?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and objective
Radiation therapy (RT) is the gold standard treatment for more than half of known tumors. Despite recent improvements in RT efficiency, the side effects of ionizing radiation (IR) in normal tissues are a dose-limiting factor that restricts higher doses in tumor treatment. One approach to enhance the efficiency of RT is the application of radiosensitizers to selectively increase the dose at the tumor site. Gold nanoparticles (GNPs) and electroporation (EP) have shown good potential as radiosensitizers for RT. This study aims to investigate the sensitizing effects of EP, GNPs, and combined GNPs-EP on the dose enhancement factor (DEF) for 6 MV photon energy.
Methods
Radiosensitizing effects of EP, GNPs, and combinations of GNPs-EP were comparatively investigated in vitro for intestinal colon cancer (HT-29) and Chinese hamster ovary (CHO) cell lines by MTT assay and colony formation assay at 6 MV photon energy in six groups: IR (control group), GNPs+IR, GNPs (24 h)+IR, EP+IR, GNPs+EP+IR, and GNPs (24 h)+EP+IR.
Results
Treatment of both cell lines with EP, GNPs, and combined GNPs-EP significantly enhanced the response of cells to irradiation. However, the HT-29 showed higher DEF values for all groups. In addition, the DEF value for HT-29 cells for GNPs+IR, GNPs (24 h)+IR, EP+IR, GNPs+EP+IR, and GNPs (24 h)+EP+IR was, respectively, 1.17, 1.47, 1.36, 2.61, and 2.89, indicating synergistic radiosensitizing effect for the GNPs (24 h)+EP+IR group. Furthermore, the synergistic effect was observed just for HT-29 tumor cell lines.
Conclusion
Combined GNPs-EP protocols induced synergistic radiosensitizing effect in HT-29 cells, and the effect is also tumor specific. This combined therapy can be beneficially used for the treatment of intrinsically less radiosensitive tumors.
Introduction
Radiation therapy is the gold standard treatment option for more than half of cancer patients due to its ability to kill malignant cells and shrink tumors.Citation1 It prevents tumor cell growth through bombardment of the tumor with ionizing radiation (IR). IR induces DNA damage by direct or indirect action through the generation of reactive oxygen species (ROS).Citation2 Unfortunately, there is no discrimination between normal and malignant tissues in absorption of IRs, and thus doses of radiation must be limited to spare healthy surrounding tissue.Citation3 One approach to increase discrimination between tumors and healthy tissues and thereby increasing the efficiency of radiation therapy is the use of radiosensitizer to preferably enhance dose at the site of tumor.Citation4–Citation6 In recent years, various radiosensitizers have been developed to increase the outcome of radiation therapy. Material with high atomic number (Z) such as gold nanoparticles (GNPs)Citation7,Citation8 and physical approaches such as electroporation (EP)Citation9,Citation10 are two important examples to achieve this goal.
GNPs have been previously shown to improve the effect of radiotherapy in vitroCitation11,Citation12 and in vivo.Citation13,Citation14 GNPs have several characteristics that make them attractive for using with radiation therapy including small size (1–100 nm), biocompatibility, preferential passive accumulation in tumor, and the feasibility of surface modification to actively target cancerous cells.Citation15–Citation18 GNPs with high Z increase the cross section of photoelectric absorption and pair production interaction in the keV and MeV energy range, respectively, and thus enhance the delivering dose to the target tumor.Citation19,Citation20 Moreover, the interaction of X-ray with GNPs can release free radicals, thereby damaging DNA. Furthermore, GNPs have the ability to enter the mitochondria and induce apoptotic death.Citation21
EP is a physical process through which applying short intense electric pulses increases cell membrane permeability.Citation22–Citation24 In normal physiological conditions, the electric conductivity of cytoplasm and extracellular medium is much higher than the conductivity of the cell membrane. Thus, when an external electric field is applied to this lipid membrane, the anode-facing and cathode-facing side become hyperpolarized and depolarized, respectively, and a transmembrane potential is induced on the exposed cell.Citation25,Citation26
EquationEquation (1)(1) is generally used to describe this induced transmembrane potential:
where Vm denotes transmembrane potential, f a factor that describes the effect of the cell on the extracellular field distribution, Eext the external electric field, r the radius of cell and ∅ is the polar angle with respect to the external field. If ∆Vm is larger than a critical value (0.2–1.0 V), the EP is occurred, and the nanoscale pores are appeared in membrane.Citation27,Citation28 This phenomenon depends on pulse parameters such as amplitude, pulse frequency, pulse duration and number of pulse, and also on experimental conditions, for example, osmotic pressure, temperature, and conductivity of EP buffer, etc.Citation27,Citation29 If these electric parameters are chosen properly, the process of EP is reversible,Citation30 and upon further increase of the electric parameters, the EP phenomenon becomes irreversible, which kills the cells.Citation31,Citation32 EP is routinely employed to transport nonpermeant molecules such as DNA, dyes, proteins, and chemotherapeutic drugs into the cell.Citation33–Citation35 However, it has been demonstrated that EP can induce oxidative jump and generate ROS.Citation36 Gabriel and TeissieCitation37 have reported that the generation of ROS is not homogenous and restricted to the electropermeabilized side of the cell membrane. Therefore, this technique can be combined with IR as a radiosensitizer to enhance the outcome of radiation therapy. WestCitation10 demonstrated that use of EP prior to irradiation can enhance the effect of irradiation by factor of 1.19.
Previous studies have demonstrated that EP and GNPs have radiosensitizing effects. However, we could not find any study investigating concurrent application of EP and GNPs to increase the sensitivity of cells to IR. Therefore, the present study was designed aiming to investigate the effects of EP and GNPs alone and in combination to increase the efficiency of radiation therapy. We hypothesized that the combination of EP and GNPs would induce synergistic radiosensitizing effect because of the following reasons:
EP can increase the uptake of GNPs by cells.
The conductivity of EP buffer is increased using GNPs, and this decreases the electric voltage consumed by the EP buffer, thereby improving the efficiency of EP.Citation38
GNPs act as microelectrodesCitation38 and the electropermeabilization of membrane and thereby generation of ROS occurs on different sites of membrane.
Materials and methods
Cell culture
Colorectal cancer (HT-29) and Chinese hamster ovary (CHO) cell lines were purchased from National Cell Bank of Pasteur Institute of Iran (NCBI, C466 and C111) and grown as monolayers in Roswell Park Memorial Institute (RPMI) 1640 medium (BIO-IDEA, B11031, Tehran, Iran) enriched with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (BIO-IDEA). The cells were routinely sub-cultured twice a week and maintained at 37°C in a humidified atmosphere with 5% CO2 in an incubator (RS Biotech Galaxy R, West Lothian, UK).
GNPs characterization
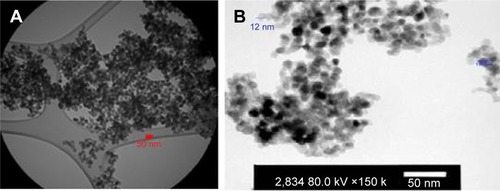
GNPs (99.95+%, 15 nm) were purchased from US Research Nanomaterials, Inc. (Houston, TX, USA). To prepare stock solution, the nanoparticles were suspended in deionized water, and other dilutions were performed in culture media immediately before use. The size and morphology of GNPs were estimated by transmission electron microscope (TEM) and scanning electron microscope. According to these images, GNPs were spherical, and the average size was 12–15 nm in diameter (). We used GNPs at concentration of 0.1 mM.
Study protocol
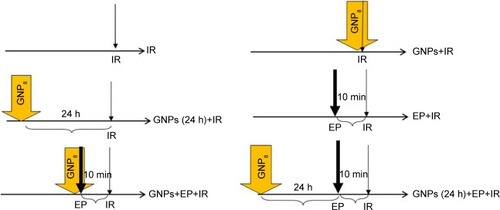
To evaluate the radiosensitizing effect of GNPs, EP, and combinations of GNPs and EP, six different experimental groups were designed in this study (): irradiation alone as a control group (IR), cells treated with GNPs immediately before irradiation (GNPs+IR), cells incubated with GNPs 24 h prior to irradiation (GNPs [24 h]+IR), cells exposed electric pulse 10 min before irradiation (EP+IR), cells treated with both GNPs and EP 10 min before irradiation (GNPs+EP+IR), and cells incubated with GNPs for 24 h and then receiving EP 10 min prior to irradiation (GNPs [24 h]+EP+IR). Different combinations of EP-GNPs with respect to time intervals between each modality were designed to study some important hypotheses on the mechanisms of actions of each modality.
Electroporation set up
The sample solution was dispensed into a 1 MM gap cuvette, and a single square pulse with voltage to distance ratio of 1,200 V/cm and 100 µs duration was delivered using a Bio-Rad Gene Pulser Xcell™ (Hercules, CA, USA) EP system at room temperature (21°C). Immediately after EP, the suspension was transferred either to 6-well or 96-well plate based on the assay, and then fresh culture medium was added to the cells. Finally, the plate was irradiated with ionization radiation after 10 min.
Irradiation setup
The cells were irradiated with megavoltage X-ray (6 MV) using Varian 2100 C/D linear accelerator (Golestan Hospital, Ahvaz, Iran) at a dose rate of 3 Gy/min with a field size of 20×20 cm2. We used a Plexiglass (water equivalent) sheet with 1.5 cm thickness on top the plate (6-well in clonogenic cell survival assay and 96-well plate in MTT assay) as a build up to produce transient charged particle equilibrium. Moreover, another Plexiglass sheet with the thickness of 6 cm was placed under the bottom of plate to sufficient production of backscatter.
Evaluation of radiosensitizing effect of GNPs, EP, and GNPs-EP
MTT assay
The cellular response to each treatment at radiation doses of 2 and 4 Gy was assessed using MTT assay kit according to the manufacturer’s protocol (Bio-Idea). In this colorimetric method, the mitochondrial dehydrogenase activity of proliferating cells reduces the MTT salt into purple MTT formazan crystals. After incubation of 104 treated cells/well for 24 h, 10 µL of MTT solution (5 mg/mL) was added to each well and incubated for additional 4 h. The MTT was reduced to blue formazan crystals due to mitochondrial dehydrogenase activity of living cells. At the end of the incubation time, formazan crystals were dissolved by adding 50 µL dimethyl sulfoxide (DMSO) and the plate was placed on the orbital shaker for 20 min. Finally, optical density (OD) at 570-nm wavelength was measured using a spectrophotometer (Bio-Rad, Model 680). The viability rate (%) of cells in different groups was calculated by the following formula: viability rate = (average OD570 nm of treated group/average OD570 nm of the control group) ×100%.
Clonogenic cell survival assay
We used “plating before treatment” protocol to perform clonogenic cell survival assay.Citation39 In this regard, the cells were washed twice with phosphate-buffered saline (PBS), trypsinized, centrifuged, and then counted. A known numbers of cells (100, 200, 400, 1,000, and 2,000 cells, respectively for irradiation dose of 0, 2, 4, 6, and 8 Gy)Citation40 were treated with predefined treatment protocol (). The treated cells were incubated for 14 days to allow them to form large colonies. Then, the cells were fixed and stained with 0.4% crystal violet, and the visible colonies with more than 50 cells were counted. The plating efficiency (PE) was determined at IR dose of 0 Gy by the following formula: colony number/plating cell number. The survival fraction (SF) of treatment groups was calculated using the equation: SF = colony number/(plating cell number × PE). The data were fit to linear quadratic model with the equation of SF = exp (−αD−βD2), and survival curve was estimated using MATLAB software. The dose enhancement factor (DEF) was calculated by dividing of LD50 (50% lethal dose) of irradiation alone group with the combined treatment group.
Statistical analysis
All experiments were performed in replicates of three, and results were reported as mean ± standard error of mean (SEM). To evaluate the cytotoxicity of GNPs, the difference between untreated control group and those treated with GNPs was assessed by paired t-test. In addition, one-way analysis of variance (ANOVA) was used to compare the differences between the treatment groups. The α and β parameters of survival curve were calculated with weighted least square regression and SigmaStat statistical software (SPSS Inc., Chicago, IL, USA). In all experiments, the statistical significance was set at P≤0.05.
Results
The cytotoxicity of GNPs
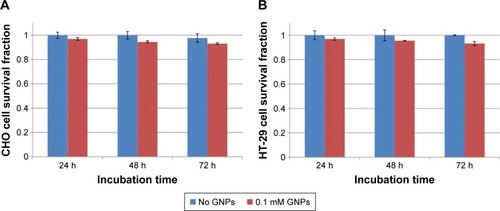
The cytotoxicity of GNPs at a concentration of 0.1 mM on HT-29 and CHO cells was assessed by MTT assay. The SFs of cells after incubation with GNPs for 24, 48, and 72 h are presented in . The analysis of data was performed with paired t-test and showed no significant cytotoxicity effect on both cell lines (P>0.05).
Figure 3 (A) Survival fraction of HT-29 cells incubated with GNPs at a concentration of 0.1 mM for 24, 48, and 72 h compared to nontreated group. None of the groups were statistically significant (P>0.05). (B) Survival fraction of CHO cells incubated with GNPs at concentration of 0.1 mM for 24, 48, and 72 h compared to nontreated group. None of the groups were statistically significant (P>0.05).
Abbreviations: CHO, Chinese hamster ovary; GNPs, gold nanoparticles.
Radiosensitivity of GNPs, EP, and GNPs-EP
Clonogenic cell survival assay
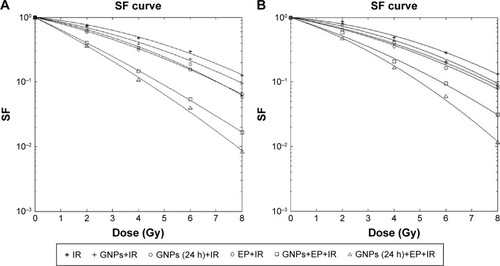
shows the survival curves of HT-29 tumor cells and CHO normal cells that were treated in different groups. In the control group, treatment of HT-29 and CHO cells with irradiation only resulted in LD50 of 3.97 Gy and 4.19 Gy, respectively (). Adding GNPs to culture medium of cells immediately before irradiation decreased the LD50 to 3.37 Gy in HT-29 and 3.63 Gy in CHO cells. In this group, GNPs could enhance treatment response by factor of 1.17 and 1.15 in HT-29 and CHO cell lines, respectively. For HT-29 cell line, the LD50 and DEF values, respectively, reached to 2.69 Gy and 1.47 in the group treated with GNPs for 24 h before IR. The corresponding values in the CHO cell lines were, respectively, 3.03 Gy and 1.38. For both cell lines, the LD50 values were significantly lower than the control group (P<0.05). When the cells were exposed to electric pulse prior to irradiation, LD50 of 2.92 Gy and DEF of 1.36 for HT-29 cells (P<0.05) and of 3.27 Gy and 1.28 for CHO cells (P<0.05) were observed. In the GNPs+EP+IR group, treatment of cells with GNPs and EP simultaneously 10 min before irradiation significantly decreased the LD50 values in both cell lines (P<0.05). For this treatment protocol, the DEF was 2.61 in HT-29 and 1.92 in CHO cell lines. In the GNPs (24 h)+EP+IR group, when the 24 h incubated HT-29 cells with GNPs were exposed to electric pulse 10 min prior to irradiation, the LD50 was further reduced to 1.37 Gy in HT-29 cells and to 1.85 Gy in CHO cell lines. Furthermore, in this group, the highest DEF was achieved (2.89 for HT-29 and 2.26 for CHO cell lines). Moreover, the DEF values demonstrated that synergistic effect was only observed in the HT-29 cells ().
Table 1 Values of α, β, LD50, DEF, and R2 values of HT-29 and CHO cells in different groups
Figure 4 (A) Radiation survival curves of cancerous HT-29 cells treated with six different protocols based on clonogenic assay. (B) Radiation survival curves of CHO cells treated with six different protocols based on clonogenic assay.
Abbreviations: IR, ionizing radiation; GNPs, gold nanoparticles; EP, electroporation; SF, survival fraction.
MTT assay
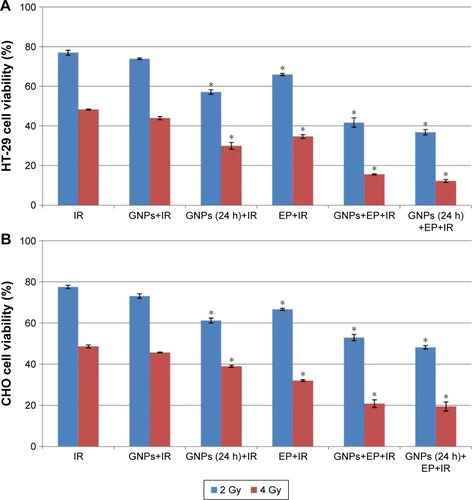
The viability of HT-29 and CHO cells in six different treatment groups was evaluated by MTT assay 24 h after irradiation with 6 MV X-ray at doses of 2 Gy and 4 Gy (). In the control group (IR), exposing the cells by 2 Gy irradiation alone yielded a viability of 76.94%± 1.2% and 77.54%±0.8% for HT-29 and CHO cell lines, respectively. The treatment of HT-29 and CHO cell lines with 4 Gy irradiation alone reduced the viability to 48.3%±0.18% and 48.67%±0.72%, respectively. No significant reduction in viability was observed when the both cells were received GNPs immediately prior to irradiation (P>0.05). The viability of HT-29 cells that were exposed to irradiation after 24-h incubation with GNPs reached 57.1%±1.1% for 2 Gy and 29.9%6±1.7% for 4 Gy irradiation (P<0.05). The treatment of CHO cells with the same protocol resulted in a viability rate of 61.19%±1.2% and 38.95%±0.5% for 2 Gy and 4 Gy irradiation (P<0.05), respectively. By delivering the electric pulse 10 min before IR, the viability rate of 65.91%±0.55% at a dose of 2 Gy and 34.71%±0.93% at a dose of 4 Gy was obtained for HT-29 cells. Almost equal values were observed for CHO cell lines. Indeed, EP could significantly enhance response of HT-29 and CHO cells to irradiation (P<0.05). In the GNPs+EP+IR group, application of both GNPs and EP 10 min before irradiation resulted in a higher radiation response of cells. After delivering of 4 Gy irradiation, only 15.61%±0.26% of HT-29 cells and 52.95%±1.5% of CHO cells were viable (P<0.05). The lowest viability in both radiation doses of 2 Gy (36.80%±1.35% for HT-29 cells and 48.19%±0.84% for CHO cells) and 4 Gy (12.29%±0.72% for HT-29 cells and 19.45%±2.19% for CHO cells) was achieved in GNPs (24 h)+EP+IR group (P<0.05). The results of these tests revealed that concurrent applications of GNPs and EP as radiosensitizer can significantly increase the efficiency of radiation therapy.
Figure 5 (A) The viability of HT-29 cells treated with IR, GNPs+IR, GNPs (24 h)+IR, EP+IR, GNPs+EP+IR, and GNPs (24 h)+EP+IR based on MTT assay. *P<0.05. (B) The viability of CHO cells treated with IR, GNPs+IR, GNPs (24 h)+IR, EP+IR, GNPs+EP+IR, and GNPs (24 h)+EP+IR based on MTT assay. *P<0.05.
Abbreviations: CHO, Chinese hamster ovary; IR, ionizing radiation; GNPs, gold nanoparticles; EP, electroporation.
Discussion
Radiosensitizing effects of GNPs and EP have been extensively investigated in several in vitro and in vivo studies. However, to our knowledge, there is no published study that investigated the radiosensitizing effects of combined GNPs-EP in any healthy or tumor cell lines. The main objective of this study was to investigate synergistic radiosensitizing effect of combined GNPs-EP to 6 MV X-ray photons. In addition, different combinations of GNPs-EP were investigated to shed more light on the mechanisms of actions of possible radiosensitizing effects.
Our results showed that EP alone could sensitize both HT-29 and CHO cell lines to 6 MV photons with a DEF of 1.36 and 1.28, respectively. Consistent with our result, Kranjc et alCitation41 reported a DEF of 1.25 for LPB sarcoma cell line. Moreover, WestCitation10 demonstrated that EP can enhance effect of 137Cs-γ radiation by a factor of 1.19. Generally, the oxidative jump at electroporated sites of membrane and production of ROS are the probable radiosensitization mechanism of EP.Citation37,Citation42 Shil et alCitation43 measured the level of generated ROS induced by EP and reported that the ROS level under the combined EP-irradiation group was significantly higher than irradiation alone group.
In the GNPs+IR group, GNPs did not significantly enhance the effect of IR due to having not enough time for GNPs to enter and accumulate in the target cells. As we know, when irradiation interacts with GNPs, free radicals are produced that further generate ROS. These ROS are very toxic for tumor cells, but have very short diffusion range about 10 nm. Therefore, the accumulation of GNPs in the target cells is essential to induce death in the tumor cells.Citation44 However, in the GNPs+IR group, there was not enough time between treatment of cells with GNPs and IR. However, when EP is added to the protocol of GNPs+IR group (GNPs+EP+IR), DEFs of 2.61 and 1.92 were observed in HT-29 and CHO cells, respectively. The observed difference between DEF values of GNPs+IR and GNPs+EP+IR groups can be attributed to the act of EP as a GNPs delivery system in GNPs+EP+IR group.
Incubation of the cells with GNPs 24 h prior to IR (GNPs (24 h)+IR) resulted in the DEF of 1.47 and 1.38, respectively, for HT-29 and CHO cells. However, these values reached to 2.89 and 2.26 in HT-29 and CHO, respectively, by introducing EP in this protocol 10 min before IR (GNPs (24 h)+EP+IR). In this group, EP showed no delivery system role. Indeed, the GNPs that could not enter to cells during 24 h were eliminated during washing cells with PBS, trypsination, and centrifuging processes prior to EP, and thus this radiosensitivity effect is related to intrinsic radiosensitivity of EP due to ROS generation.
Our findings support the previous studies that have investigated the radiosensitizing effect of GNPs alone. Different mechanisms have been proposed in these studies for radiosensitivity induced by GNPs. The cell cycle regulation or accumulation of DU-145 prostate cancer cells in G2/M phase as a most radiosensitive phase of cell cycle due to activation of CDK kinase was reported by Roa et alCitation45 and Wang et al.Citation40 Wang et alCitation40 demonstrated that Glu-GNPs increased apoptosis by overexpression of Bax and caspase 3 and under-expression of Bcl-2 proteins. Other probable mechanisms are increasing of ROS production and DNA double strand break, and these have been proposed by Geng et alCitation46 and Chithrani et al,Citation7 respectively.
Radiosensitizing effect of GNPs is dependent on three important factors: size, concentration of GNPs, and energy of the ionization radiation source.Citation47 The effects of size and concentration of PEG-coated GNPs have been comprehensively investigated by Zhang et al.Citation48 Their in vitro and in vivo studies revealed that for all sizes of 4.8, 12.1, 27.3, and 46.6 nm, the concentration of 0.1 mM was safe and nontoxic. The strongest radiosensitizing effect with SER of 2.07 was obtained with 12.1 nm GNPs at concentration of 0.1 mM. Similarly, in vivo radiotherapy demonstrated that all sizes of GNPs can enhance the effect of 5 Gy radiation.Citation48 Similarly, we used 12–15 nm GNPs at concentration of 0.1 mM, and the results of MTT assay revealed that this dose of GNPs is safe for all incubation times of 24, 48, and 72 h.
Several studies have been conducted on radiosensitivity effect of GNPs with KV radiations due to a Z4 relationship between photoelectric cross section and atomic number (Z).Citation49,Citation50 However, Jain et alCitation51 comprehensively evaluated the impact of type and energy of radiation (kV and MV photons and MV electrons) on GNPs radiosensitizing. They reported that 1.9 nm GNPs could significantly sensitize MDA-MB-231 breast cancer cells by a factor of 1.41, 1.29, 1.16, and 1.35 using 160 kVp, 6 MV, and 15 MV X-ray photons, and 16 MeV electrons, respectively.Citation51 Furthermore, Wang et alCitation40 reported that treatment of A549 cells with 13 nm Glu-GNPs and 6 MV photons resulted in a DEF of 1.49. According to these results and because of the extensive uses of MV photons to treat deep tumors as well as spare skin of patients in clinic, we decided to use 6 MV photons for irradiation.
In addition, the cell type is other factor that can influence the outcome of treatment with GNPs. During the recent years, the radiosensitizing feature of GNPs has been used to treat several cell lines such as prostate,Citation11 breast,Citation50,Citation51 lung,Citation40 ovarian,Citation46 and colorectalCitation52 cancer cell lines. Arab-Bafrani et alCitation52 observed that the response of HT-29 colorectal cancer cell line to 9 MV photons was increased by a factor of 1.4 using GNPs. Similarly, in our study using only GNPs 24 h prior to irradiation, a DEF of 1.47 was observed. However, the GNPs (24 h)+EP+IR protocol resulted in a DEF of 2.89 in the HT-29 cancer cell line. In addition, the synergistic effect was only observed in the HT-29 cell.
Conclusion
In conclusion, to our knowledge, this study was the first to use both GNPs and EP simultaneously to sensitize cells to 6 MV radiations. The most radiosensitizing effect was achieved with GNPs (24 h)+EP+IR protocol in HT-29 cell with intermediate intrinsic radiosensitivity. Therefore, this protocol has a potential to sensitize less radiosensitive tumor cells, and thus there is need for more in vivo studies to translate this approach into the clinic.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
This study was a part of the MSc thesis registered in the Medical Physics department, School of Medicine, Ahvaz Jundishapur University of Medical Sciences (AJUMS), conducted in the Cellular and Molecular Research Center (CMRC) of AJUMS. Financial support for the study was provided by AJUMS (Grant No: CMRC-9428). The authors thank Mr Sadegh Saremi, the technical expert of the CMRC, for his technical supports during the conducting of this study. In addition, the authors thank Dr Mohammad Javad Tahmasebi Birgani for his help in arranging the using of the irradiation set up.
Disclosure
The authors report no conflicts of interest in this work.
References
- HainfeldJFDilmanianFASlatkinDNSmilowitzHMRadiotherapy enhancement with gold nanoparticlesJ Pharm Pharmacol200860897798518644191
- HallEJGiacciaAJRadiobiology for the RadiologistPhiladelphia, PALippincott Williams & Wilkins2006
- DrouetFLagrangeJNormal tissue tolerance to external beam radiation therapy: bone marrowCancer Radiother2010144–5392404 French20598617
- MatsudairaHUenoAMFurunoIIodine contrast medium sensitizes cultured mammalian cells to X rays but not to γ raysRadiat Res19808411441487454977
- NishiokaAOhizumiYLamGKThe effects of nicotinamide plus carbogen or pions for microscopic SCCVII tumorsOncol Rep1999658358610203595
- ShibamotoYZhouLHattaHMoriMNishimotoSIIn vivo evaluation of a novel antitumor prodrug, 1-(2′-oxopropyl)-5-fluorouracil (OFU001), which releases 5-fluorouracil upon hypoxic irradiationInt J Radiat Oncol Biol Phys200149240741311173134
- ChithraniDBJelvehSJalaliFGold nanoparticles as radiation sensitizers in cancer therapyRadiat Res2010173671972820518651
- HainfeldJFDilmanianFAZhongZSlatkinDNKalef-EzraJASmilowitzHMGold nanoparticles enhance the radiation therapy of a murine squamous cell carcinomaPhys Med Biol20105511304520463371
- ShilPSanghviSHVidyasagarPBMishraKPEnhancement of radiation cytotoxicity in murine cancer cells by electroporation: in vitro and in vivo studiesJ Environ Pathol Toxicol Oncol200524429129816393122
- WestCMA potential pitfall in the use of electroporation: cellular radiosensitization by pulsed high-voltage electric fieldsInt J Radiat Biol19926133293341347065
- ZhangXXingJZChenJEnhanced radiation sensitivity in prostate cancer by gold-nanoparticlesClin Invest Med2008313160167
- JohDYSunLStanglMSelective targeting of brain tumors with gold nanoparticle-induced radiosensitizationPLoS One201384e6242523638079
- HainfeldJFSlatkinDNSmilowitzHMThe use of gold nanoparticles to enhance radiotherapy in miceCancer Res200565Suppl 9287
- AnijdanSHMahdaviSRShiraziAZarrinfardMAHajatiJMegavolt-age X-ray dose enhancement with gold nanoparticles in tumor bearing miceInt J Mol Cell Med20132311812324551801
- ConnorEEMwamukaJGoleAMurphyCJWyattMDGold nanoparticles are taken up by human cells but do not cause acute cytotoxicitySmall20051332532717193451
- MironavaTHadjiargyrouMSimonMJurukovskiVRafailovichMHGold nanoparticles cellular toxicity and recovery: effect of size, concentration and exposure timeNanotoxicology20104112013720795906
- ShuklaRBansalVChaudharyMBasuABhondeRRSastryMBiocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overviewLangmuir20052123106441065416262332
- PanYNeussSLeifertASize-dependent cytotoxicity of gold nanoparticlesSmall20073111941194917963284
- ChoSHEstimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo studyPhys Med Biol20055015N16316030374
- RoeskeJCNuñezLHoggarthMLabayEWeichselbaumRRCharacterization of the theorectical radiation dose enhancement from nanoparticlesTechnol Cancer Res Treat20076539540117877427
- ChangMYShiauALChenYHChangCJChenHHWuCLIncreased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing miceCancer Sci20089971479148418410403
- JordanCANeumannESowersAEElectroporation and Electrofusion in Cell BiologyNew York, NYSpringer Science & Business Media2013
- TsongTYElectroporation of cell membranesBiophys J19916022971912274
- PavlinMLebenVMiklavčičDElectroporation in dense cell suspension – theoretical and experimental analysis of ion diffusion and cell permeabilizationBiochim Biophys Acta200717701122316935427
- WeaverJCMolecular basis for cell membrane electroporationaAnn N Y Acad Sci199472011411528010633
- HaltiwangerSWhy electroporation is a useful technique for cancer treatmentsSundararajanRElectroporation-Based Therapies for Cancer: From Basics to Clinical ApplicationsCambridge, UKElsevier2014103
- MirLMOrlowskiSThe basis of electrochemotherapyJaroszeskiMJHellerRGilbertRElectrochemotherapy, Electrogenetherapy, and Transdermal Drug Delivery: Electrically Mediated Delivery of Molecules to CellsTotowa, NJHumana Press200099117
- YadollahpourARezaeeZElectroporation as a new cancer treatment technique: a review on the mechanisms of actionBiomed Pharmacol J2014715362
- KandušerMMiklavčičDElectroporation in biological cell and tissue: an overviewElectrotechnologies for Extraction from Food Plants and BiomaterialsNew York, NYSpringer2009137
- KinositaKHibinoMItohHEvents of membrane electroporation visualized on a time scale from microsecond to secondsPaper presented at: Elsevier Inc2012
- OnikGMikusPRubinskyBIrreversible electroporation: implications for prostate ablationTechnol Cancer Res Treat20076429530017668936
- RubinskyBIrreversible electroporation in medicineTechnol Cancer Res Treat20076425525917668932
- WideraGAustinMRabussayDIncreased DNA vaccine delivery and immunogenicity by electroporation in vivoJ Immunol200016494635464010779767
- ZhangGHTanXFShenDGene expression and antitumor effect following im electroporation delivery of human interferon 2 geneActa Pharmacol Sin200324989189612956937
- QuaglinoPMorteraCOsella-AbateSElectrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastasesAnn Surgical Oncol200815822152222
- GabrielBTeissieJGeneration of reactive-oxygen species induced by electropermeabilization of Chinese hamster ovary cells and their consequence on cell viabilityEur J Biochem1994223125338033899
- GabrielBTeissieJSpatial compartmentation and time resolution of photooxidation of a cell membrane probe in electropermeabilized Chinese hamster ovary cellsEur J Biochem199522837107187737168
- ZuYHuangSLiaoWCLuYWangSGold nanoparticles enhanced electroporation for mammalian cell transfectionJ Biomed Nanotechnol201410698299224749393
- FrankenNARodermondHMStapJHavemanJVan BreeCClonogenic assay of cells in vitroNat Protoc2006152315231917406473
- WangCLiXWangYLiuZFuLHuLEnhancement of radiation effect and increase of apoptosis in lung cancer cells by thio-glucose-bound gold nanoparticles at megavoltage radiation energiesJ Nanoparticle Res2013155112
- KranjcSCemazarMGroselASentjurcMSersaGRadiosensitising effect of electrochemotherapy with bleomycin in LPB sarcoma cells and tumors in miceBMC Cancer200551115631637
- BonnafousPVernhesMCTeissiéJGabrielBThe generation of reactive-oxygen species associated with long-lasting pulse-induced electropermeabilisation of mammalian cells is based on a non-destructive alteration of the plasma membraneBiochim Biophys Acta19991461112313410556494
- ShilPSanghviSHVidyasagarPBMishraKPEnhancement of radiation cytotoxicity in murine cancer cells by electroporation: In vitro and in vivo studiesJ Environ Pathol Toxicol Oncol200524429129816393122
- SongKXuPMengYSmart gold nanoparticles enhance killing effect on cancer cellsInt J Oncol201342259760823229536
- RoaWZhangXGuoLGold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycleNanotechnology2009203737510119706948
- GengFSongKXingJZThio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancerNanotechnology2011222828510121654036
- BrunESancheLSicard-RoselliCParameters governing gold nanoparticle X-ray radiosensitization of DNA in solutionColloids Surf B Biointerfaces200972112813419414242
- ZhangXDWuDShenXSize-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapyBiomaterials201233276408641922681980
- KhoshgardKHashemiBArbabiARasaeeMJSoleimaniMRadiosensitization effect of folate-conjugated gold nanoparticles on HeLa cancer cells under orthovoltage superficial radiotherapy techniquesPhys Med Biol2014599224924733041
- KongTZengJWangXEnhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticlesSmall2008491537154318712753
- JainSCoulterJAHounsellARCell-specific radiosensitization by gold nanoparticles at megavoltage radiation energiesInt J Radiat Oncol Biol Phys201179253153921095075
- Arab-BafraniZSaberiABirganiMJTShahbazi-GahroueiDAbbasianMFesharakiMGold nanoparticle and mean inactivation dose of human intestinal colon cancer HT-29 cellsJundishapur J Nat Pharm Prod2015104e29153