor-liquid equilibrium for the binary systems carbon dioxide-ethanol, carbon dioxide-acetone and carbon dioxide-dichloromethaneJ Supercritical Fluids200533714
- GeiserMSchurchSGehrPInfluence of surface chemistry and topography of particles on their immersion into the lung’s surface-lining layerJ Appl Physiol2003941793180112547838
- WattenbergLWWiedmannTSEstensenRDChemoprevention of cancer of the upper respiratory tract of the Syrian golden hamster by aerosol administration of difluoromethylornithine and 5-fluorouracilCancer Res2004642347234915059884
- TatsumuraTKTsujimotoMKitagawaMFurther study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: Fundamental and clinicalBr J Cancer199368114611498260366
- HwangSMKimDDChungSJDelivery of ofloxacin to the lung and alveolar macrophages via hyaluronan microspheres for the treatment of tuberculosisJ Control Release200812910010618538437
- ByronPRPrediction of drug residence time in regions of the human respiratory tract following aerosol inhalationJ Pharm Sci1986754334383735078
- CourrierHMButzNVandammeTFPulmonary drug delivery systems: Recent developments and prospectsCrit Rev Ther Drug Carrier Syst20021942549812661699
- CorriganDOCorriganOIHealyAMPhysicochemical and in-vitro deposition properties of salbutamol sulphate/ipratropium bromide and salbutamol sulphate/excipient spray dried mixtures for use in dry powder inhalersInt J Pharm2006322223016815654
- YangaWPetersJIWilliamsROInhaled nanoparticles – A current reviewInt J Pharm200835623924718358652