Abstract
Silica nanoparticles (SiNPs) are being studied and used for medical purposes. As nanotechnology grows rapidly, its biosafety and toxicity have frequently raised concerns. However, diverse results have been reported about the safety of SiNPs; several studies reported that smaller particles might exhibit toxic effects to some cell lines, and larger particles of 100 nm were reported to be genotoxic to the cocultured cells. Here, we investigated the in vivo toxicity of SiNPs of 150 nm in various dosages via intravenous administration in mice. The mice were observed for 14 days before blood examination and histopathological assay. All the mice survived and behaved normally after the administration of nanoparticles. No significant weight change was noted. Blood examinations showed no definite systemic dysfunction of organ systems. Histopathological studies of vital organs confirmed no SiNP-related adverse effects. We concluded that 150 nm SiNPs were biocompatible and safe for in vivo use in mice.
Introduction
Due to their widespread distribution and abundance,Citation1 as well as their chemical and physical properties, silicon-based materials have been used in many industries, including construction or building, electronics, food industry, consumer products, and medical uses.Citation2 Many products containing silicon have been manufactured for human use, which can be applied on the skin or inside the body, such as bandages, lens, dietary supplements, dental fillers, catheters, and implants.Citation3–Citation5 In addition, micro/nanoscale silicone-based materials were used to manufacture consumer products. Due to their basic features, such as size, high specific surface area, low density, optical properties, capacity for absorption, encapsulation capacity, biocompatibility, and low toxicity, silica nanoparticles (SiNPs) attained an important role in the rapidly growing nanotechnologies.Citation6 These characteristics of SiNPs result in their wide utilization as an inert substance entrapping or supporting matrix.Citation7 Consequent research on biomedical applications using SiNPs was undertaken intensively through decades, including diagnosing and controlling disease, identifying and correcting genetic disorders, and increasing longevity.Citation8 SiNPs were used to innovate newer biomedical applications, such as biosensors,Citation9 enzyme supporters,Citation10 controlled drug release and delivery,Citation11,Citation12 and cellular uptake.Citation12
As these particles are being applied to humans, concerns about biocompatibility and harm to body health raise. These abovementioned macroscopic devices, including silica and other materials, are generally known to be safe and biocompatible. When the size of particles was decreased to nanoscale, toxicity has been discovered and reported, such as silver and gold, which have been earlier utilized in biomedical field. Owing to its antibacterial property, silver is used for the production of SiNPs containing medical products, such as wound dressings, devices, and catheters, to lower the incidence of bacterial infections.Citation13 However, Paddle-Ledinek et alCitation14 found that extracts from wound dressings containing SiNPs were more toxic to keratinocytes among those nanomaterials tested. SiNPs are well known to be toxic to various tissues, such as lung, liver, brain, vessels, and reproductive organs.Citation15 Gold is inert and considered as biocompatible, and its nanoparticles are used in medical applications, including drug carrier, biosensor, tumor detector, photothermal agent, and dose enhancer in radiotherapy,Citation16 but a study had shown that gold ions caused suicidal death of erythrocytes.Citation17 Hematological alterations, a common hallmark of toxicity, had been demonstrated in mice that were intravenously given gold nanoparticles (AuNPs).Citation18 Cytotoxic effect was noted in both SiNP- and AuNP-treated mice by Shrivastava et al,Citation19 and increased reactive oxygen species resulting in oxidative stress damage was demonstrated to be the reason for the noxious effect. However, a recent study performed by Fraga et alCitation20 to observe the short- and long-term toxicities after a single-dose intravenous AuNPs to rats showed no severe acute or delayed toxicity. Size-dependent cytotoxicity of AuNPs was reported, and 1.4 nm nanoparticles induced necrosis of the studied cells, but 15 nm nanoparticles exhibited no toxicity with up to 60-fold higher concentration.Citation21
Although some data found that SiNPs are biocompatible, a recent in vitro study with various cell lines showed side effects to some investigated cells depending on nanoparticle size and cell type as well as dosing of the particles.Citation22 Inflammatory responses presenting as elevated interleukin-1β were elicited more by smaller particles when different size, dose, concentration, and surface area mixtures of SiNPs were internalized by mouse bone marrow-derived macrophages.Citation23 Sohaebuddin et alCitation24 reported that SiO2 nanoparticles of 30 nm diameter induced apoptosis of the cocultured cells with increasing percentages in 3T3 fibroblasts, human bronchiolar epithelial cells, and RAW macrophages, reaching ~10%, 50%, and 90%, respectively; however, little necrosis was observed in these studied cells. In contrast, limited cytotoxicity, measured as global metabolism activity, was seen when human epithelial intestinal HT-29 cells were exposed to both 25 and 100 nm nano-SiO2 particles for 24 hours.Citation25 Surprisingly, larger nanoparticles in lower dose resulted in a more unfavorable effect in terms of genotoxicity in the abovementioned study, which was opposite to the concept that smaller particles in higher concentration are usually more toxic to studied subjects.
In in vivo studies, in which SiNPs were administered to animals, discordant results have been reported in various experiments. Inhalation of SiNPs was noted to result in pulmonary tract inflammation and myocardial ischemia in rats, especially in old individuals.Citation26 Oxidative stress biomarkers and proinflammatory cytokines were found in rat lungs when 30 nm SiNPs were instilled in the trachea for 5 weeks by Lin et al,Citation27 and oxidation damage with inflammation was thought to be the rationale of pulmonary toxicity caused by the nanoparticles. Pulmonary inflammation was detected by microcomputed tomography in mice lungs after intratracheal injection with the SiNPs of 30 or 3,000 nm, and smaller particles caused more severe complications.Citation23 Many studies demonstrated that most porous SiNPs deposited in liver and spleen when administered via blood and most of them were cleared in <1 month.Citation28,Citation29 Delayed clearance was noted when nonporous amorphous SiNPs were given intravenously to mice, and elevated aminotransferases, increased inflammatory cytokines, and hepatocytic necrosis were also found.Citation30 SiNPs accumulated in various organs in mice, but no sign of toxicity to those organs was seen with the particle sizes of 20–25 and 80 nm while giving 3 and 2 mg/kg to mice, respectively.Citation31,Citation32 A study by Liu et alCitation33 in 2011, administering 110 nm SiNPs to Institute of Cancer Research mice, reported that 2 of the 10 mice that received >1,000 mg/kg of the SiNPs died, but this lethal dose was much higher than that given to mice receiving 64 nm SiNPs.Citation34 Moreover, as in the report mentioned earlier published in 2012, 100 nm SiNPs produced more genotoxic effect to human epithelial intestinal HT-29 cells than did 25 nm SiNPs;Citation25 it is doubtful whether larger SiNPs are really safer when administered in vivo.
Owing to the diverse results of various investigations, we carried out an in vivo study to evaluate the toxic events as well as the dose-related effects of intravenous SiNPs in mice. In this study, we gave regimens in different concentrations of 150 nm SiNPs to the subjects and attempted to determine the lethal dose. In order to determine the general toxicity of SiNPs, we observed for any depressed activity, disability, or disorder in these treated animals. Multiple blood and serum indicators were measured to detect the systemic dysfunction caused by SiNPs in different dosages. Histopathological analysis of various vital organs was performed to understand the extent of cellular effects produced by the nanoparticles.
Methods
Preparation of SiNPs
SiNPs were synthesized by tetraethylorthosilicate (TEOS), ammonium hydroxide, ethanol, and deionized water according to the methods previously reported.Citation35,Citation36 At first, 3 mL of ammonium hydroxide (30%, J.T. Baker, Center Valley, Pennsylvania, USA) was added to deionized water and 50 mL of ethanol (EtOH, 99.8%; Sigma-Aldrich, St. Louis, Missouri, USA) to make an ammonium–water–EtOH solution, and the solution was stirred vigorously for 5 minutes after admixture. Then, 1.5 mL of TEOS (Sigma Aldrich) was added dropwise to the ammonium–water–EtOH solution with stirring for 1 hour, giving a milky white suspension. This mixture was stood for 17 hours to coarsen the SiNPs. The solidified SiNPs were collected after centrifugation and ethanol washing.
Animal and treatment
Thirty male Balb/C mice aged 7 weeks were purchased from the National Laboratory Animal Center (Taiwan). All mice were housed to a 12-hour light/dark cycle at the animal care center; and environmental condition was maintained at a constant temperature of 22°C±1°C and the humidity of 55%±10%. Water and autoclaved food (Laboratory Autoclavable Rodent Diet 5010; LabDiet, St. Louis, Missouri, USA) were provided ad libitum. The mice were acclimatized for 1 week prior to the experiments. After 1 week, the mice weight was between 24 and 26 g. They were divided into eight groups, each group consisting of three to four mice. A series of doses (1, 2.5, 5, 10, 100, 200, and 300 mg/kg) were set to process the in vivo toxicologic study of SiNPs. The mice received 100 μL of SiNPs in different concentrations by intravenous injection via the tail vein. The control group received 100 μL saline instead of SiNPs, ie, the 0 mg/kg group. Every day, the mice were weighed and behavioral changes were assessed. Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of MacKay Memorial Hospital, Taiwan (IACUC Number: MMH-A-S-101-43). The guidelines of animal welfare were instituted by the National Laboratory Animal Center (Taiwan) and supervised by the Laboratory Animal Center of MacKay Memorial Hospital.
Sample collection
To detect any systemic dysfunction, we examined the blood for changes in hematological and biochemical parameters. Blood samples were collected via the ocular vein 14 days after the exposure to SiNPs. Complete blood counts were analyzed by HEMAVET® (Drew Scientific, Dusseldorf, Germany). Blood samples were centrifuged at 3,000 rpm for 15 minutes in order to obtain serum. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, total protein, albumin, blood urea nitrogen (BUN), and creatinine were measured by FUJI DRI-CHEM 4000i (FujiFilm, Dusseldorf, Germany).
To examine the cellular effect or pathological events, all mice were sacrificed after blood withdrawal. Organs, including brain, heart, lungs, liver, spleen, and kidneys, were harvested. The organs were fixed in 10% formalin and subjected to further histopathological examinations by the Research Center for Animal Medicine, National Chung Hsing University (Taiwan).
Statistics
The quantitative data were expressed as mean ± standard deviation (SD) for measurements. Statistical analyses were performed with independent t-test using SPSS 12.0. Statistical significance was defined as a P-value of <0.05.
Results
Size of synthesized nanoparticles
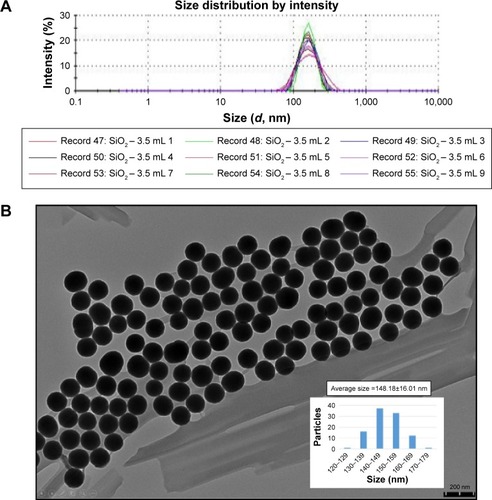
The size and size distribution of the synthesized nanoparticles were analyzed by a dynamic light scattering (DLS) particle size analyzer (Zetasizer NANO-ZS90; Malvern Instruments, Malvern, Worcestershire, UK). The nanoparticle size was 153.8 nm in average (peak 164.4 nm and width 43.58 nm) (). TEM images showed that the SiNPs exhibited a near-spherical shape and dispersed well, with the average size of 148.18±16.01 nm ().
Figure 1 Characterization of SiNPs.
Notes: (A) DLS size distribution with an average diameter of ~153.8 nm. (B) TEM images showed the SiNPs in near-spherical shape with well dispersibility with an average diameter of ~148.18 nm.
Abbreviations: DLS, dynamic light scattering; SiNPs, silica nanoparticles.
Passive behaviors and toxic symptoms
Thirty male Balb/C mice were divided into eight groups, each group consisting of three or four mice. Each group of mice received different dosages of SiNPs (0, 1, 2.5, 5, 10, 100, 200, and 300 mg/kg) through the tail vein. All mice tolerated one dose injection of SiNPs from 1 to 300 mg/kg. No mice showed any passive behavior, such as hypopnea, tremor, and arching of back, or any symptoms of poisoning, such as loss of appetite, diarrhea, and vomiting, to various dosages up to 14 days after treatment. Faintness was noted on the first day of observation when the mice received >100 mg/kg of SiNPs, but the symptom did not occur on the following days (). All mice survived before they were sacrificed for histopathological analysis after the observation period of 14 days. During the experimental period, no significant difference in body weight change was noted among those mice ().
Table 1 Passive behaviors and acute symptoms of poisoning after the injection of SiNPs with various dosages
Table 2 Body weight changes (%) after the injection of SiNPs with various dosages
Blood cells
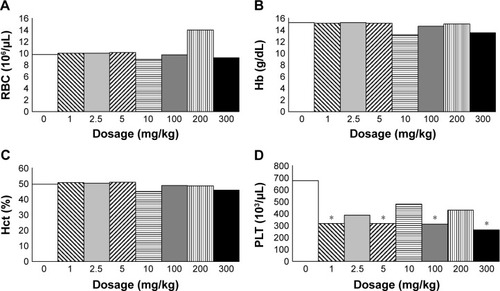
Blood examination was performed 14 days after the mice were exposed to SiNPs. Increase in total white blood cell (WBC) as well as the differentiated WBCs was noted in all groups, except the neutrophils that showed various elevations. Total WBC was increased most in the 200 mg/kg group when compared with the control (), and increased lymphocytes and monocytes were responsible for the result. No obvious difference was noted regarding the red blood cell (RBC) counts, hemoglobin, and hematocrit (). Platelet decreased in all groups and significantly in the 1, 5, 100, and 300 mg/kg groups ().
Table 3 White blood cells and differentiation at 14 days after the injection of SiNPs
Figure 2 Hemogram of RBCs and PLTs.
Notes: Hemogram at 14 days after the injection of SiNPs: (A) RBC count, (B) Hb, (C) Hct, and (D) PLT count. *P<0.05 when compared with control group, 0 mg/kg.
Abbreviations: Hb, hemoglobin; Hct, hematocrit; PLT, platelet; RBC, red blood cell; SiNPs, silica nanoparticles.
Liver and spleen
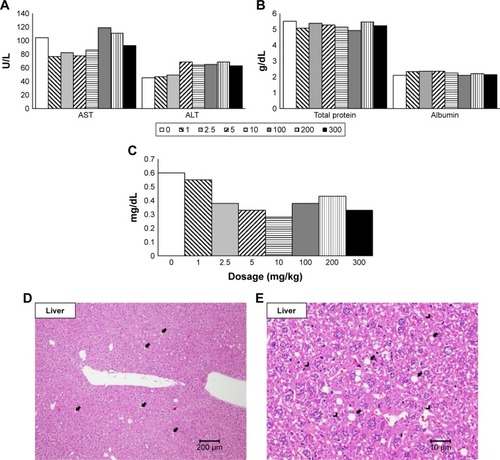
Serum AST and ALT, which represent the liver cell damage,Citation37 were not elevated significantly (). Total bilirubin, total protein, and albumin, which represent the liver function,Citation37 were not different to the control (). These results indicated that SiNPs did not hurt the liver cells. There was no gross pathological lesion seen in the livers and in the spleens. However, in the livers, various degrees of diffuse glycogen infiltration were seen in all groups and minimal-to-mild focal infiltration of fat was seen in all groups except the 200 and 300 mg/kg groups under the microscopic examination, which was thought to be due to nonfasting status before sacrifice ().Citation38 No microscopic abnormality was seen in the spleens.
Figure 3 Blood biochemistry test and histopathological analysis of liver.
Notes: Serum biochemical parameters indicating liver cell damage with (A) AST and ALT, liver function with (B) total protein and albumin and (C) total bilirubin at 14 days after the injection of SiNPs. Histopathological analysis with H&E staining of liver at (D) 20× and (E) 400× magnifications with mild focal fat deposition (arrow) and mild glycogen deposition (arrow head).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; SiNPs, silica nanoparticles.
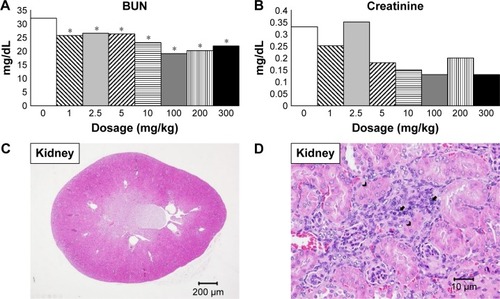
Kidneys
BUN and creatinine were not increased after the injection of SiNPs, indicating no kidney dysfunction ().Citation37 No gross lesion was seen within the kidneys. Microscopically, minimal to mild focal infiltration of mononuclear cells as well as focal tubular regeneration was seen in all groups except the 200 mg/kg group ().
Figure 4 Blood biochemistry test and histopathological analysis of kidney.
Notes: Serum biochemical parameters indicating kidney function with (A) BUN and (B) creatinine. BUN was significantly lower in all treatment groups. Histopathological analysis with H&E staining of kidney at (C) 20× and (D) 400× magnifications with mild focal monocyte infiltration (arrow) and tubular regeneration (arrow head). *P<0.05 when compared with control group, 0 mg/kg.
Abbreviation: BUN, blood urea nitrogen.
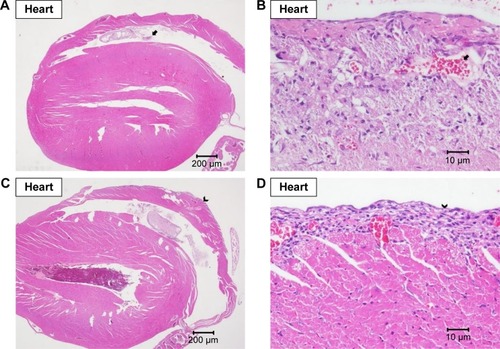
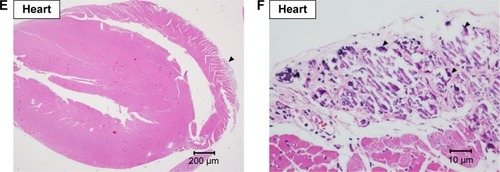
Heart and lungs
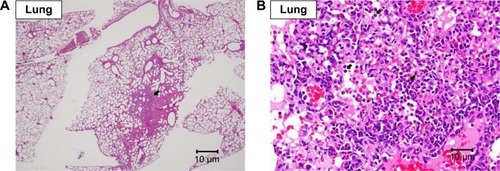
Grossly, no lesions were noted in the hearts and in the lungs. Under the microscopic examination, mild-to-moderate focal fibrosis and mild focal fibrotic embolism were seen in some individual hearts and epicardial mineralization was seen in all groups but not in the 10 and 200 mg/kg groups (). No pulmonary lesion was seen in all mice except one mouse in the 1 mg/kg group showing moderate focal inflammation in lungs ().
Figure 5 Histopathological analysis of heart.
Note: Histopathological analysis with H&E staining of heart at (A) 20× and (B) 400× magnifications with focal fibrotic emboli (arrow), at (C) 20× and (D) 400× magnifications with focal fibrosis (arrow head), and at (E) 20× and (F) 400× magnifications with epicardial mineral deposition (triangle).
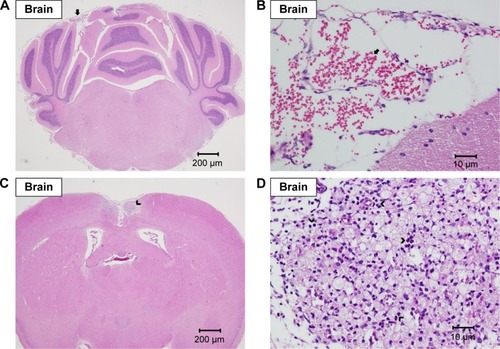
Brain
No gross lesion was seen in the brain. Mild-to-moderate focal hemorrhage was seen in some brains of all groups, which was thought to be resulted due to the method of their sacrifices ().Citation39 One mouse in the 100 mg/kg group showed moderate granuloma in cerebral cortex ().
Figure 7 Histopathological analysis of brain.
Note: Histopathological analysis with H&E staining of brain showing focal hemorrhage in the cortex of cerebellum (arrow) at (A) 20× and (B) 400× magnifications and focal granuloma in the cortex of the cerebrum (arrow head) at (C) 20× and (D) 400× magnifications.
These lesions found in the hearts, lungs, and kidneys were related to aging rather than to adverse effects from SiNPs. The incidence of the microscopic lesions in the organs is listed in .
Table 4 The case number of histopathological lesions of various organs in each group
Discussion
According to the literature, SiNPs were considered safe and biocompatible.Citation8,Citation31,Citation32,Citation40 The lethal dose 50 (LD50) of single injected 64 and 110 nm SiNPs that have been demonstrated in ICR mice were 262.45±33.78 and >1,000 mg/kg, respectively.Citation33,Citation34 No mice died in our study received a single injection of 150 nm SiNPs up to 300 mg/kg. Acute toxicity, even death, was encountered with very large amount of application of SiNPs, easier in those with a single dose with larger amount.Citation33 In addition, smaller nanoparticles caused death easier than larger nanoparticles.
According to the result from the in vitro test that lysis of mice erythrocyte, hemolysis, occurred while those cells were incubated with SiNPs, the authors supposed that SiNPs might lead to anemia when administered in vivo.Citation41 Such condition was not observed in our study as there was no obvious change with the data about RBCs. Platelet aggregation was demonstrated by Corbalan et alCitation42 applying SiNPs to isolated human platelets, and they also stated that the effects on platelet aggregation were inversely proportional to the nanoparticle size. The effect of SiNPs on platelet aggregation was noted to be induced through the thromboxane A2-mediated and matrix metalloproteinase-2-mediated pathways.Citation43 In our study, platelet count decreased in those mice received SiNPs, which was supposed to be compatible with the aggregation of platelets. In a cell model utilizing mice platelets, larger dose of SiNPs aggregated more platelets,Citation44 but dosing effect of the SiNPs on platelet aggregation was not observed in current experiment. The discrepancy may be due to the difference of in vitro and in vivo, as SiNPs contribute to system organs following injection in the blood stream, resulting in a loss of the nanoparticles from the circulation.Citation32 Further studies should be performed to understand if both conditions, erythrocyte hemolysis and platelet aggregation, may be caused by the administration of SiNPs. SiNPs of 70 nm had been demonstrated to induce coagulopathy as well as fatality in Balb/C mice but not larger sizes, including 100, 300, and 1,000 nm;Citation45 no such disaster was seen in our mice that received nanoparticle size of 150 nm.
Several studies demonstrated that injected SiNPs accumulated mainly in liver and spleen, accounting ~75% of the injected 20–25 nm multimodal organically modified silica (ORMOSIL) nanoparticles Citation31 or 80% of the injected rod-shaped mesoporous SiNPs.Citation29 Most of these nanoparticles were excreted via the hepatobiliary system, and ~100% of the injected ORMOSIL taken up by liver was cleared out in 15 days.Citation31 However, ~38%–42% of these SiNPs (20 or 80 nm) accumulated in liver and 30%–37% of that accumulated in spleen remained after 30 days followed by a single injection to ICR mice.Citation32 It is reasonable to consider that SiNPs may damage the liver and spleen when given intravenously. Inflammatory cell infiltration was found in the liver tissue of ratsCitation46 and miceCitation32 when these animals were injected 15 and 20 nm and 80 nm SiNPs, respectively. After injection into the blood stream, these substances may diffuse through the endothelial wall of capillaries into tissues or may be blocked by the endothelial barrier. Other than larger molecules, nanoparticles passed through the gaps between the cells of endothelium.Citation47 Small NPs passed through more easily than large NPs and, thus, accumulated more in certain tissues.Citation48 In liver, hepatocytes did not uptake SiNPs, but the Kupffer cells, macrophage in the liver, endocytosed the nanoparticles.Citation32 Kupffer cells were activated, and the released cytokines induced inflammatory responses in liver.Citation32,Citation46 However, most of the in vivo studies showed no or minimal toxicity to these two organs,Citation2 as in our current study.
Most of the in vivo studies investigating the biodistribution of SiNPs found little renal accumulation of such particles.Citation29,Citation31,Citation49 It is because kidney is not a reticuloendothelial system (RES) organ, but excretion via kidney was an important way for the clearance of injected SiNPs from the body.Citation29,Citation49 Borak et alCitation50 reported that 36% of the introduced amount would be excreted from urine in rats administered with 150 nm SiNPs. Another report by He et alCitation49 stated that most of the mesoporous SiNPs were excreted in 30 minutes after an intravenous injection to ICR mice, then the excretion rate lowered gradually, and a total of up to 54% of these SiNPs were excreted in urine after 30 days. Usually, there was no or minimal damage to kidney when SiNPs were administered in vivo.Citation40,Citation49,Citation51
It had been reported that SiNPs induced cardiotoxicity to zebrafish embryo because these particles inhibited angiogenesis and disturbed heart formation and development;Citation52 neutrophil-mediated cardiac inflammationCitation53 was assumed to be the cause. However, no definite cardiac injury was noted in the studiesCitation40,Citation49,Citation51 of mature mice. Studies investigating the adverse effects about inhalation or intratracheal instillation with SiNPs had been performed, and pulmonary inflammation was common.Citation27,Citation54,Citation55 As the lung is an RES organ, SiNPs may be endocytosed by the pulmonary macrophages and accumulation of SiNPs in lungs was noted in oral,Citation56 inhaled, and intravenousCitation32 administrations. In contrast, least complication was found in those mice receiving SiNPs intravascularly.Citation31,Citation32
SiNPs were shown to be able to pass through the blood–brain barrier of mice when injected into the carotid arteryCitation57 or instillated into the nasal cavities,Citation58 and inflammation of the brain was proposed in these studies. However, when administered intravenously, only scanty amount of SiNPs was deposited in mice brainCitation59 and no studies reported brain damage in the tested animals.Citation31,Citation51
All the histopathological abnormal findings in our study were nonspecific, and the incidence did not correlate to the dosage of the SiNPs administered proportionally.
Conclusion
No definite toxic effect was noted in our study. The lesions seen in those vital organs were not contributed by SiNPs injected because those lesions might also be seen in mice received no nanoparticles in this study and were not closely related to the given amount of nanoparticles. We concluded that SiNPs of 150 nm diameter were safe to Balb/C mice when administered intravascularly.
Acknowledgments
The authors acknowledge funding supported by the National Taipei University of Technology (grant no NTUT-MMH-102-10), MacKay Memorial Hospital (grant nos MMH-TT-102-10 and MMH-TT-103-08), and MacKay Memorial Hospital Hsinchu Branch (grant no MMH-E-102). They also thank the Research Center for Animal Medicine, National Chung Hsing University, for their work on the histopathological study of the mice.
Disclosure
The authors report no conflicts of interest in this work.
References
- MaJFPlant root responses to three abundant soil minerals: silicon, aluminum and ironCRC Crit Rev Plant Sci2005244267281
- JaganathanHGodinBBiocompatibility assessment of Si-based nano-and micro-particlesAdv Drug Deliv Rev201264151800181922634160
- Van DyckKVan CauwenberghRRobberechtHDeelstraHBioavailability of silicon from food and food supplementsFresenius J Anal Chem19993635–6541544
- BraleySThe chemistry and properties of the medical-grade siliconesJ Macromol Sci Chem197043529544
- LührsA-KGeurtsenWThe application of silicon and silicates in dentistry: a reviewMüllerWEGGrachevMABiosilica in Evolution, Morphogenesis, and NanobiotechnologyHeidelbergSpringer2009359380
- HalasNJNanoscience under glass: the versatile chemistry of silica nanostructuresACS Nano20082217918319206616
- AngelosSLiongMChoiEZinkJIMesoporous silicate materials as substrates for molecular machines and drug deliveryChem Eng J20081371413
- BitarAAhmadNMFessiHElaissariASilica-based nanoparticles for biomedical applicationsDrug Discov Today20121719–201147115422772028
- TalluryPPaytonKSantraSSilica-based multimodal/multifunctional nanoparticles for bioimaging and biosensing applicationsNanomedicine (Lond)20083457959218694319
- CollCMondragonLMartinez-ManezREnzyme-mediated controlled release systems by anchoring peptide sequences on mesoporous silica supportsAngew Chem Int Ed Engl20115092138214021344569
- Vivero-EscotoJLSlowingIITrewynBGLinVSMesoporous silica nanoparticles for intracellular controlled drug deliverySmall20106181952196720690133
- ChuZHuangYTaoQLiQCellular uptake, evolution, and excretion of silica nanoparticles in human cellsNanoscale2011383291329921743927
- ChopraIThe increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern?J Antimicrob Chemother200759458759017307768
- Paddle-LedinekJENasaZClelandHJEffect of different wound dressings on cell viability and proliferationPlast Reconstr Surg20061177 suppl110S118S discussion 9S–20S16799377
- AhamedMAlsalhiMSSiddiquiMKSilver nanoparticle applications and human healthClin Chim Acta201041123–241841184820719239
- LiuAYeBApplication of gold nanoparticles in biomedical researches and diagnosisClin Lab2013591–2233623505903
- SopjaniMFollerMLangFGold stimulates Ca2+ entry into and subsequent suicidal death of erythrocytesToxicology20082442–327127918207621
- ZhangXDWuDShenXSize-dependent in vivo toxicity of PEG-coated gold nanoparticlesInt J Nanomedicine201162071208121976982
- ShrivastavaRKushwahaPBhutiaYCFloraSOxidative stress following exposure to silver and gold nanoparticles in miceToxicol Ind Health20163281391140425548373
- FragaSBrandaoASoaresMEShort- and long-term distribution and toxicity of gold nanoparticles in the rat after a single-dose intravenous administrationNanomedicine20141081757176624941462
- PanYNeussSLeifertASize-dependent cytotoxicity of gold nanoparticlesSmall20073111941194917963284
- WilczewskaAZNiemirowiczKMarkiewiczKHCarHNanoparticles as drug delivery systemsPharmacol Rep20126451020103723238461
- KusakaTNakayamaMNakamuraKIshimiyaMFurusawaEOgasawaraKEffect of silica particle size on macrophage inflammatory responsesPLoS One201493e9263424681489
- SohaebuddinSKThevenotPTBakerDEatonJWTangLNanomaterial cytotoxicity is composition, size, and cell type dependentPart Fibre Toxicol201072220727197
- SergentJAPagetVChevillardSToxicity and genotoxicity of nano-SiO2 on human epithelial intestinal HT-29 cell lineAnn Occup Hyg201256562263022378843
- ChenZMengHXingGAge-related differences in pulmonary and cardiovascular responses to SiO2 nanoparticle inhalation: nanotoxicity has susceptible populationEnviron Sci Technol200842238985899219192829
- LinZMaLZGXZhangHLinBA comparative study of lung toxicity in rats induced by three types of nanomaterialsNanoscale Res Lett20138152124321467
- LiYSunLJinMSize-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cellsToxicol In Vitro20112571343135221575712
- HuangXLiLLiuTThe shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivoACS Nano2011575390539921634407
- LuXTianYZhaoQJinTXiaoSFanXIntegrated metabonomics analysis of the size-response relationship of silica nanoparticles-induced toxicity in miceNanotechnology201122505510121178262
- KumarRRoyIOhulchanskkyTYIn vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticlesACS Nano20104269970820088598
- XieGSunJZhongGShiLZhangDBiodistribution and toxicity of intravenously administered silica nanoparticles in miceArch Toxicol201084318319019936708
- LiuTLiLTengXSingle and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed miceBiomaterials20113261657166821093905
- YuYLiYWangWAcute toxicity of amorphous silica nanoparticles in intravenously exposed ICR micePLoS One201384e6134623593469
- PhamTJacksonJBHalasNJLeeTRPreparation and characterization of gold nanoshells coated with self-assembled monolayersLangmuir2002181249154920
- SainiAMaurerTLorenzoIISynthesis and SERS application of SiO2@Au NanoparticlesPlasmonics2015104791796
- BishopMLFodyEPSchoeffLEClinical Chemistry: Principles, Techniques, and CorrelationsPhiladelphia, PAWolters Kluwer Health2013
- ThoolenBMaronpotRRHaradaTProliferative and nonproliferative lesions of the rat and mouse hepatobiliary systemToxicol Pathol2010387 suppl5s81s21191096
- LiuCHAn Atlas and Manual of Histopathological Staining Methods: HistochemistryMaoli, TaiwanPig Research Institute Taiwan1996
- TanakaTGodinBBhavaneRIn vivo evaluation of safety of nanoporous silicon carriers following single and multiple dose intravenous administrations in miceInt J Pharm20104021–219019720883755
- NemmarABeegamSYuvarajuPYasinJShahinAAliBHInteraction of amorphous silica nanoparticles with erythrocytes in vitro: role of oxidative stressCell Physiol Biochem201434225526525033832
- CorbalanJJMedinaCJacobyAMalinskiTRadomskiMWAmorphous silica nanoparticles aggregate human platelets: potential implications for vascular homeostasisInt J Nanomedicine2012763163922334785
- Santos-MartinezMJTomaszewskiKAMedinaCBazouDGilmerJFRadomskiMWPharmacological characterization of nanoparticle- induced platelet microaggregation using quartz crystal microbalance with dissipation: comparison with light aggregometryInt J Nanomedicine2015105107511926316743
- NemmarAYuvarajuPBeegamSIn vitro platelet aggregation and oxidative stress caused by amorphous silica nanoparticlesInt J Physiol Pathophysiol Pharmacol201571273326069526
- NabeshiHYoshikawaTMatsuyamaKAmorphous nanosilicas induce consumptive coagulopathy after systemic exposureNanotechnology201223404510122214761
- ChenQXueYSunJKupffer cell-mediated hepatic injury induced by silica nanoparticles in vitro and in vivoInt J Nanomedicine201381129114023515466
- LiSDHuangLPharmacokinetics and biodistribution of nanoparticlesMol Pharm20085449650418611037
- TorchilinVPLukyanovANGaoZPapahadjopoulos-SternbergBImmunomicelles: targeted pharmaceutical carriers for poorly soluble drugsProc Natl Acad Sci U S A2003100106039604412716967
- HeQZhangZGaoFLiYShiJIn vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylationSmall20117227128021213393
- BorakBBiernatPPreschaABaszczukAPlutaJIn vivo study on the biodistribution of silica particles in the bodies of ratsAdv Clin Exp Med2012211131823214294
- NishimoriHKondohMIsodaKTsunodaSTsutsumiYYagiKHistological analysis of 70-nm silica particles-induced chronic toxicity in miceEur J Pharm Biopharm200972362662919341796
- DuanJYuYLiYYuYSunZCardiovascular toxicity evaluation of silica nanoparticles in endothelial cells and zebrafish modelBiomaterials201334235853586223663927
- DuanJYuYLiYLow-dose exposure of silica nanoparticles induces cardiac dysfunction via neutrophil-mediated inflammation and cardiac contraction in zebrafish embryosNanotoxicology201610557558526551753
- KaewamatawongTKawamuraNOkajimaMSawadaMMoritaTShimadaAAcute pulmonary toxicity caused by exposure to colloidal silica: particle size dependent pathological changes in miceToxicol Pathol200533774374916306027
- ChoWSChoiMHanBSInflammatory mediators induced by intratracheal instillation of ultrafine amorphous silica particlesToxicol Lett20071751–3243317981407
- LeeJAKimMKPaekHJTissue distribution and excretion kinetics of orally administered silica nanoparticles in ratsInt J Nanomedicine20149suppl 225126025565843
- LiuDLinBShaoWZhuZJiTYangCIn vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood–brain barrierACS Appl Mater Interfaces2014632131213624417514
- WuJWangCSunJXueYNeurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathwaysACS Nano2011564476448921526751
- DecuzziPGodinBTanakaTSize and shape effects in the biodistribution of intravascularly injected particlesJ Control Release2010141332032719874859