?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A novel amphiphilic pH-sensitive triblock polymer brush (poly(β-amino esters)-g-cholesterol)-b-poly(ethylene glycol)-b-(poly(β-amino esters)-g-cholesterol) ((PAE-g-Chol)-b-PEG-b-(PAE-g-Chol)) was designed and synthesized successfully through a three-step reaction, and their self-assembled polymeric micelles were used as hydrophobic anticancer drug delivery carriers to realize effectively controlled release. The critical micelle concentrations were 6.8 μg/mL, 12.6 μg/mL, 17.4 μg/mL, and 26.6 μg/mL at pH values of 7.4, 6.5, 6.0, and 5.0, respectively. The trend of critical micelle concentrations indicated that the polymer had high stability that could prolong the circulation time in the body. The hydrodynamic diameter and zeta potential of the polymeric micelles were influenced significantly by the pH values. As pH decreased from 7.4 to 5.0, the particle size and zeta potential increased from 205.4 nm to 285.7 nm and from +12.7 mV to +47.0 mV, respectively. The pKb of the polymer was confirmed to be approximately 6.5 by the acid–base titration method. The results showed that the polymer had sharp pH-sensitivity because of the protonation of the amino groups, resulting in transformation of the PAE segment from hydrophobic to hydrophilic. Doxorubicin-loaded polymeric micelles were prepared with a high loading content (20%) and entrapment efficiency (60%) using the dialysis method. The in vitro results demonstrated that drug release rate and cumulative release were obviously dependent on pH values. Furthermore, the drug release mechanism was also controlled by the pH values. The polymer had barely any cytotoxicity, whereas the doxorubicin-loaded system showed high toxicity for HepG2 cells as free drugs. All the results proved that the pH-sensitive triblock polymer brush and its self-assembled micelle might be a potential delivery carrier for anticancer drugs with sustained release.
Introduction
With the increasing number of cancer patients, chemotherapy, widely used in cancer therapy, has attracted greater attention in recent years.Citation1,Citation2 Traditional antitumor drugs, such as doxorubicin (DOX), camptothecin (CPT), and paclitaxel (PTX), show high cytotoxicity for various tumor cells, but their clinical use is limited by many fatal drawbacks, such as poor solubility in water, uncontrolled release behavior, acquired multidrug resistance, and severe side effects.Citation3–Citation5 To overcome these obstacles, various drug delivery systems (DDSs) have been designed and developed to efficiently deliver anticancer drugs to tumor sites and reduce accumulation in normal sites. For instance, nanoparticles, liquid emulsions, films, and liposomes have been prepared as drug carriers in recent decades.Citation6–Citation11 Compared with these nanoscale carriers, polymeric micelles self-assembled from amphiphilic polymers have captured increasing attention.Citation12–Citation14 Polymeric micelles used as hydrophobic anticancer drug carriers have been proved to have a multitude of advantages, such as a convenient synthesis, high drug loading capacity and biocompatibility, low cytotoxicity, efficient drug delivery, and controlled release.Citation15–Citation17 As an example, polymeric micelles self-assembled from diblock copolymer MPEG-PCL were used as hydrophobic honokiol carriers by Gou et al,Citation18 and the drug-loaded micelles were easy to prepare, stable, safe, and showed potential clinical application. Xue et al also used methyl ether poly(ethylene glycol)-polycaprolactone (MPEG-PCL) micelles to entrap the hydrophobic oridonin to prepare ORI-micelles, which have great potential as a transdermal drug delivery system in cancer chemotherapy.Citation19
In the past decades, many stimuli-response polymers have been synthesized and used as drug carriers based on the differences between normal and targeted sites, including temperature, enzyme, pressure, etc.Citation20–Citation25 As reported, extracellular pH of tumor is weakly acidic (about 6.5–7.2), and the pH values in endosome and lysosome are much lower (4.5~6.5), compared with the normal physiological pH of 7.4.Citation26–Citation28 Therefore, increasing attention has been directed at pH-sensitive polymeric micelles.Citation29–Citation34 It is well known that pH-responsive polymeric micelles exhibit core-shell architecture constructed with a hydrophobic core and a hydrophilic shell as well as a pH-sensitive segment.Citation35 The internal core could load anticancer drugs by hydrophobic interaction. The surrounding shell could maintain the stability of the system and escape from the reticuloendothelial system as well as protect internal drugs from degradation in the biological environment. The pH-sensitivity of the system could be used to realize the drug controlled release.Citation32,Citation34,Citation36,Citation37 In summary, an ideal pH-sensitive polymeric micelle used as an anticancer drug delivery carrier should possess high drug loading content (LC), long-time circulation, low cytotoxicity, and sharp pH-sensitivity. For example, amphiphilic diblock pH-sensitive polymer poly(β-amino ester)-b-poly(ethylene glycol) (MPEG-b-PAE) was synthesized successfully, and its self-assembled micelle was developed as a carrier for co-delivery of CPT and DOX. The results showed that the drug release behavior was influenced significantly by the pH values, and the drug release rate increased with decreasing pH.Citation33,Citation38 Laskar’s group designed and synthesized pH-sensitive cholesterol-containing polymeric micelles for use as CPT carriers. The CPT release could be triggered by hydrolysis of the ester linkages under acidic conditions, indicating good chemotherapeutic activity of the CPT-loaded polymeric micelles.Citation39
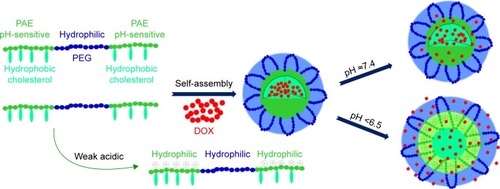
In this work, amphiphilic triblock polymer brush cholesterol grafted PAE-b-PEG-b-PAE with pH-sensitivity was designed, and their self-assembled polymeric micelles were used as carriers for anticancer drug delivery and controlled release. PAE, which was first synthesized by Lynn et al using Michael-type step polymerization, was chosen as a pH-sensitive segment because of its superior pH-sensitivity.Citation40,Citation41 The pKb values of PAE were about 6.0~6.5 as reported, indicating the beneficial pH-sensitive range.Citation42,Citation43 The hydrophilic PEG was selected as a hydrophilic segment to form the shell, maintaining stability of the system during long-time biological circulation.Citation44,Citation45 The pH-sensitive PAE segment was conjugated on the two terminals of PEG, resulting in triblock polymer PAE-b-PEG-b-PAE. For the purpose of high stability and drug loading capacity, cholesterol with high hydrophobic sterol group, reported as an important part of mammalian cell membranes that showed high biocompatibility and pathogenicity, was grafted on the tails of the PAE segment, resulting in triblock polymer brush (poly(β-amino esters)-g-cholesterol)-b-poly(ethylene glycol)-b-(poly(β-amino esters)-g-cholesterol) ((PAE-g-Chol)-b-PEG-b-(PAE-g-Chol)). DOX, used extensively in chemotherapy for cancer, was selected as a model anticancer drug. demonstrates the schematic micellization, drug loading, and controlled release of DOX-loaded (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol) micelles dependent on pH values. We hypothesize that DOX-loaded polymeric micelles (DOX-PMs) could remain stable with a compact structure at normal sites with well-protected drug molecules in the micellar core, when DOX-PMs were under weak acidic conditions, the micellar structure was transfered to swollen and loose because of protonation of the tertiary amino groups, leading to accelerated drug release rate and accumulative amount. Furthermore, the synthesized polymer should be barely cytotoxic. The physicochemical properties of the polymer and drug-loaded micelles, such as hydrodynamic diameter, zeta potential, pH-sensitivity, drug controlled release behavior and mechanism, would be measured by a variety of methods.
Figure 1 Scheme of drug loading and controlled release from triblock polymer brush (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol) dependent on pH values.
Abbreviations: (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol), (poly(β-amino esters)-g-cholesterol)-b-poly(ethylene glycol)-b-(poly(β-amino esters)-g-cholesterol); DOX, dox orubicin; PAE, poly (β-amino esters); PEG, poly(ethylene glycol).
Materials and methods
Materials
PEG with amine modified on the terminals (NH2-PEG-NH2, Mn =4,000), 3-amino-1-propanol (AP, 99%), 4-(dimethyl amino)pyridine (DMAP, 95%), pyrene (99%), cholesteryl chloroformate (Chol, 99%), and 1,4-butylene diacrylate (BD, 99%) were purchased from Alfa Aesar (Haverhill, MA, USA). DOX hydrochloride was purchased from Wuhan Yuan Cheng Gong Chuang Co. (Wuhan, People’s Republic of China) and hydrochloric acid was removed before use. Methylthiazoltetrazolium (MTT) was purchased from Sigma (St Louis, MO, USA). Phosphate-buffered saline (PBS) buffers with different pH values were purchased from 1st BASE (Selangor, Malaysia) and diluted to the intended concentration before use. HepG2 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured under the recommended conditions according to the supplier. Ultrapure water of chromatography grade was obtained from J.T. Baker (Center Valley, PA, USA). Anhydrous dichloromethane (DCM), anhydrous triethylamine (TEA), and other materials were prepared as described in previous work.Citation42,Citation44 All other reagents were used as received except additional description.
Synthesis of poly(β-amino esters) (PAE)
PAE homopolymer was synthesized via Michael-type step polymerization as reported in our previous work with quite a few modifications.Citation42 In brief, BD (1 mol) was dissolved in anhydrous DCM and immersed in an ice bath. AP (0.9 mol) was added dropwise slowly into the flask. The reaction was carried out at 100°C for 24 h under nitrogen with stirring. Later, the resultant mixture was cooled to room temperature and precipitated with n-hexane to obtain the solids. After being dried under vacuum for 48 h, a light yellow powder, PAE, was received.
Synthesis of PAE-b-PEG-b-PAE
The triblock polymer PAE-b-PEG-b-PAE was synthesized using Michael-type step polymerization as previously described. In brief, PAE (1 mol) was dissolved in anhydrous DCM, and NH2-PEG-NH2 (0.9 mol) was added dropwise into the flask. The resulting solution was carried out at 100°C for 24 h under nitrogen with stirring. After the reaction, the products were then precipitated with diethyl ether and n-hexane. The precipitate was dissolved in dimethyl sulfoxide (DMSO) and dialyzed against deionized water in a dialysis bag (molecular weight cutoff [MWCO] 8,000~12,000) for another 72 h to remove unreacted PAE and PEG. The triblock polymer PAE-b-PEG-b-PAE was obtained after lyophilization.
Synthesis of (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol) triblock polymer brush
The synthesized triblock polymers PAE-b-PEG-b-PAE (1 mmol) and TEA (15 mmol) were dissolved in anhydrous DCM. DMAP (10 mmol) was then added into the flask with moderate stirring. Excess cholesteryl chloroformate (10 mmol) was dissolved in anhydrous DCM and added into the flask. The reaction mixture was stirred at room temperature for 24 h. The resulting solution was precipitated in n-hexane three times, and then the solids obtained were dialyzed in DMSO and deionized water successively. After lyophilization, cholesterol grafted polymer brush was received.
Characterization
1H NMR analysis (AVANCE III 400, Bruker, Fällande, Switzerland) was performed on the polymer samples in deuterated chloroform (CDCl3-d) with tetramethylsilane (TMS) as an internal standard operating at 400 MHz and 25°C.
Fourier transform infrared spectra measurements were executed on a Nicolet Nexus for Euro (America) spectrometer to confirm the structure of samples composed of KBr and freeze-dried polymer. The spectra were scanned from 400 to 4,000 cm−1 with a resolution of 2 cm−1.
The number average molecular weight (Mn) and the polydispersity index (Mw/Mn, PDI) were determined by the GPC method, and executed by the Agilent 1,200 series GPC system with THF as mobile phase (1.0 mL/min at 30°C) for 45 min.
The polymeric micelles self-assembled from synthesized polymers were prepared by dialysis for further study. The hydrodynamic diameter and zeta potential of micelles were measured by dynamic light scattering. The measurements were performed on a Malvern Zetasizer Nano S (Malvern Instruments, Malvern, UK) with a diode laser of 800 nm and a scattering angle 90° at room temperature.
The morphological shapes of the polymeric micelles treated at different pH values were confirmed using a transmission electron microscope (TEM, Hitachi H-7650, Tokyo, Japan) operating at 80 kV.
Potentiometric titration
To evaluate the pH-sensitivity of the polymer brush, potentiometric titrations were used to confirm the base dissociation constant (pKb), as reported in our previous work with a few modifications.Citation42,Citation43 In brief, the polymer brush (20 mg) was dissolved in deionized water (20 mL), and the pH value of the solution was adjusted with dilute hydrochloric acid to 3. The polymer solution was titrated using NaOH aqueous solution (0.1 mol/L), and the real-time pH values were recorded by an automatic titrator (Hanon T-860, Jinan, People’s Republic of China) at room temperature. The plots of pH value against the volume of NaOH solution were calculated to confirm the pKb value.
Critical micelle concentration (CMC) measurement
The CMCs of synthesized polymer brush in different PBS buffer solutions were estimated by fluorescence spectroscopy using pyrene as a probe.Citation42–Citation44 In brief, a series of polymer solutions at various concentrations of 0.0001~0.1 mg/mL (ca. 20 samples) containing required pyrene (6×10−7 mol/L) in PBS buffer solutions at pH 7.4, 6.5, 6.0, and 5.0 were prepared in advance. The sample solutions mixed with polymer brush and pyrene were preserved in the dark overnight at room temperature before measurement. A fluorescence spectrophotometer (F-4500, Hitachi, Tokyo, Japan) was used to receive the fluorescence spectra for the calculation of CMCs of samples at different pH values.
Preparation of blank and drug-loaded polymeric micelles
The blank and drug-loaded polymeric micelles were prepared using the dialysis method. In brief, polymer brush (50 mg) was dissolved in DMF (50 mL) with 2 mL deionized water with gentle stirring. The scheduled DOX-HCl (0, 5, 10, 25, 40 mg) was treated with overdose of TEA (0.01 mL per 10 mg of DOX-HCl) to remove HCl. Then the DOX was mixed with polymer solution. The mixture was dialyzed against 1 L of deionized water for 48 h at ambient temperature using a preswollen cellulose membrane bag (MWCO 3,500~4,000). The deionized water was replaced every 4 h. After filtration and lyophilization, the blank or drug-loaded polymeric micelles were obtained in white or red powder, respectively, for further research. The drug LC and entrapment efficiency (EE) were confirmed using a UV-vis spectrophotometer (UV-2450, Shimadzu, Kyoto, Japan) at 479 nm. In a typical experiment, 1 mg DOX-loaded polymeric micelles was dissolved in 10 mL DMSO under vigorous vortex. The micelles were broken and the DOX molecules were dissolved in DMSO. The solution was measured, and the concentration of DOX was calculated according to a standard curve of pure DOX/DMSO solution. The LC and EE were calculated using the following equations, respectively:
where mloaded drug is the amount of drug loaded in the micelle, mdrug-loaded polymeric micelle is the weight of drug-loaded polymeric micelles, and mdrug in feed is the weight of drug in feed.
In vitro release of DOX from drug-loaded polymeric micelles
The in vitro drug release profiles from polymeric micelles were studied using the dialysis method. In brief, DOX-PMs (5 mg) were dispersed in respective PBS buffer solutions (5 mL, Ve) at different pH values of 7.4, 6.5, 6.0, and 5.0, and the resulting solution was placed into a dialysis bag (MWCO 3,500~4,000) and immersed in corresponding PBS solution (45 mL, Vb) in a flask. The flask was immersed in 37°C water with stirring at 110 rpm. A 2 mL sample was drawn using a syringe at desired time intervals and analyzed with UV-vis spectrophotometry. The cumulative drug release percent (Er) was calculated using the following equation. The experiments for each PBS buffer were repeated three times.
where mloaded drug is the amount of drug loaded in the micelle, and Ci is the concentration of DOX in the ith sample.
Cytotoxicity test
The in vitro cytotoxicity of free DOX, blank polymeric micelles, or DOX-PMs for HepG2 cells was evaluated by the standard MTT assay.Citation46,Citation47 The cell culture and cytotoxicity assay were similar to those described in previous work.Citation42–Citation44 A series of free DOX, blank, and DOX-PMs were added into wells with the preprepared cells and incubated for 24 h or 48 h, while fresh medium was used as control. After the addition of MTT (20 μL, 5 mg/mL in PBS buffer), the plates were shaken at 150 rpm for 5 min, and incubated for another 4 h. DMSO (200 μL) was added into the well after the medium was removed, and then the plate was stirred moderately for 20 min and measured with a microplate reader (Multiskan Spectrum, Thermo Scientific, Vantaa, Finland). The cell viability (%) was calculated on the basis of the following equation:
where Ablank was the absorbance at 490 nm without cells. Asample and Acontrol were the absorbance at 490 nm in the presence and in the absence of samples treatment, respectively. The experiment was performed in replicates of six wells.
Statistical analysis
The experimental data were presented as the mean ± standard deviation (SD). Student’s t-test (Excel, 2007) was used to analyze the data. P-values of less than 0.05 (P<0.05) were considered to be significant.
Results and discussion
Synthesis and characterization of polymer (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol)
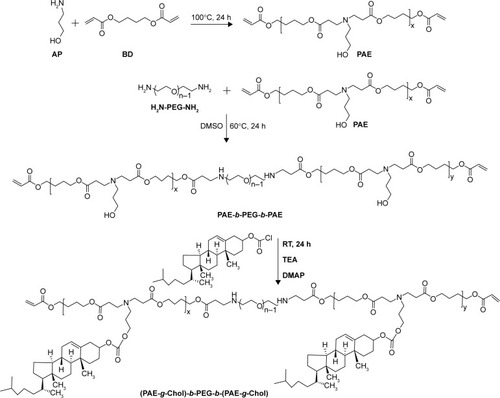
The amphiphilic pH-sensitive polymer brush conjugated with cholesterol was synthesized by Michael-type step polymerization and acylation reaction. As shown in , macromonomer PAE with diacrylate esters on the two terminals was synthesized at first. Subsequently, the precursor PAE-b-PEG-b-PAE was prepared by further Michael-type step polymerization using macromonomer PAE and NH2-PEG-NH2 as diacrylate esters and amine, respectively. For these two steps, the mole ratio of diacrylate esters and amine was 1:0.9, indicating the double-bond-ends on the two terminals. Finally, cholesterol was grafted on the pendant of the PAE segment through acylation reaction using TEA and DMAP as catalyst, resulting in polymer brush (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol). In this work, PEG was selected as the hydrophilic segment which could form the protective shell distributed outside of the polymeric micelles. The macromonomer PAE was conjugated on the two terminals of PEG and used as a pH-sensitive and hydrophobic segment in normal physiological condition. The cholesterol was grafted on the side to enhance the drug LC and stability of the system.
Figure 2 The synthetic route of the polymer (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol).
Abbreviation: (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol), (poly(β-amino esters)-g-cholesterol)-b-poly(ethylene glycol)-b-(poly(β-amino esters)-g-cholesterol).
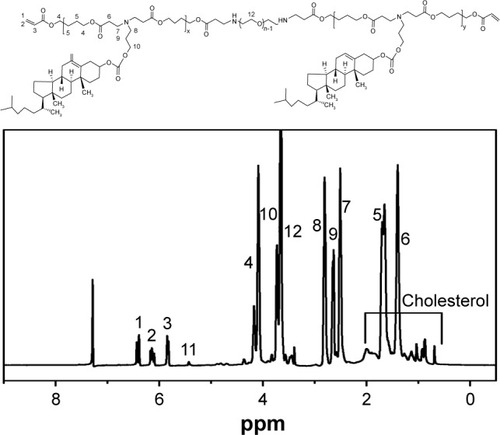
The products were purified by precipitation process and by two-steps dialysis, and the polymer was received for further study after lyophilization. The molecular structure of polymer brush was confirmed by 1H NMR, as shown in . The signals at 6.41–6.50 (1), 6.12~6.23 (2), and 5.81~5.90 (3) ppm were the characteristic peaks of three protons at the end of C=C. The signals at 4.0~4.15 (4) and 1.45~1.65 (5) ppm were ascribed to the -O-CH2- and -CH2-, respectively. The signals at 1.25~1.43 (6) and 2.20~2.30 (7) ppm were ascribed, respectively, to the -CH2- and -CH2-N-. The three characteristic peaks of 2.35~2.45 (9), 2.51~2.65 (8), and 3.66~3.85 (10) ppm were ascribed to the -N-CH2-, -CH2-, and -CH2-O- on the tail of amine. The signals at 0.5~2.0 ppm were due to the protons of cholesterol group, and the peak of 5.38 (11) ppm was the characteristic signal that could be clearly found in the 1H NMR spectrum. The signal at 3.65 (12) ppm due to the -CH2-CH2-O- protons was the characteristic PEG peak of the polymer brush. The peak area of 5.81–6.50 ppm or 5.38 ppm was the characteristic delegates of PAE or cholesterol, respectively. This ratio was used to calculate the grafted ratio of cholesterol (about 48 %).
Characterization of pH-sensitive polymer
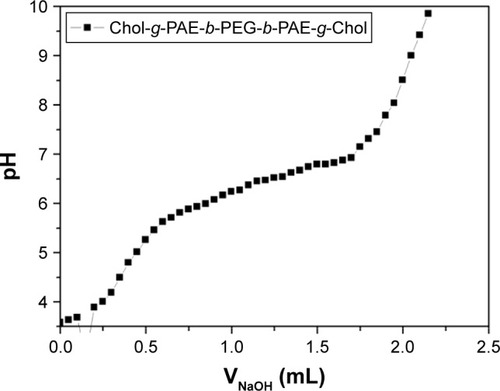
To evaluate the pH-sensitivity of polymer brush, the pKb value was determined by an acid–base titration. As shown in , the pH value increased rapidly after NaOH solution was added. Then the trend changed gently in the range of pH 5.8~6.8. With the continuous addition of base solution, the pH value increased sharply again presumably because the protonated PAE segment was converted to the deprotonated form during the titration process. The results indicated that the pH-sensitive range was about 5.8~6.8 shown as the plateau. The pKb value was defined as the pH value at 50% neutralization of protonated amine groups according to the reference.Citation48 Therefore, the pKb value of synthesized polymer brush was confirmed to be about 6.5, which showed the pH-sensitivity of polymer brush.
Micelle formation
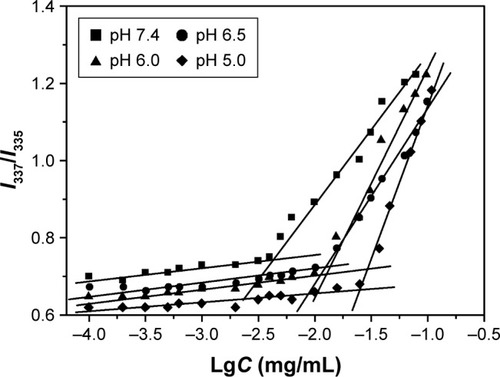
The CMCs of polymeric micelles self-assembled from polymer brush in different PBS buffer solutions at pH values of 7.4, 6.5, 6.0, and 5.0 were investigated by fluorescence spectroscopy to confirm the micellization process and the stability of the system. The I337/I335 ratios were plotted against the logarithm of polymer concentrations at different pH values, as shown in . With the decrease in pH values from 7.4, 6.5, 6.0 to 5.0, the CMCs of (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol) increased from 6.8 to 12.6, 17.4, and 26.6 mg/L, which was lower than those reported in previous studies.Citation43,Citation44 There were several reasons behind the pH-dependent CMCs. First, the amine groups in the PAE segment were ionized gradually in weakly acidic environment since the pKb value was confirmed to be about 6.5, as already mentioned, leading to the transformation of the PAE segment from hydrophobic to hydrophilic, which could require greater driving force to offset the enhanced repulsive forces caused by longer hydrophilic segments. A further reason is that the zeta potential increased markedly caused by the protonation of the PAE segment, resulting in stronger positive-charge repulsion. In summary, the extremely low CMC value (6.8 mg/L) at a pH of 7.4 indicated that the micelles self-assembled from polymer brush implied a high stability in aqueous media during the long-time circulations. The pH-dependent CMCs of polymeric micelles proved the pH-sensitivity of synthesized amphiphilic polymer brush.
Characterization of blank and DOX-loaded polymeric micelles
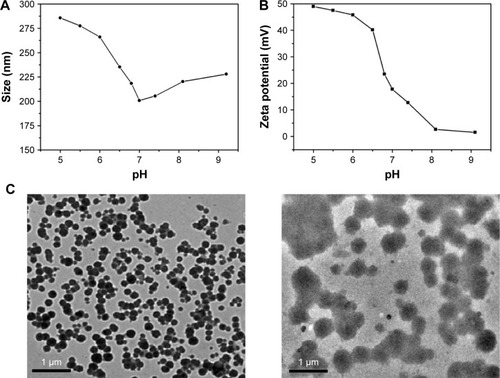
The blank polymeric micelles or DOX-PMs were prepared using the dialysis method. The hydrodynamic diameter and zeta potential of blank polymeric micelles at different pH values were determined to further investigate the pH-sensitivity of polymer brush, as shown in . With regard to the hydrodynamic diameter (), when the pH value was higher than 7.4, the particle size increased slightly (from 200 nm to 225 nm) with an increase in the pH values (from 7.4 to 9.2), resulting from the increased aggregation number of polymeric micelles in basic condition. When the pH value was lower than 7.4, the particle size increased sharply with the decrease in pH value (285 nm at pH 5.0). The reason could be that the amine groups of the PAE segment were protonated in an acidic environment, which enlarged the hydrophilic chain and enhanced the repulsive force, resulting in more difficulty for micellization. The change in the trend of particle size indicated that the polymeric micelles stayed in different shapes, depending on pH values, showing the pH-sensitivity of polymer brush. As shown in , the zeta potential of polymeric micelles was slightly positive in basic condition. As pH value decreased, the zeta potential increased rapidly, particularly from pH 7.4 to 6.0. It then increased slowly with pH value decreasing continuously from 6.0 to 5.0, because of the complete protonation of the amine groups of the PAE segment. As already mentioned, the enhanced positive zeta potential generated a higher repulsive force because of the electrostatic effect, leading to the higher hydrodynamic diameter of polymeric micelles in acidic condition. In conclusion, the change in the trends of particle size and zeta potential dependent on pH values demonstrated the pH-sensitivity of polymer brush. presented the TEM images of the blank polymeric micelles in the buffer solution with pH 7.4 and 5.0. It was found that the micelles exhibited a spherical morphology at a pH of 7.4. The dispersion in the size of the micelles was characterized using PDI. The PDI was 0.124 at a pH of 7.4, indicating the narrow size distribution of the polymeric micelles. After incubation at pH 5.0, the PDI was confirmed as 0.958, and there were two or more peaks measured by dynamic light scattering, because the polymeric micelles were swelling and eroding under weakly acidic conditions. This could be attributed to the transformation of PAE residues from water-insoluble to water-soluble ones as pH decreased.
Figure 6 Particle size (A) and zeta potential (B) of polymeric micelles dependent on pH values. Typical TEM images (C) of micelles in different solutions with pH 7.4 (left) and 5.0 (right).
Abbreviation: TEM, transmission electron microscopy.
The model anticancer drug DOX was entrapped into polymeric micelles using the membrane dialysis method for further study. To confirm the optimal drug feed, a series of DOX-PMs with different weight ratios of polymer brush and drug were prepared. The details are shown in . As the amount of DOX increased, the LC and EE were enhanced generally. When the ratio increased from 10:1 to 10:8, the LC increased from 4.2% to 24.3%. However, when this ratio was 10:5, the EE was the maximum (59.5%). The results showed that when the ratio of polymer and DOX was 10:5, the drug loading efficiency was optimal. The particle size of drug-loaded polymeric micelles increased with LC increasing, attributed to the entrapped hydrophobic drug molecule in the micellar core. The other characteristics of DOX-PMs are found in .
Table 1 Characterization of DOX-loaded (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol) micelles at pH 7.4, T =37°C
In vitro release of DOX from polymeric micelles
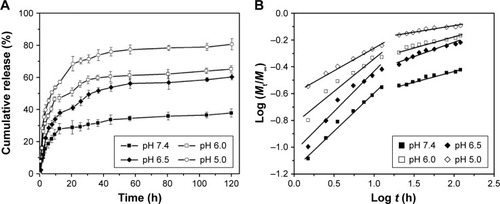
Based on the pH-sensitivity of polymer brush, in vitro DOX release profiles from DOX-PMs in various PBS buffer solutions with pH values of 7.4 (simulate normal physiological conditions), 6.5, 6.0, and 5.0 (simulate weakly acidic environment) were performed. The plots of DOX cumulative release as a function of time in different PBS buffer solutions are shown in . As expected, the DOX release rates were severely influenced by the pH value of the solutions. At pH 7.4, the DOX release rate from the polymeric micelles was slow, and the cumulative releases were only about 15%, 29%, and 37% after 3 h, 24 h, and 120 h, respectively, indicating that the DOX molecules could be protected in the micellar core and the DOX-PMs remained tight and compact for a long time. When the pH value reduced (ie, 6.5), the DOX release rate was accelerated compared with that at a pH of 7.4, and the cumulative releases were, respectively, more than 22%, 45%, and 60% for 3 h, 24 h, and 120 h. When the pH value was much lower (ie, 5.0), the DOX release rate and cumulative release were enhanced sharply as seen in . More than 40%, 70%, and 80% of the drugs were released for 3 h, 24 h, and 120 h, respectively. The key reason for this phenomenon could be that the structure of DOX-PMs became porous, swollen, and loose because of stronger and stronger protonation of the amine groups, which led to a water-soluble PAE segment under weakly acidic conditions. Moreover, the zeta potential was enhanced when the amino groups in PAE block were ionized which led to increased charge density on the micellar surface, facilitating the swelling of the DOX-PMs. As seen in , the drug burst release behavior was reduced effectively presumably because of the hydrophobic cholesterol in the core, which could have enhanced the drug loading capacity. The special brush structure with the central hydrophilic PEG segment was also different from the linear chain.
Figure 7 In vitro drug release profiles (A) and mechanism analysis (B) of DOX-loaded (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol) micelles.
The drug release mechanism has not been understood well until now,Citation49 but it could be roughly analyzed by the following comprehensive semiempirical equations established by Siepmann and Peppas:Citation50
where Mt and M∞ were drug cumulative release amount at time t and infinite time, respectively. n was used to confirm the drug release mechanism type, and k corresponded to drug release rate from polymeric micelles. n=0.43 or 0.85, respectively, indicated the mechanism was Fickian diffusion or swelling controlled. Otherwise, the mechanism was decided as a combination of diffusion and erosion control (n<0.43) or anomalous transport mechanism (0.43<n<0.85).Citation51 The theoretical profiles fitted on the basis of experimental release data and calculated drug release exponent n and rate constant k at different pH values are shown in and . The DOX release process was divided into two stages, the first being from 0~12 h, and the second from 12 h~120 h. displays good linearity for two stages in PBS buffer solutions with different pH values. In the cases of 7.4, 6.5, and 6.0, the n values were higher than 0.43 and lower than 0.85 in the first stage and much less than 0.43 in the second stage, indicating the DOX release behaviors corresponded to anomalous transport mechanism in the first 12 h and switched to a combination of diffusion and erosion control for the second stage. For a pH of 5.0, the n values were lower than 0.43 in two stages, which demonstrated a combination of diffusion and erosion control in the whole DOX release process from drug-loaded polymeric micelles. The reason could be that the DOX-PMs remained tight and compact in the first stage to protect drug molecules in the micellar core well, resulting in a stable system. With the extension of time and decrease in pH value, DOX-PMs became swollen and loose, resulting in a change in the DOX release mechanism. With regard to k values in different pH solutions for both stages, the k values increased with decreasing pH values, suggesting that the DOX release rates enhanced as pH value decreased (). This could be ascribed to protonation of the amine groups as previously mentioned, resulting in porous and loose DOX-PMs and facilitating DOX release from micelles. In a word, DOX release rate and mechanism from drug-loaded polymeric micelles were dependent on the pH values of the solution and on time. The DOX release rate and cumulative release could be enhanced significantly by decreasing the pH value from 7.4 to 5.0.
Table 2 n and k values for DOX-PMs at pH 7.4, 6.5, 6.0, and 5.0, T =37°C
Cytotoxicity test
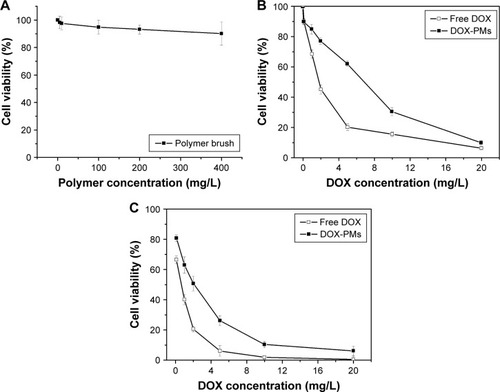
The cytotoxicity of polymer brush, free DOX, or DOX-PMs against HepG2 cells was performed by MTT assay, as shown in . The cytotoxicity of polymer brush increased slightly with the increase in concentration, and the cell viability was higher than 90% even at the highest concentration of polymer brush for 48 h (400 mg/L), showing that the synthesized polymer brush was bare and negligible toxicity for HepG2 cells (). The toxicity of free DOX or DOX-PMs for HepG2 cells was executed for 24 h and 48 h, and the results are shown in . The half maximal inhibitory concentration values of free DOX or DOX-PMs were 1.65 μg/mL or 5.10 μg/mL after 24 h, and 0.71 μg/mL or 2.15 μg/mL after 48 h, respectively. The reason could be that only about 40% and 53% of drug had to be released from the polymeric micelles in the medium at pH 6.5 after 24 h and 48 h, respectively. The cytotoxicity of DOX-PMs was much lower than that of free DOX at low DOX concentrations. But with increasing DOX concentration, the toxic effect of DOX-PMs was close to that of free DOX. For treatment of 48 h, the trend of cytotoxicity of DOX-PMs was similar to that of free DOX, indicating that DOX released from the DOX-PMs still had a high capacity for killing tumor cells.
Conclusion
The amphiphilic pH-sensitive polymer brush (PAE-g-Chol)-b-PEG-b-(PAE-g-Chol) was designed and synthesized successfully via Michael-type step polymerization and esterification reaction, and its self-assembly polymeric micelle was used in hydrophobic anticancer drug delivery with sustained controlled release. The system had low CMC, which increased with decreasing pH value, indicating high stability and extending applications. The particle size of polymeric micelles increased markedly with decreasing pH value, demonstrating sharp pH-sensitivity. DOX was loaded in polymeric micelles by the membrane dialysis method with high drug LC and EE. The DOX release rate and cumulative release were significantly accelerated and enhanced as the pH value decreased from 7.4, 6.5, 6.0 to 5.0, achieving the purpose of controlled release with reduced drug burst release. The DOX release mechanism from polymeric micelles was investigated using mathematical theory models. Moreover, the cytotoxicity of polymer brush was barely observed for HepG2 cells, whereas the DOX-PMs showed similar ability of cell death to free DOX. The overall result demonstrated that this polymer brush and its self-assembly polymeric micelle might be a potential hydrophobic drug carrier for controlled release.
Acknowledgments
This work was financially supported by National Natural Science Foundation project (21606045), funded by People’s Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
- Pérez-HerreroEFernández-MedardeAAdvanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapyEur J Pharm Biopharm201593527925813885
- WongHLBendayanRRauthAMLiYWuXYChemotherapy with anticancer drugs encapsulated in solid lipid nanoparticlesAdv Drug Deliver Rev2007596491504
- AlakhovVKlinskiELiSBlock copolymer-based formulation of doxorubicin. From cell screen to clinical trialsColloid Surface B1999161–4113134
- AmnaTHassanMSNamKTPreparation, characterization, and cytotoxicity of CPT/Fe(2)O(3)-embedded PLGA ultrafine composite fibers: a synergistic approach to develop promising anticancer materialInt J Nanomedicine201271659167022615530
- DuFMengHXuKCPT loaded nanoparticles based on beta-cyclodextrin-grafted poly(ethylene glycol)/poly (l-glutamic acid) diblock copolymer and their inclusion complexes with CPTColloid Surface B20141131230236
- HuaMYYangHWLiuHLSuperhigh-magnetization nanocarrier as a doxorubicin delivery platform for magnetic targeting therapyBiomaterials201132348999901021872924
- KopečekJSmart and genetically engineered biomaterials and drug delivery systemsEur J Pharm Sci200320111613678788
- SmartTLomasHMassignaniMFlores-MerinoMVPerezLRBattagliaGBlock copolymer nanostructuresNano Today200833–43846
- SarkerDKEngineering of nanoemulsions for drug deliveryCurr Drug Deliv20052429731016305433
- KraftJCFreelingJPWangZHoRJEmerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systemsJ Pharm Sci20141031295224338748
- NguyenTXHuangLGauthierMYangGWangQRecent advances in liposome surface modification for oral drug deliveryNanomedicine20161191169118527074098
- NishiyamaNOkazakiSCabralHNovel cisplatin-incorporated polymeric micelles can eradicate solid tumors in miceCancer Res200363248977898314695216
- PeerDKarpJMHongSFarokhzadOCMargalitRLangerRNanocarriers as an emerging platform for cancer therapyNat Nanotechnol2007275176018654426
- UppuDSSamaddarSHoqueJSide chain degradable cationic–amphiphilic polymers with tunable hydrophobicity show in vivo activityBiomacromolecules20161793094310227442617
- KataokaKHaradaANagasakiYBlock copolymer micelles for drug delivery: design, characterization and biological significanceAdv Drug Deliv Rev200147111313111251249
- RöslerAVandermeulenGWKlokHAAdvanced drug delivery devices via self-assembly of amphiphilic block copolymersAdv Drug Deliv Re200153195108
- AlexandridisPHolzwarthJFHattonTAMicellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer associationMacromolecules199427924142425
- GouMZhengXMenKSelf-assembled hydrophobic honokiol loaded MPEG-PCL diblock copolymer micellesPharma Res200926921642173
- XueBWangYTangXBiodegradable self-assembled MPEG-PCL micelles for hydrophobic oridonin delivery in vitroJ Biomed Nanotechnol201281808922515096
- FleigeEQuadirMAHaagRStimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and applicationsAdv Drug Deliv Rev201264986688422349241
- MuraSNicolasJCouvreurPStimuli-responsive nanocarriers for drug deliveryNat Mater201312991100324150417
- WeiXLuoQSunLEnzyme- and pH-sensitive branched polymer–doxorubicin conjugate-based nanoscale drug delivery system for cancer therapyACS Appl Mater Interfaces2016818117651177827102364
- BuiQNLiYJangMSHuynhDPLeeJHLeeDSRedox- and pH-sensitive polymeric micelles based on poly (β-amino ester)-grafted disulfide methylene oxide poly(ethylene glycol) for anticancer drug deliveryMacromolecules2015481240464054
- ThambiTParkJHLeeDSStimuli-responsive polymersomes for cancer therapyBiomater Sci201641556926456625
- GantaSDevalapallyHShahiwalaAAmijiMA review of stimuli-responsive nanocarriers for drug and gene deliveryJ Control Release2008126318720418261822
- SchmaljohannDThermo- and pH-responsive polymers in drug deliveryAdv Drug Deliv Rev200658151655167017125884
- SchmidSLFuchsRMalePMellmanITwo distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomesCell198852173833345561
- EnginKLeeperDBCaterJRThistlethwaiteAJTupchongLMcFarlaneJDExtracellular pH distribution in human tumoursInter J Hyperther199511211216
- FelberAEDufresneMHLerouxJCpH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylatesAdv Drug Deliv Rev2012641197999221996056
- LiuZZhangNpH-Sensitive polymeric micelles for programmable drug and gene deliveryCurr Pharm Des201218233442345122632817
- HanSSLiZYZhuJYDual-pH sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug deliverySmall201511212543255425626995
- LimEKHuhYMYangJLeeKSuhJSHaamSpH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by MRIAdv Mater201123212436244221491515
- MinKHKimJHBaeSMTumoral acidic pH-responsive MPEG-poly(beta-amino ester) polymeric micelles for cancer targeting therapyJ Control Release2010144225926620188131
- WuWZhangQWangJTumor-targeted aggregation of pH-sensitive nanocarriers for enhanced retention and rapid intracellular drug releasePolym Chem2014556685679
- SavicRLuoLEisenbergAMaysingerDMicellar nanocontainers distribute to defined cytoplasmic organellesScience2003300561961561812714738
- QiuLYBaeYHSelf-assembled polyethylenimine-graft-poly(ε-caprolactone) micelles as potential dual carriers of genes and anticancer drugsBiomaterials200728284132414217582489
- GuJChengWPLiuJpH-triggered reversible “stealth” polycationic micellesBiomacromolecules20089125526218095651
- KimMSHwangSJHanJKpH-responsive PEG-poly(β-amino ester) block copolymer micelles with a sharp transitionMacromol Rapid Comm2006276447451
- LaskarPSamantaSGhoshSKDeyJIn vitro evaluation of pH-sensitive cholesterol-containing stable polymeric micelles for delivery of camptothecinJ Colloid Interface Sci201443030531424974243
- LynnDMLangerRDegradable poly (β-amino esters): synthesis, characterization, and self-assembly with plasmid DNAJ Am Chem Soc2000122441076110768
- LynnDMAmijiMMLangerRpH-responsive polymer microspheres: rapid release of encapsulated material within the range of intracellular pHAngew Chem Int Ed Engl20014091707171011353487
- ZhangCYXiongDSunYZhaoBLinWJZhangLJSelf-assembled micelles based on pH-sensitive PAE-g-MPEG-cholesterol block copolymer for anticancer drug deliveryInt J Nanomedicine201494923493325364250
- ZhangCYWuWSYaoNZhaoBZhangLJpH-sensitive amphiphilic copolymer brush Chol-g-P(HEMA-co-DEAEMA)-b-PPEGMA: synthesis and self-assembled micelles for controlled anti-cancer drug releaseRSC Adv201444023240240
- ZhangCYYangYQHuangTXSelf-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug deliveryBiomaterials201233266273628322695069
- HussainHMyaKYHeCSelf-assembly of brush-like poly [poly(ethylene glycol) methyl ether methacrylate] synthesized via aqueous atom transfer radical polymerizationLangmuir20082423132791328618986178
- MalarvizhiGLRetnakumariAPNairSKoyakuttyMTransferrin targeted core-shell nanomedicine for combinatorial delivery of doxorubicin and sorafenib against hepatocellular carcinomaNanomedicine20141081649165924905399
- WangYIbrahimNLJiangJGaoSErathodiyilNYingJYConstruction of block copolymers for the coordinated delivery of doxorubicin and magnetite nanocubesJ Control Release2013169321121923499717
- ShenYTangHZhanYVan KirkEAMurdochWJDegradable poly (β-amino ester) nanoparticles for cancer cytoplasmic drug deliveryNanomedicine20095219220119223244
- YoungCRDietzschCCereaMPhysicochemical characterization and mechanisms of release of theophylline from melt-extruded dosage forms based on a methacrylic acid copolymerInt J Pharm20053011–211212016055285
- SiepmannJPeppasNAModeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC)Adv Drug Deliv Rev2001482–313915711369079
- LiYLiHWeiMLuJJinLpH-Responsive composite based on prednisone-block copolymer micelle intercalated inorganic layered matrix: structure and in vitro drug releaseChem Eng J20091511–3359366