?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Fatal Candida albicans infections in the mucosal system can occur in association with immune-compromised diseases and dysbacteriosis. Currently, amphotericin B (AmB) is considered to be the most effective antibiotic in the treatment of C. albicans infections, but its clinical application is limited by side effects and poor bioavailability. In order to use AmB in the local treatment of oral C. albicans infections, AmB/MPEG-PCL-g-PEI (monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone)-graft-polyethylenimine, MPP) micelles were prepared. A series of characterizations were performed. The micelles allowed a sustained in vitro release in both normal oral conditions (pH 6.8) and C. albicans infection conditions (pH 5.8). Then, buccal tablets containing freeze-dried powder of AmB/MPP micelles were produced by direct compression process and evaluated as regards to weight variation, hardness, and friability. In vitro drug release of the buccal tablets was measured in both the United States Pharmacopeia dissolution apparatus and the dissolution rate test apparatus, which was previously designed for simulating in vivo conditions of the oral cavity. The buccal tablets could sustainably release within 8 h and meet the antifungal requirements. Regarding safety assessment of AmB/MPP micelles, in vivo histopathological data showed no irritation toward buccal mucosa of the rats in both optical microscopy and ultrastructure observation of the tissues. MTT experiment proved that AmB/MPP micelles reduced the cytotoxicity of AmB. The micelles delivered through the gastrointestinal route were also found to be non-systemic toxicity by liquid chromatography-mass spectrometry analysis. Furthermore, the antifungal action of AmB/MPP micelles was evaluated. Although AmB/MPP had no obvious improvement as compared to AmB alone in the antifungal effect on planktonic C. albicans, the micelles significantly enhanced the antifungal activity against the biofilm state of C. albicans. Thus, it was concluded that AmB/MPP micelles represent a promising novel drug delivery system for the local treatment of oral C. albicans infections.
Introduction
Candida albicans is one of the major human fungal pathogens, which usually causes oral mucosal infections.Citation1 Such infections are difficult to eradicate and may emerge as lethal depending on the immune status of patients. Particularly, patients with immune-compromised diseases such as AIDS and cancer are life-threatened by the infection of C. albicans.Citation2,Citation3 In these patients, C. albicans invade their oral cavities and make them even more painful. In general, Candida infections begin with the adherence and colonization of yeast cells on a biotic host surface to form microbial communities, the so-called “biofilms”. Biofilms are heterogeneous communities of microorganisms entrapped in an extracellular matrix that limits the penetration of antimicrobial drugs and antibodies.Citation4 The formation of C. albicans biofilms is a crucial virulence factor that allows C. albicans 30–2,000 times more resistant to several antifungal agents compared with their planktonic (or free-living) counterparts.Citation5 Therefore, the development of novel approaches to inactivate C. albicans biofilms has a great clinical significance in treating C. albicans infections.
Amphotericin B (AmB) is considered to be the most effective antibiotic in the treatment of chronic fungal infections, even effective against azole-resistant fungus. However, the clinical use of this antifungal drug is limited by side effects and poor bioavailability deriving from its inherent low solubility in water (<0.001 mg/mL).Citation6 Reported adverse effects of AmB also mainly result from its low solubility, and include renal failure, hypokalemia, hypomagnesemia, and polyuria.Citation7 Thus, much effort has been expended to increase the water solubility of AmB.Citation6,Citation8–Citation10 Fungizone® (Bristol-Myers Squibb, Rueil-Malmaison, France) has been launched first as a colloidal dispersion combined with sodium deoxycholate (DOC) to develop a micellar dispersion for encapsulating AmB, which improves the solubility of AmB. However, the infusion toxicity and nephrotoxicity, which may ascribe to the dimeric form obtained with DOC, using a technological process with pH changes, still frequently cause troublesome complications.Citation11–Citation13 Commercial products, including a lipid complex (Abelcet®, Sigma-Tau, Gaithersburg, MD, USA) and a liposomal product (Ambisome®, Astellas, Tokyo, Japan) that encapsulate AmB with hydrogenated soybean lecithin, phosphatidylcholine, and cholesterol (polyaggregate forms), have already come to the market. The toxicity incidence of these formulations becomes lower, and they are accepted well when administered in higher doses.Citation14,Citation15 Nevertheless, their widespread use has been limited on account of high costs and high-dose requirements. Thus, extensive research has been done to develop other cost-efficient delivery systems (such as micelles, nanospheres, and conjugates) with lower toxicity.
Micelles show great superiority in aspects of solubilizing, permeability, and lower toxicity. Amphiphilic block copolymers have been in use as pharmaceutical excipients in drug delivery for a long time, and their application is developing rapidly in pharmaceutics and nanomedicine.Citation16 These amphiphilic polymeric molecules can spontaneously form core–shell structures as micelles in an aqueous medium. The hydrophobic cores serve as reservoirs for poorly water-soluble drugs. Encapsulation of hydrophobic drugs into micelles contributed to substantially increasing a drug’s solubility/dispersibility in aqueous media.Citation17 Previously, we have synthesized monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone) (MPEG-PCL) amphiphilic block copolymer and verified that the solubility of some poorly water-soluble drugs could be enhanced by encapsulating into MPEG-PCL copolymer.Citation18 Wiegand et alCitation19–Citation21 have suggested that cationic homopolymers polyethylenimine (PEI) possessed a significant inhibition to C. albicans and even influenced the growth of C. albicans biofilms.Citation22 However, an increasing degree of branching and molecular weight of PEI is confirmed to raise its cytotoxicity both in vitro and in vivo. In order to facilitate the antifungal efficiency and decrease toxicity, low-molecular-weight (800 or 1,800 Da) PEIs can be connected with other groups through short biodegradable linkages.Citation23 Therefore, MPEG-PCL could be linked with PEI forming biodegradable triblock copolymer as a new drug delivery system. In this research, PEI with molecular weight 1,800 Da is utilized, and the connection with MPEG-PCL lowers the toxicity of PEI as well.
Buccal tablet is a mucoadhesive system that represents a good recent alternative. The mucoadhesive buccal drug delivery system offers several advantages as compared with traditional methods of systemic drug administration. The tablets are designed to release the drug either unidirec-tionally by targeting buccal mucosa or multidirectionally into the saliva.Citation24,Citation25 In addition to this, the drug can be easily applied and localized to the application site and can be removed directly by patients from there if necessary.Citation26,Citation27 We prepared buccal tablet as a controlled and effective dosage form to let the drug release to oral saliva. The bioadhesive polymers were employed to increase the residence time of the drug in the oral cavity and the localization of the drug in a particular region.
Hence, in this work, we aimed to prepare AmB/monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone)-graft-polyethylenimine (MPP) micelles to improve the solubility, safety, and antifungal effect of AmB in the oral buccal application. The characteristics of micelles and buccal tablets were investigated. Safety and gastrointestinal toxicity were also explored. Furthermore, we examined the antifungal activity of AmB/MPP micelles against planktonic and biofilm state of C. albicans.
Materials and methods
Materials
AmB (98% purity) was purchased from Shanghai Ruichu Biological Technology Co., Ltd., Shanghai, China. Milk protein concentrate (MPC85) was from Jinan Heluo Trade Company Ltd., Jinan, China. Hydroxypropyl methylcellulose K4M, corn starch, anhydrous lactose, and magnesium stearate were all obtained from Xi’an Yue Lai Medical Technology Co., Ltd., Shaanxi, China. Methanol was obtained from Tianjin Tianli Chemical Reagent Co., Ltd., Tianjin, China. MPEG-PCL diblock copolymer with a designed molecular weight of 2k-2k was obtained from our laboratory. All other chemicals used were of analytical or high-performance liquid chromatography (HPLC) grade. C. albicans strains (ATCC 76485) were kindly provided by the Department of Microbiology and Immunology, Xi’an Jiaotong University.
Synthesis of MPEG-PCL-g-PEI copolymer
The diblock copolymer MPEG-PCL was synthesized previously by our laboratory.Citation18 After dissolving the copolymer, a few drops of triethylamine and an excess amount of acryloyl chloride were added dropwise to the MPEG-PCL anhydrous dichloromethane solution. The mixture was stirred for 8 h at 45°C under the protection of nitrogen, then the copolymer MPEG-PCL containing C=C was collected. Subsequently, the earlier copolymer was dissolved in chloroform and a certain amount of PEI chloroform solution was added dropwise. The mixture was stirred for another 48 h at 45°C under nitrogen atmosphere. The products were obtained by cooled petroleum ether co-precipitation and purified through dialysis. The chemical composition was confirmed by 1H-NMR spectra.
Preparation of AmB/MPP micelles
AmB/MPP micelles were prepared by the thin-film dispersion method.Citation28–Citation30 First, 10 mg of AmB and 100 mg of MPP triblock copolymer were co-dissolved in 200 mL methanol with the help of ultrasound. The methanol was recovered by evaporating at 55°C under reduced pressure (RE-52A; Shanghai Ya Rong Biochemistry Instrument Factory, Shanghai, China) to form a uniform thin film. To obtain the solution of drug-loaded micelles, AmB/MPP film was rehydrated in 100 mL of deionized water at 50°C with gentle agitation. Afterward, the solution was filtered using a 0.22 μm filter, and the filtrate was lyophilized as the sample for further characterization research.
Characterizations of micelles
Size and zeta potential
Particle size, size distribution, and zeta potential values were measured by Malvern Zetasizer Nano-ZS (Malvern Instruments Ltd., Malvern, UK) at 25°C. Measurements were performed in triplicate for every prepared sample.
Transmission electron microscopy (TEM)
The morphological feature of AmB/MPP micelles was observed by transmission electron microscope (H-600; Hitachi, Tokyo, Japan). Prior to examination, one drop of micelles suspension was loaded on a copper grid and left to adhere for 2 min. The excess suspension was sucked with a filter paper and air-dried before observation.
Drug loading (DL%) and entrapment efficiency (EE%)
For quantitative assay, AmB/MPP micelles were broken up by dimethylformamide (DMF) with the help of sonication and analyzed by HPLC.Citation31–Citation33 DL% and EE% were calculated as follows:Citation34,Citation35
Differential scanning calorimetry (DSC)
The formation of the inclusion complex between AmB and MPP was confirmed by DSC (DSC822e; Mettler Toledo, Greifensee, Switzerland). Each sample weighing 3 mg was heated in hermetically sealed aluminum pans at a rate of 10°C/min up to 250°C.
In vitro release
The release profile of AmB was analyzed in phosphate-buffered saline (PBS; pH 5.8 and 6.8, each containing Tween-80, 0.5:100, w/v). A total of 1 mL of each sample was placed in a dialysis bag (molecular weight cut off 3.5 KDa). The dialysis bag was inserted in 20 mL of PBS with gentle shaking (100 rpm) in a water bath at 37°C. At specific time intervals, 0.5 mL of each sample was withdrawn from the external aqueous solution, and an equal amount of the dissolution medium was added in order to maintain sink conditions. Subsequently, the concentration of AmB released at various times up to 8 h was determined by HPLC. This analysis was performed on a Dikma C18 column (250×4.6 mm, 5 μm) with the injection volume of 20 μL, with acetonitrile:10 mmol sodium acetate aqueous solution (40:60) as mobile phase at a flow rate of 1 mL/min. The detector was at the wavelength of 408 nm, and the column temperature was set at 30°C. All release experiments were repeated three times.
Preparation of buccal tablets with AmB/MPP micelles
Prior to production, micelle solution was lyophilized to obtain freeze-dried powder, which can be compressed with other excipients. The freeze-dried powder and excipients without lubricant were blended in the order of ascending weights with gentle shaking. Then magnesium stearate was added and mixed again. Buccal tablets were prepared by direct compression process using a single punch tablet press (DP-30; Beijing Gylongli Sci. & Tech. Co., Ltd., Beijing, China) at 1 ton pressure. Three formulations were made including AmB+DOC physical mixtures (T1), AmB+MPP physical mixtures (T2), and AmB/MPP micelles (T3). Details of the compositions are listed in . Evaluation of buccal tablets is given in the Supplementary materials.
Table 1 Composition of different buccal tablets
In vitro release of buccal tablets
Using the United States Pharmacopeia (USP) dissolution apparatus
In vitro release characteristics of the mucoadhesive buccal tablets were investigated by basket stirring method using the USP dissolution test apparatus, and the accumulative release percentage was calculated. A total of 200 mL of sodium dodecyl sulfate (SDS) aqueous solution (0.5%, w/v) was selected as the release medium at 37°C and shaken at 50 rpm. At predetermined periods, 2.5 mL of the release medium was withdrawn and an equal volume of fresh medium (37°C) was added back to maintain sink condition. The samples were filtered and analyzed by HPLC. All release experiments were repeated three times.
Using the dissolution rate test apparatus
To evaluate drug release in vivo, the dissolution rate test apparatus (China Patent no ZL201320445613.7) was designed for simulating in vivo conditions of the oral cavity.Citation18 Each tablet was adhered to the cap of the dissolution cell using 20 μL of artificial saliva (500 mL containing 5 mmol/dm3 sodium bicarbonate, 7.36 mmol/dm3 sodium chloride, 20 mmol/dm3 potassium chloride, 6.6 mmol/dm3 sodium dihydrogen phosphate monohydrate, and 1.5 mmol/dm3 calcium chloride dehydrate, adjusted to pH 6.8 or 5.8) and lightly pressed for 30 s.Citation36 After assembling the cap to the dissolution cell, artificial saliva was pumped through the cell with a peristaltic pump to control the velocity at ~0.6 mL/min.Citation26,Citation37 A total of 1.5 mL continuous flow of artificial saliva suffused the dissolution cell and was agitated with a magnetic stirrer at 50 rpm. The apparatus was maintained at 37°C in a thermostat bath. Every fractional sample was collected at predetermined periods and analyzed by HPLC. Six tablets of each formulation were analysed. The accumulative release amount (Qn) of the drug at different times was calculated as follows:
where Cn indicated the drug concentration of sample n at determined times; Vn showed the volume of the collected artificial saliva; and W was the labeled amount (mg) of the buccal tablet.
Cytotoxicity assay of the micelles
To evaluate the cytotoxicity of AmB/MPP micelles by MTT assay, KB cells were inoculated at a density of 1×104 cells/well in a 96-well plate overnight. Then, the culture medium was removed by pipette. Fungizone (a micellar dispersion of AmB with DOC),Citation38,Citation39 AmB/MPP micelles, and MPP blank micelles were added to each well and incubated at 37°C for a certain time. Following incubation, 20 μL of 5 mg/mL MTT in PBS was put into each well and then incubated for 4 h at 37°C. To dissolve the formazan crystal, 150 μL of dimethyl sulfoxide (DMSO) was added. After shaking the 96-well plate for 30 s, the absorbance was measured at 492 nm by a microplate reader (BioTek Epoch, Winooski, VT, USA). The 100% value as the standard to assess cyto-toxicity was obtained from the optical density (OD) values assayed in untreated cells.
Histopathology of oral buccal mucosal tissues
Male Sprague Dawley rats (230±10 g) were obtained from the Lab Animal Center of Xi’an Jiaotong University (Shaanxi, China). The experimental protocol was in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and was approved by the Institutional Animal Care Committee of Xi’an Jiaotong University. Before the experiments, rats were raised in an environmentally controlled breeding room for 1 week and fed with standard laboratory food and water. The control group was the 5% dextrose sterile solution, and other groups (including Fungizone, AmB/MPP micelles, and MPP blank micelles) were dissolved in the 5% dextrose sterile solution. Then, 50 μL of each formulation was injected into the oral buccal mucosal tissues of the rats. At the end of treatments (after injection for 5, 15, and 30 days), some rats were sacrificed by injecting air into the thoracic cavity. Oral buccal mucosal tissues were isolated and fixed in 10% neutral carbonated-buffered formaldehyde. The fixed tissues were optimal cutting temperature-embedded and sliced using a freezing microtome. Hematoxylin and eosin-stained sections of the oral buccal mucosal tissues were evaluated.
Ultrastructure observation of the tissues
Before the observation, the isolated oral buccal mucosal tissues were cut into small pieces of size 0.5–1 mm3 and directly placed into the precooling glutaraldehyde solution at 4°C. At least 4 h later, samples were rinsed with 0.1 mol/L PBS three times and then fixed with 1% osmic acid. Epon812 was the embedding medium. Ultrastructure observation of the oral buccal mucosal tissues was analyzed under transmission electron microscope (H-7650; Hitachi).
Intragastric administration of the micelles
For toxicity analysis of the micelles through the gastrointestinal tract, AmB/MPP micelles in the 5% dextrose sterile solution were intragastrically administrated through the stomach sondle needle at the dose of 5 mL/kg. Then, blood samples (0.2 mL) were withdrawn in heparinized tubes from the femoral artery using cannulation at 0, 15, 30, 60, 120, 240, and 480 min after oral administration. The blood samples were centrifuged at 3,000× g for 5 min, and the plasma samples were harvested and stored at −20°C until liquid chromatography-mass spectrometry (LC-MS) analysis (LC-MS method is given in the Supplementary materials).
Antifungal activity
The minimal inhibitory concentration (MIC) for planktonic C. albicans was determined using the microdilution method according to the guidelines of the M27-A3 document of the Clinical and Laboratory Standards Institute. The fungal cells were seeded on a 96-well microtiter plate in Roswell Park Memorial Institute 1640 medium at a density of 1×105 cells (100 μL per well), and the final drug concentrations were 0.098–50 μg/mL for AmB/MPP micelles and AmB. Plates were incubated at 30°C for 48 h. Then, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT)-menadione solution was added and finally measured at the absorbance of 490 nm using a microplate reader (BioTek Epoch). Experiments were performed three times with six replicate wells for each experiment.
Formation and quantification of biofilms
Biofilms were prepared in flat-bottomed 96-well microplates adapted from a method of O’Toole and Kolter,Citation40 which was based on the ability of microorganisms to form biofilms on solid surfaces and used crystal violet (CV) to stain biofilms. Briefly, 100 μL of 1×105 cells/mL suspension in Roswell Park Memorial Institute 1640 medium was inoculated at 37°C without shaking for 48 h, forming the biofilms. The morphology of biofilms was observed under scanning electron microscope (SEM; TH-1000; Hitachi). Biofilms were washed with sterile PBS and incubated at 37°C for 48 h with different concentrations of drugs. Plates were gently rinsed with PBS and air-dried for 24 h prior to CV staining. Adherent films of each well were stained with 200 μL per well of 1% (w/v) CV (5 min) and then washed with sterile PBS to remove unbound dye. Afterward, CV was extracted with 200 μL of the bleaching solution: ethanol/glacial acetone (70:30), and biofilms were quantified by measuring the OD at 595 nm with the microplate reader. The biofilm biomass unit (BBU) was arbitrarily defined with 0.1 OD595 equal to 1 BBU.
Statistical analysis
The statistical analyses were performed using Student’s t-test with a level of significance at P<0.05.
Results and discussion
Preparation and characterization of micelles
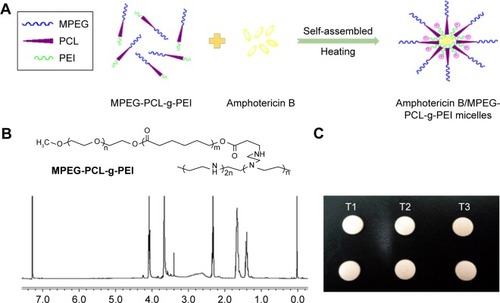
The copolymer MPEG-PCL-g-PEI was synthesized, and the chemical composition was confirmed by 1H-NMR spectra (). In the aqueous solution, this triblock copolymer could self-assemble into nanoscale micelles when heated to 50°C (). Poorly water-soluble AmB was encapsulated into the inner hydrophobic core of the copolymer on account of the hydrophobic interaction. The encapsulation efficiency and drug loading were 94.11%±1.25% and 8.60%±0.21%, respectively.
Figure 1 (A) Spontaneous AmB-loaded micelles formation from amphiphilic molecules in aqueous media; (B) the chemical formula and the 1H-NMR spectra (400 MHz, CDCl3) of MPEG-PCL-g-PEI; (C) buccal tablets containing AmB+DOC (T1), AmB+MPEG-PCL-g-PEI physical mixtures (T2), and AmB/MPEG-PCL-g-PEI micelles (T3).
Abbreviations: AmB, amphotericin B; MPEG-PCL-g-PEI, monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone)-graft-polyethylenimine; DOC, sodium deoxycholate.
The average size of blank micelles and drug-loaded micelles () was 149.7±0.7 nm (polydispersity index [PDI] =0.297±0.017) and 148.1±0.9 nm (PDI =0.279±0.018), respectively. These size distribution spectrums suggested that there was no significant variation in the particle size between the drug-loaded micelles and blank micelles. As shown in , zeta potential of blank micelles and drug-loaded micelles was ~25.5±1.67 and 17.2±0.62 mV, respectively. These micelles showed positive charge, which indicated the linkage of cationic polymer PEI. The reduction of zeta potential may be related to the encapsulation of AmB, which was identical to that of the previous studies.Citation41 Although zeta potential of drug-loaded micelles was lower than that of blank micelles, they all presented good dispersion and stability during the period of the experiment. exhibits the morphological observation of AmB/MPP micelles. Most of the micelles appeared spherical in shape and dispersed uniformly. It could be inferred that the hydrophobic terminals of PCL chains formed the micellar core and the hydrophilic terminals of MPEG and PEI chains composed the hydrophilic corona.
Figure 2 (A) The particle size distribution spectrum of blank micelles and AmB-loaded micelles; (B) zeta potential of blank micelles and AmB-loaded micelles; (C) morphology of AmB-loaded micelles determined by TEM (scale bar 100 nm); (D) DSC analysis of (a) MPP, (b) AmB, (c) AmB/MPP micelles, and (d) AmB and MPP physical mixtures.
Abbreviations: AmB, amphotericin B; TEM, transmission electron microscopy; DSC, differential scanning calorimetry; MPP, monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone)-graft-polyethylenimine.
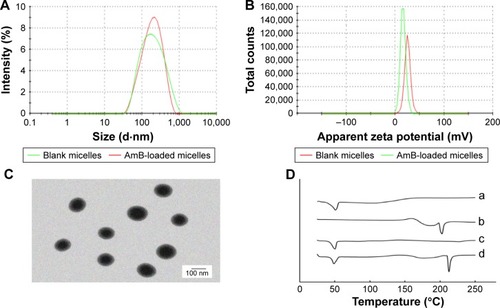
DSC analysis is an effective identification method to validate the state of incorporated drugs. The DSC curves of AmB, MPEG-PCL-g-PEI, AmB and MPEG-PCL-g-PEI physical mixture, and AmB/MPP micelles are shown in . There was no evidence of melting in AmB up to 250°C, because this antibiotic has already decomposed, which could be observed in the DSC thermogram for AmB (double broad endotherms at ~170 and 210°C) and was identical to that of the previous report.Citation42 MPEG-PCL-g-PEI showed a broad endothermic peak at ~47°C, while the DSC scan of AmB/MPEG-PCL-g-PEI micelles did not show any signs of AmB endothermic peak, indicating the formation of an amorphous state and the molecular encapsulation of the drug inside the core–shell structure.
In vitro release of micelles
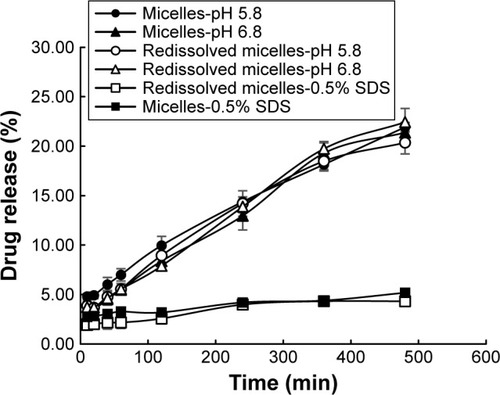
The pH value in the oral cavity for healthy humans was found to be ~6.3–7.4,Citation43,Citation44 while it decreased to weak acid condition for patients who suffered from oral C. albicans. In this research, in vitro release profile of AmB/MPP micelles and redissolved micelles (lyophilized powder redissolved in water) in different mediums (pH 5.8 and 6.8 and 0.5% SDS aqueous solution) was investigated (). Mediums (pH 5.8 and 6.8) contained 0.5% Tween-80 (w/v). It could be observed that there was no significant difference between micelles and redissolved micelles release; thus, lyophilized powder redissolved in water was utilized in further research. In the pH 5.8 and 6.8 buffer solutions, AmB release was dramatically faster than that in the 0.5% SDS aqueous solution (pH 8.0); probably the different ionic strength of the media could influence the release. It seems that pH 5.8 and 6.8 buffer solutions were more suitable for the evaluation of the micelles release. Besides, no obvious difference was observed between pH 5.8 and 6.8 buffer solutions for AmB release. As well known, AmB was insoluble in water, and its solubility could be enhanced when encapsulated into the MPEG-PCL-g-PEI micelles in both normal conditions (pH 6.8) and pathological conditions (pH 5.8) without dependence on the pH changes.
Evaluation of buccal tablets
The buccal tablets were produced according to the formulation composition in , and the evaluation results are shown in . The weight variation, hardness, and friability parameters of the prepared buccal tablets were within the range of Chinese pharmacopoeial limit. The parameters of buccal tablets’ weight, size, and hardness were selected on the basis of Oravig® (Miconazole buccal tablet, approved by the Food and Drug Administration [FDA] since 2010), which take into consideration patients’ tolerance.Citation45 The picture of the tablets is shown in .
Table 2 Evaluation results of buccal tablets
In vitro drug release studies of buccal tablets
For USP dissolution apparatus release, as shown in , there was no burst release phenomenon in any AmB buccal tablet formulation. MPC85 and HPMC K4M in the prescription were employed as mucoadhesive polymers. A 0.5% SDS aqueous solution was used in the dissolution medium for miconazole buccal tablet in FDA dissolution methods. T1 was the control group mainly including AmB and DOC, which had the same ingredient with Fungizone.Citation46,Citation47 T2 consisted of the physical mixtures of AmB and MPP. AmB/MPP micelles took up the major component of T3. T2 was designed to prove the solubilization capacity of the core–shell structure of the micelles compared with the physical mixtures. It was obvious that AmB was able to release ~100% at 8 h for T3, and the cumulative release amount was significantly higher than T1. Thus, it could be inferred that this core–shell structure of the micelles has advantages on dissolution and improves the release of hydrophobic drugs. The buccal tablet T3 we prepared could meet the release requirements and exert medicinal effect effectively and continuously.
Figure 4 The release profiles of AmB from buccal tablets using different dissolution apparatuses.
Notes: (A) USP dissolution apparatus (in 0.5% SDS solution) and (B) the dissolution rate test apparatus (in pH 5.8 and pH 6.8 medium). T1, AmB+DOC; T2, AmB+MPP; T3, AmB/MPP micelles.
Abbreviations: AmB, amphotericin B; SDS, sodium dodecyl sulfate; DOC, sodium deoxycholate; MPP, monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone)-graft-polyethylenimine; USP, United States Pharmacopeia.
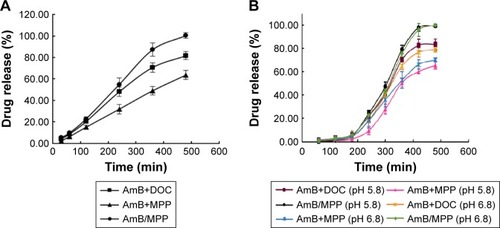
The in vitro release results of the buccal tablets were similar between our designed dissolution rate test apparatus () and USP dissolution apparatus. Both the apparatuses could be utilized in the evaluation of in vitro release for the buccal tablets. All the results demonstrated that MPEG-PCL-g-PEI micelles could improve the solubility of AmB.
Cytotoxicity assay of the micelles
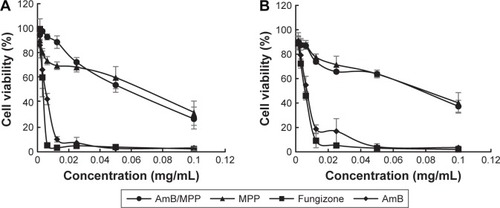
The cytotoxicities of AmB/MPP micelles were evaluated by MTT assay using KB cells (). Cell viabilities (%) of different concentrations of free AmB, AmB+DOC, AmB/MPP micelles, and MPP blank micelles were investigated. Although AmB reduced the viability of the cells in a dose-dependent manner, the use of MPP micelles significantly lowered the cytotoxicity of AmB. The cell viabilities of AmB/MPP micelles and MPP blank micelles were much higher than those of free AmB and AmB+DOC. It could be observed that the MTT results after dosing for 12 or 24 h had no obvious difference. Both these results proved that AmB/MPP micelles and MPP blank micelles were less cytotoxic than free AmB and AmB+DOC.
Histopathology of oral buccal mucosal tissues
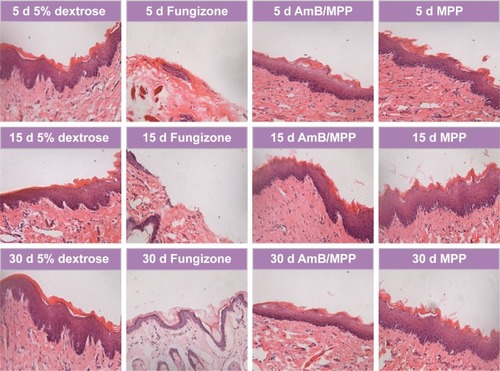
Safety is a critical issue driving the design or evaluation for a drug delivery system. Given the cell cytotoxicity results, we next examined the histology of the oral buccal mucosal tissues after administration. All the drugs were dissolved in 5% dextrose solution and filtered before use. Right buccal tissues of the rats were treated with 50 μL of AmB+DOC, AmB/MPP, and MPP micelles through injection into the buccal mucosal tissues, and left buccal tissues were instilled with an equal volume of 5% dextrose solution as negative control groups. After administration over 5, 15, and 30 days, there were no discernible effects on the histopathology of oral mucosa for 5% dextrose solution, AmB/MPP, and MPP micelle groups (). Histopathology data showed neither inflammation nor tissue necrosis after administration for 5 days. However, for AmB+DOC group, visible signs of break at the surface, middle, and base level of buccal epithelium membrane emerged from the oral mucosa.Citation48 Until administration over 30 days, there was a slight recovery in the oral mucosa. As compared to the 5% dextrose solution group, AmB/MPP- and MPP-treated ones showed no visible sign of pathological changes.
Figure 6 Histopathological observation of oral buccal tissues through injection with 5% dextrose solution, Fungizone (AmB+DOC), AmB/MPP micelles, and MPP blank micelles under a light microscope (400×) after dosing for 5, 15, and 30 days.
Abbreviations: AmB, amphotericin B; DOC, sodium deoxycholate; MPP, monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone)-graft-polyethylenimine; d, days.
Ultrastructure observation of the tissues
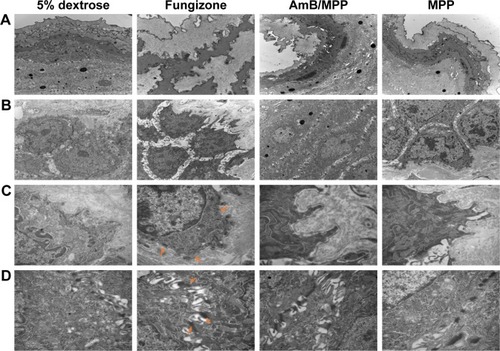
The histopathological examination of oral buccal mucosal tissues was done in order to evaluate the changes of the intermediate layer in mucosa epithelium and base layer cells; so ultrastructure observation of the tissues was also required.Citation49–Citation53 We analysed the buccal tissues after dosing for 30 days with 5% dextrose solution, AmB+DOC, AmB/MPP, and MPP groups (). In , the mucosal epithelial surface layer of the AmB+DOC group showed apparent swelling, so that only a partial surface layer could be observed in the same view compared with other groups. The epithelial intermediate layer (spinous layer) containing several prickle cells is shown in . For AmB+DOC group, the prickle cells were loose and the gap of intercellular space became bigger than other groups. shows the basement membrane of the mucosa; some parts in the basement membrane had disappeared in the AMB+DOC group, while other groups were intact. Basement membrane played a major role on support, connection, and fix effect to the tissue. Besides, it was also a barrier structure for permeability, facilitating blood and material exchange between epithelial cells. Therefore, the basilar membrane had vital significance for the physiological functions of buccal mucosa tissue. In , desmosome junctions between epithelial cells were able to reflect the stability state of the tissue, namely, the more desmosome junctions the greater stability of the tissue. However, desmosome junctions in AmB+DOC group were less than those in other groups. As compared to the 5% dextrose solution group, the AmB/MPP- and MPP-treated ones exhibited less pathological changes. The inner components of prickle cells and basal cells in the AmB+DOC group showed no obvious lesion, which may be attributed to the recovery of the animals after dosing.
Figure 7 Ultrastructure observation of oral buccal tissues through injection with 5% dextrose solution, Fungizone (AmB+DOC), AmB/MPP micelles, and MPP blank micelles after dosing for 30 days under transmission electron microscope.
Notes: (A) Epithelial surface layer of the oral buccal tissues (10,000×); (B) epithelial middle layer containing prickle cells (10,000×); (C) basement membrane (30,000×, orange arrows pointed to the disappeared parts in the basement membrane of the AMB+DOC group); (D) desmosome junctions between epithelial cells (30,000×, orange arrows pointed to the residual desmosome junctions in AmB+DOC group).
Abbreviations: AmB, amphotericin B; DOC, sodium deoxycholate; MPP, monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone)-graft-polyethylenimine.
Gastrointestinal administration of the micelles
When AmB/MPP micelles were employed in the buccal application, it seems possible that the micelles could be swallowed into the gastrointestinal environment. To analyze the toxicity of the micelles through the gastrointestinal tract, AmB/MPEG-PCL-g-PEI micelles were intragastrically administrated to the rats. In this experiment, the blood samples were withdrawn at 0, 15, 30, 60, 120, 240, and 480 min after oral administration. After LC-MS detection of the plasma samples, no AmB absorption was detected until 8 h (Supplementary materials). This verified that AmB/MPP micelles in the buccal application would not be ingested into the blood if unnecessary swallowing occurred. In consequence, AmB/MPP micelles in the oral cavity, local application, would not cause systemic toxicity, especially for nephrotoxicity.
Antifungal effect of the micelles
The biological studies showed that AmB/MPP micelles maintain high antimicrobial activity, which is similar to that of free AmB in DMSO (), whereas empty micelles had a significantly higher MIC value. This indicates that AmB-incorporated micelles have a similar antimicrobial potential to free AmB itself. In spite of the introduction of cation polymer, the antifungal effect to planktonic cells did not improve significantly. This phenomenon may be ascribed to the drug release process from the micelles, while the drug in DMSO is immediately available. Moreover, considering that the mechanism for AmB activity seems to be related to the formation of multimeric drug associations, it could be possible that the drug released from the micelles is partially in conformation with the target interaction.
Figure 8 (A) MIC values of AmB/MPP and AmB; (B) observation of Candida albicans mature biofilms under SEM; (C) C. albicans BBU, concentrations of drugs were μg/mL.
Notes: *Statistical difference at P,0.05. All data represent the mean ± SD (n=6).
Abbreviations: MIC, minimum inhibitory concentration; AmB, amphotericin B; MPP, monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone)-graft-polyethylenimine; SEM, scanning electron microscope; BBU, biofilm biomass unit; SD, standard deviation; C.A., Candida albicans.
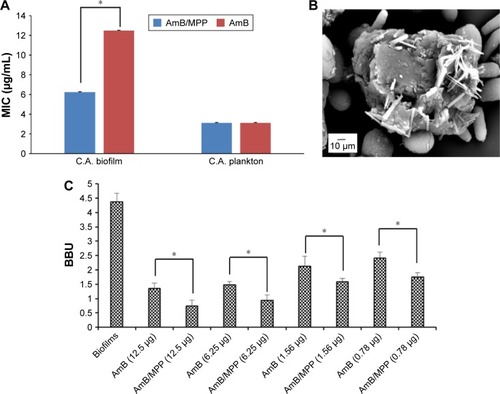
In this study, C. albicans were incubated for 48 h in order to form the mature biofilms, which could be verified through the observation under SEM (). From the picture, we could see the extra-polymeric matrix wrapped around the cells. The effects were assessed against C. albicans strains using XTT reduction assay that showed a concentration-dependent reduction in biofilm biomass of Candida strains. The increased resistance of sessile cells to antibiotic treatment is mainly due to the biofilm formation. It was obvious that AmB-incorporated micelles have significantly improved the anti-biofilm effect of C. albicans (), which was similar to another report.Citation54 To directly quantify the effect of micelles on biofilms, CV staining was used expressed in BBU. The results showed a correlation between the CV assay and XTT reduction assay. This confirms that AmB-loaded micelles were more effective in inhibiting the growth of C. albicans biofilms. It may indicate that these micelles presented a superior penetration ability to pass through the extra-polymeric matrix and interact with the membrane of C. albicans. On the other hand, the cation polymer PEI with positive charge had more affinity to the biofilm that showed negative charge on the surface. Fungus would favor the adhesion over the surface of the cation polymer PEI; thus, MPP may influence the adhesion of C. albicans on the biotic host surface. Apart from that, there have been many reports that self-aggregated AmB is toxic to both mammalian and fungal cell membranes, while the monomeric form of the drug is toxic to fungal cell membrane only.Citation55,Citation56 The favorable result with cell and tissue toxicity of AmB-incorporated micelles appears to be associated with the existence of the drug molecules in the monomeric form in the micelles, whereas the antifungal effect did not weaken, suggesting an interesting practical application.
Conclusion
In this study, we prepared AmB/MPEG-PCL-g-PEI micelles and improved the aqueous solubility of AmB. The micelles were spherical shaped, nano-sized, and stably dispersed with high DL% (8.60%±0.21%) and EE% (94.11%±1.25%). DSC assay suggested that AmB was encapsulated into the core–shell structure. The micelles allowed a sustained in vitro release in both normal conditions (pH 6.8) and C. albicans infection pathological conditions (pH 5.8) without pH dependence. Then, the buccal tablets containing AmB/MPP micelles were produced by direct compression process and evaluated as regards to weight variation, hardness, and friability. In vitro drug release of the buccal tablets was measured in both the USP dissolution apparatus and the dissolution rate test apparatus, which were previously designed for in vitro release assessment to simulate in vivo conditions of the oral cavity. The buccal tablets could sustainably release within 8 h. Regarding safety examination of AmB/MPP micelles, in vivo histopathological data showed no irritation toward buccal mucosa of the rats in both optical microscopy and ultrastructure observation of the tissues. MTT experiment proved that AmB/MPP micelles reduced the cytotoxicity of AmB. In addition, the toxicity of micelles delivered through the gastrointestinal route was also found to be non-systemic toxicity. Furthermore, the antifungal action of AmB/MPP micelles was evaluated. Although AmB/MPP had no obvious improvement as compared to AmB alone in the antifungal effect of planktonic C. albicans, the micelles significantly enhanced the antifungal activity against the biofilm state of C. albicans. Thus, it was concluded that the AmB/MPP micelles could have a superior potential to be developed as a more convenient and effective oral buccal delivery system.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81470549, No 81402925, and No 81400233) and the Fundamental Research Funds for the Central Universities.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Evaluation of buccal tablets
Tablet weight variation
Twenty tablets were weighed by an electronic scale (AY 120; Shimadzu Co., Ltd., Kyoto, Japan), and the average weight of the tablets was calculated.
Hardness
Hardness was measured using a hardness tester (YD-1; Shanghai Develop Machinery Co., Limited, Shanghai, China). Six tablets from three formulations were randomly tested. The mean and standard deviation values were obtained.
Friability
Friability was determined through a friability tester (CJY-300B; Shanghai Hongyi, Shanghai, China). After removing the superfluous powder, 60 preweighed tablets were placed in the tester and rotated 4 min at 25 rpm. Then, the powder of the tablets was gently loosened with a blower and reweighed. The percentage loss was calculated as follows:
LC-MS analysis
LC 20AD HPLC system (Shimadzu Co., Ltd.) was coupled to a triple–quadrupole tandem mass spectrometer (API3200; AB SCIEX, Foster City, CA, USA) in electron spray ionization mode to monitor positive molecular ions [M+H]+. High-performance liquid chromatography (HPLC) analysis was performed on a Dikma C18 column (250×4.6 mm, 5 μm) at 30°C with the injection volume of 20 μL. The mobile phase (acetonitrile: 10 mmol ammonium acetate aqueous solution =40:60, 1 mL/min) from the HPLC column was delivered directly into the mass spectrometer with parameters as follows: optimized ion spray voltage 5,000 V, optimized temperature 600°C, and N2 pressure GS1 =40 psi, GS2 =15 psi. The operating conditions were as follows: collision energy, declustering potential, entrance potential, and collision cell exit potentials were 129, 41, 9, and 38 V for AmB. The mass transition used for AmB was m/z 922.608/81 Da.
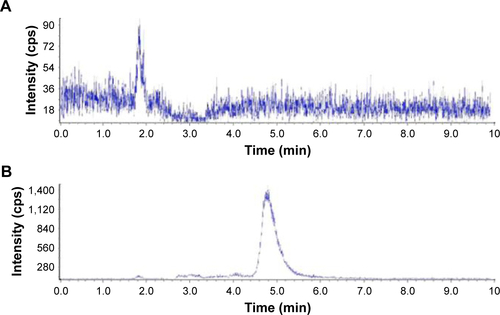
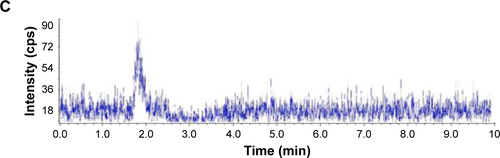
Figure S1 LC-MS spectrum for the toxicity of AmB/MPP micelles through (A) gastrointestinal tract, (B) blank plasma, and (C) AmB in blank plasma, after 8 h dosing of a plasma sample.
Abbreviations: AmB, amphotericin B; MPP, monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone)-graft-polyethylenimine; LC-MS, liquid chromatography-mass spectrometry.
References
- GhannoumMARiceLBAntifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistanceClin Microbiol Rev1999121250151710515900
- CassoneACaudaRCandida and candidiasis in HIV-infected patients: where commensalism, opportunistic behavior and frank pathogenicity lose their bordersAIDS201226121457147222472853
- KimJSudberyPCandida albicans, a major human fungal pathogenJ Microbiol201149217117721538235
- HawserSPDouglasLJResistance of Candida albicans biofilms to antifungal agents in vitroAntimicrob Agents Chemother1995399212821318540729
- OlsenIBiofilm-specific antibiotic tolerance and resistanceEur J Clin Microbiol2015345877886
- QasimMBaipaywadPUdomluckNNaDParkHEnhanced therapeutic efficacy of lipophilic amphotericin B against Candida albicans with amphiphilic poly(N-isopropylacrylamide) nanogelsMacromol Res2014221011251131
- Laniado-LaborinRCabrales-VargasMNAmphotericin B: side effects and toxicityRev Iberoam Micol200926422322719836985
- PippaNMariakiMPispasSDemetzosCPreparation, development and in vitro release evaluation of amphotericin B-loaded amphiphilic block copolymer vectorsInt J Pharm20144731–2808624998505
- GuptaUAgasheHBJainNKPolypropylene imine dendrimer mediated solubility enhancement: effect of pH and functional groups of hydrophobesJ Pharm Pharm Sci200710335836717727799
- BansalKKKakdeDPurdieLNew biomaterials from renewable resources – amphiphilic block copolymers from delta-decalactonePolym Chem201564071967210
- VandermeulenGRouxhetLArienABrewsterMEPreatVEncapsulation of amphotericin B in poly(ethylene glycol)-block-poly(epsilon-caprolactone-co-trimethylenecarbonate) polymeric micellesInt J Pharm20063091–223424016406402
- LegrandPRomeroEACohenBEBolardJEffects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytesAntimicrob Agents Chemother19923611251825221489196
- BarwiczJChristianSGrudaIEffects of the aggregation state of amphotericin B on its toxicity to miceAntimicrob Agents Chemother19923610231023151444311
- LemkeAKiderlenAFKayserOAmphotericin BAppl Microbiol Biot2005682151162
- ChenYCSuCYJhanHJHoHOSheuMTPhysical characterization and in vivo pharmacokinetic study of self-assembling amphotericin B-loaded lecithin-based mixed polymeric micellesInt J Nanomedicine2015107265727426664117
- AdamsMLLavasanifarAKwonGSAmphiphilic block copolymers for drug deliveryJ Pharm Sci20039271343135512820139
- GaucherGSatturwarPJonesMCFurtosALerouxJCPolymeric micelles for oral drug deliveryEur J Pharm Biopharm201076214715820600891
- WangKLiuTTLinRPreparation and in vitro release of buccal tablets of naringenin-loaded MPEG-PCL nanoparticlesRSC Adv20144643367233679
- WiegandCBauerMFrischmuthTFischerDHiplerUCEvaluation of the antifungal effect of polyethylenimine (PEI) by laser nephelometryMycoses2009525427427
- WiegandCBauerMFrischmuthTFischerDHiplerUCEvaluation of the antifungal effect of polyethylenimine (PEI) using microplate laser nephelometry (MLN)J Dtsch Dermatol Ges201191225225
- WiegandCBauerMFischerDHiplerUCDetermination of the antifungal effect of polyethylenimine (PEI) by microplate laser nephelometry in vitroMycoses201255425725721914003
- Soncini-JúniorGFranchettiSMMMarconatoJCArchitecture and relevance of several strongly adhered biofilms over a polyester imide (PEI) surfaceBraz J Microbiol20033434105107
- ShiSZhuXCGuoQFSelf-assembled mPEG-PCL-g-PEI micelles for simultaneous codelivery of chemotherapeutic drugs and DNA: synthesis and characterization in vitroInt J Nanomedicine2012791749175922619525
- HaoJSHengPWSBuccal delivery systemsDrug Dev Ind Pharm200329882183214570303
- ZhangHZhangJStreisandJBOral mucosal drug delivery – clinical pharmacokinetics and therapeutic applicationsClin Pharmacokinet200241966168012126458
- ReddyPCChaitanyaKSCRaoYMA review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methodsDaru201119638540323008684
- MrsnyRJOral drug delivery research in EuropeJ Control Release2012161224725322342473
- DabholkarRDSawantRMMongaytDADevarajanPVTorchilinVPPolyethylene glycol-phosphatidylethanolamine conjugate (PEG-PE)-based mixed micelles: some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated effluxInt J Pharm20063151–214815716616818
- CasaDMCarraroTCde CamargoLEADalmolinLFKhalilNMMainardesRMPoly(L-lactide) nanoparticles reduce amphotericin B cytotoxicity and maintain its in vitro antifungal activityJ Nanosci Nanotechnol201515184885426328449
- LavasanifarASamuelJSattariSKwonGSBlock copolymer micelles for the encapsulation and delivery of amphotericin BPharm Res200219441842212033373
- DiazILParraCLinarezMPerezLDDesign of micelle nanocontainers based on PDMAEMA-b-PCL-b-PDMAEMA triblock copolymers for the encapsulation of amphotericin BAAPS PharmSciTech20151651069107825669917
- TangXLLiangYZhuYQAnti-transferrin receptor-modified amphotericin B-loaded PLA-PEG nanoparticles cure Candidal meningitis and reduce drug toxicityInt J Nanomedicine2015106227624126491294
- DuanYCaiXDuHZhaiGNovel in situ gel systems based on P123/TPGS mixed micelles and gellan gum for ophthalmic delivery of curcuminColloids Surf B Biointerfaces201512832233025707750
- ButtAMAminMCIMKatasHSarisutaNWitoonsaridsilpWBenjakulRIn vitro characterization of pluronic F127 and D-alpha-tocopheryl polyethylene glycol 1000 succinate mixed micelles as nanocarriers for targeted anticancer-drug deliveryJ Nanomater2012201211
- ZhangWShiYChenYZHaoJGShaXYFangXLThe potential of pluronic polymeric micelles encapsulated with paclitaxel for the treatment of melanoma using subcutaneous and pulmonary metastatic mice modelsBiomaterials201132255934594421596432
- KeeganGMSmartJDIngramMJBarnesLMBurnettGRReesGDChitosan microparticles for the controlled delivery of fluorideJ Dent201240322924022212236
- SchneyerLHLevinLKRate of secretion by individual salivary gland pairs of man under conditions of reduced exogenous stimulationJ Appl Physiol19557550851214367237
- JainVGuptaAPawarVKChitosan-assisted immunotherapy for intervention of experimental leishmaniasis via amphotericin B-loaded solid lipid nanoparticlesAppl Biochem Biotechnol201417441309133025106894
- GangadharKNAdhikariKSrichanaTSynthesis and evaluation of sodium deoxycholate sulfate as a lipid drug carrier to enhance the solubility, stability and safety of an amphotericin B inhalation formulationInt J Pharm20144711–243043824907597
- O’TooleGAKolterRInitiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysisMol Microbiol19982834494619632250
- AsthanaSGuptaPKJaiswalAKDubeAChourasiaMKTargeted chemotherapy of visceral leishmaniasis by lactoferrin-appended amphotericin B-loaded nanoreservoir: in vitro and in vivo studiesNanomedicine20151071093110925929567
- KimYTShinBKGarripelliVKA thermosensitive vaginal gel formulation with HPgammaCD for the pH-dependent release and solubilization of amphotericin BEur J Pharm Sci201041239940620654712
- AframianDJDavidowitzTBenolielRThe distribution of oral mucosal pH values in healthy saliva secretorsOral Dis200612442042316792729
- XiaBFYangZZhouHYDevelopment of a novel oral cavity compartmental absorption and transit model for sublingual administration: illustration with ZolpidemAAPS J201517363164225716146
- LallaRVBensadounRJMiconazole mucoadhesive tablet for oropha-ryngeal candidiasisExpert Rev Anti Infect Ther201191131721171872
- TorradoJJEspadaRBallesterosMPTorrado-SantiagoSAmphotericin B formulations and drug targetingJ Pharm Sci20089772405242517893903
- Van de VenHPaulussenCFeijensPBPLGA nanoparticles and nanosuspensions with amphotericin B: potent in vitro and in vivo alternatives to Fungizone and AmBisomeJ Control Release2012161379580322641062
- BurgessBLCavigiolioGFannucchiMVIllekBForteTMOdaMNA phospholipid-apolipoprotein A-I nanoparticle containing amphotericin B as a drug delivery platform with cell membrane protective propertiesInt J Pharm20103991–214815520696226
- ThomasPHeckerJFauntJFenechMBuccal micronucleus cytome biomarkers may be associated with Alzheimer’s diseaseMutagenesis200722637137917709794
- GandhiRBRobinsonJROral cavity as a site for bioadhesive drug-deliveryAdv Drug Deliv Rev1994131–24374
- DowtyMEKnuthKEIronsBKRobinsonJRTransport of thyrotropin-releasing-hormone in rabbit buccal mucosa in vitroPharm Res199299111311221409390
- SquierCAWertzPWStructure and function of the oral mucosa and implications for drug deliveryDrugs Pharm Sci199674126
- RathboneMJTuckerIGMechanisms, barriers and pathways of oral mucosal drug permeationAdv Drug Deliv Rev1993121–24160
- RandhawaMAGondalMAAl-ZahraniAHRashidSGAliASynthesis, morphology and antifungal activity of nano-particulated amphotericin-B, ketoconazole and thymoquinone against Candida albicans yeasts and Candida biofilmJ Environ Sci Health A Tox Hazard Subst Environ Eng201550211912425560257
- BolardJLegrandPHeitzFCybulskaBOne-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the mediumBiochemistry19913023570757152043613
- BrajtburgJElbergSTravisSJKobayashiGSTreatment of murine candidiasis and cryptococcosis with amphotericin B incorporated into egg lecithin-bile salt mixed micellesAntimicrob Agents Chemother19943822942998192455