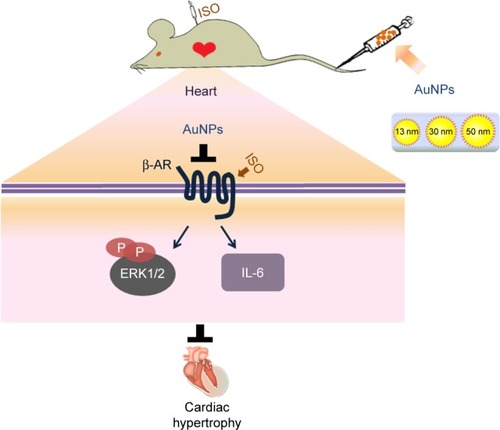
Abstract
Gold nanoparticles (AuNPs) are widely used as a drug delivery vehicle, which can accumulate in the heart through blood circulation. Therefore, it is very important to understand the effect of AuNPs on the heart, especially under pathological conditions. In this study, we found that PEG-coated AuNPs attenuate β-adrenergic receptor (β-AR)-mediated acute cardiac hypertrophy and inflammation. However, both isoproterenol, a non-selective β-AR agonist, and AuNPs did not induce cardiac function change or cardiac fibrosis. AuNPs exerted an anti-cardiac hypertrophy effect by decreasing β1-AR expression and its downstream ERK1/2 hypertrophic pathway. Our results indicated that AuNPs might be safe and have the potential to be used as multi-functional materials (drug carrier systems and anti-cardiac hypertrophy agents).
Background
Along with the advances in nanotechnology, nanoparticles have been applied widely in biomedicine.Citation1,Citation2 In particular, gold nanoparticles (AuNPs) are a potential candidate for the development of diagnostic and therapeutic methods, due to their unique physical, chemical, optical, and pharmacological properties. For example, gold cores are inert, stable, biocompatible, and have low toxicity. In addition, AuNPs are easy to prepare and functionalize.Citation3,Citation4 Therefore, AuNPs have great potential for many biomedical applications, such as drug or protein delivery, gene transfection, cancer therapy, biomedical imaging, bio-labeling, and molecular diagnostic tools. Furthermore, the surface of AuNPs can be modified to improve specificity and safety of its application in clinical or research.Citation5,Citation6 Since AuNPs have shown great potential for wide application in diagnosis and treatment of diseases, it is important to evaluate the safety of AuNPs in the diseases.
Cardiac diseases have been the leading cause of death worldwide.Citation7 AuNPs are widely used in the diagnosis and treatment of cardiac diseases.Citation8–Citation11 However, there have been only a few studies that examined the effect of AuNPs on the heart. So it is necessary to investigate the safety of AuNPs for the heart under both physiological and pathological conditions. Our previous study proved the safety of AuNPs under physiological conditions,Citation12 so we focused on the safety of AuNPs for the heart under pathological conditions in the present study.
Cardiac hypertrophy is an important pathological basis for various heart diseases and is an independent risk factor for morbidity and mortality of heart failure.Citation13 The safety of AuNPs in cardiac hypertrophy is still unclear. A key factor in cardiac hypertrophy is the over-activation of β-adrenergic receptor (β-AR)Citation14 and β-blockers have been one of the standardized therapeutic drugs for heart failure in clinical settings.Citation15 Thus isoproterenol (ISO), the agonist of β-AR, has been widely used to establish the animal model of cardiac hypertrophy.Citation16,Citation17 ISO is reported to induce both chronic and acute cardiac hypertrophy in animal experiments. The chronic cardiac hypertrophy model is used to simulate the chronic progression of heart remodeling in some chronic cardiovascular diseases such as hypertension.Citation18 The acute cardiac hypertrophy model reflects cardiac remodeling following acute cardiac injuries.Citation19 The safety of AuNPs in ISO-induced chronic cardiac hypertrophy model has been demonstrated in our previous study.Citation20 Thus, in the present study, the safety of AuNPs in ISO-induced acute cardiac hypertrophy model was investigated.
The results in this study indicated that the accumulation of AuNPs in the heart depends on their size and the accumulation of AuNPs in the heart attenuates β-AR-mediated acute cardiac hypertrophy and inflammation through decrease of β1-AR expression and its downstream ERK1/2 hypertrophy signaling pathway. These novel findings will be helpful for the wider application of AuNPs in cardiac diseases.
Methods
Characterization of the AuNPs
Three different sizes (13, 30, and 50 nm) of PEG-coated AuNPs were used in this experiment (Nanocs Inc., New York, NY, USA). The morphology size and aggregation state of the AuNPs were evaluated using transmission electron microscopy (JEM-200CX; Jeol, Ltd., Tokyo, Japan) and Multiskan GO (Thermo Scientific, Ltd, Waltham, MA, USA). The PEG-coated AuNP suspension was sonicated for 5 min before use.
Establishment of animal model
Our investigation was approved by the Biomedical Research Ethics Committee of Peking University (LA 2010-048) and strictly adhered to the American Physiological Society’s “Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training”. Male FVB/N mice (10–12 weeks) were obtained from laboratory animal department of Peking University Health Science Center. Mice were housed in groups of four and maintained on a 12 h dark/light cycle in a room with controlled temperature (25°C±2°C). Mice had free access to food and water. Cardiac hypertrophy model was established by subcutaneous injection of ISO (200 mg/kg/day, dissolved in saline [Sigma-Aldrich, St Louis, MO, USA]) for 3 consecutive days.
There were five groups in total (eight to ten mice in each group): i) control group: daily administration of saline for 3 days; ii) ISO group: daily subcutaneous administration of ISO (200 mg/kg/day) for 3 days; iii) ISO +13 nm AuNPs group: daily subcutaneous administration of ISO (200 mg/kg/day) for 3 days, 540 μg/kg AuNPs of 13 nm were injected into the tail vein on the same days after ISO injections; iv) ISO +30 nm AuNPs group: daily subcutaneous administration of ISO (200 mg/kg/day) for 3 days, 540 μg/kg AuNPs of 30 nm were injected into the tail vein on the same days after ISO injections; v) ISO +50 nm AuNPs group: daily subcutaneous administration of ISO (200 mg/kg/day) for 3 days, 540 μg/kg AuNPs of 50 nm were injected into the tail vein on the same days after ISO injections. The content of AuNPs of all three sizes (13, 30, and 50 nm) was 0.01% Ag/mL. The volume of injection was adjusted to 5 μL/g of mice weight. The PEGylated AuNP suspension was sonicated for 5 min before use to make the AuNPs disperse adequately. The treatment of animals in each group is shown in .
Table 1 Treatment of animals in each group
Echocardiographic analysis
Echocardiography analysis was performed 1 day after the last injection. Mice were anesthetized with 1.5% isoflurane (Baxter International Inc., Deerfield, IL, USA). Echocardiographic images were obtained by the Visualsonics high-resolution Vevo 770 system (VisualSonics, Inc., Toronto, ON, Canada). Two-dimensional short-axis views were obtained at the level of the papillary muscle. The diastolic left ventricular posterior wall thickness (LVPW;d) and systolic left ventricular posterior wall thickness (LVPW;s) were measured to calculate the ejection fraction (EF) and fractional shortening (FS). All measurements were averaged from three consecutive cardiac cycles. Cardiac systolic function was represented by the values of EF and FS. Doppler echocardiograms were captured via an apical four chamber view. Transmitral flow Doppler was obtained through mitral flow center and two characteristic E wave and A wave were obtained. Tissue Doppler images were obtained through the mitral annulus and Doppler velocities E′ and A′ were obtained. E/A, E′/A′, and E/E′ ratios were calculated to evaluate cardiac diastolic function. The average of three consecutive cardiac cycles was taken for each parameter. Echocardiography procedures were performed in accordance with the guideline of American Society of Echocardiography.
Quantitative histological analysis
Mice were anesthetized and sacrificed after echocardiography analysis. The hearts were excised and weighed immediately after being washed with cold PBS. The cardiac tissues for histological and immunohistochemistry analysis were fixed with 4% paraformaldehyde for 12 h, dehydrated in 20% sucrose for 24 h, and then embedded in paraffin. Serial sections (5 μm thick) were stained with hematoxylin and eosin stain (H&E) for morphological analysis and picrosirius red was used for the detection of fibrosis. For morphometric analysis, photographs of left ventricular sections cut from the same location of each heart were observed under 4×, 200×, and 400× magnification, respectively (Leica Microsystems Imaging Solutions Ltd., Wetzlar, Germany). Myocyte cross-sectional area was measured by Image Pro Plus using the photographs under 400× magnification. Interstitial fibrosis was visualized with picrosirius red staining, and the cardiac fibrosis volume fraction was calculated as the ratio of the stained fibrotic area to the total myocardial area.
Western blot analysis
The cardiac tissues were cracked by tissue homogenizer and ultrasonic unit. After centrifugation (12,000 rpm, 15 min, 4°C), the protein content of supernatant was determined by BCA protein quantitative method. The loading quantity of samples was 30 μg. Samples were separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes. After being blocked, blots were probed with the appropriate primary antibodies overnight at 4°C, then washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody. Bands were visualized with a super Western blot sensitivity chemiluminescence detection system (Thermo Fisher Scientific). The conditions of primary and secondary antibodies used for immunoblot analysis are summarized in .
Table 2 Conditions of first and second antibodies for Western blot
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated from heart tissues using Trizol Reagent (Thermo Fisher Scientific). The complementary DNA was synthesized using the kit (017317; Promega Corporation, Fitchburg, WI, USA). Relative quantitation by real-time PCR was performed using SYBR Green to detect PCR products in real time with ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Thermo Fisher Scientific). Primer sequences were as follows: EIF5:5′-CAGAAAAACAAGAGCGAAGACAG-3′, 5′-GCTTCCAGAGACAACTCCTCC-3′; TNF-α:5′-CCCACGTCGTAGCAAACCA-3′, 5′-ACAAGGTACAACCCATCGGC-3′; IL-1β:5′-TGCCACCTTTTGACAGTGATG-3 ′, 5′-AAGGTCCACGGGAAAGACAC-3′; IL-6:5′-CGGCCTTCCCTACTTCACAA-3′, 5′-TTCTGCAAGTGCATCATCGT-3′; β1-AR:5′-GCCCTTTCGCTACCAGAGTT-3′, 5′-ACTTGGGGTCGTTGTAGCAG-3′; β2-AR:5′-TCGAGCGACTACAAACCGTC-3′, 5′-AAGTCCAGAACTCGCACCAG-3′, ANF: 5′-CTTCCAGGCCATATTGGAG-3′, 5′-GGGGGCATGACCTCATCTT-3′; BNP: 5′-ACAAGATAGACCGGATCGGA-3′,5′-AGCCAGGAGGTCTTCCTACA-3′; CollagenI:5′-GTAACTTCGTGCCTAGCAACA-3′, 5′-CCTTTGTCAGAATACTGAGCAGC-3′; CollagenIII:5′-CCTGGCTCAAATGGCTCAC-3′; 5′-CAGGACTGCCGTTATTCCCG-3′. PCR was performed under the following conditions: 95°C for 2 min, followed by 40 amplification cycles (95°C for 15 s, 60°C for 1 min). The CT (threshold cycle) values obtained for genes of interest were normalized to concurrent measurement of EIF5 mRNA level, and fold changes were compared to the control.
Inductively coupled plasma-mass spectrometry (ICP-MS)
The concentrations of AuNPs in tissues were assessed by quantitative inductively coupled plasma mass spectrometry (ICP-MS). Cardiac tissues (approximately 30 mg each) were digested in aqua fortis (nitric acid:hydrochloric acid 3:1). After adjusting the solution volume to 2 mL using 2% nitric acid and 1% hydrochloride acid (1:1), Au content assays were performed using an ELAN DRC-e ICP-MS instrument (PerkinElmer Inc., Waltham, MA, USA).
Statistical analysis
Data were summarized as means ± SEM. Differences in data between groups were compared using Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) with one-way ANOVA followed by Dunnett’s test. Data with P<0.05 were considered statistically significant.
Results
The accumulation of AuNPs in the mouse heart
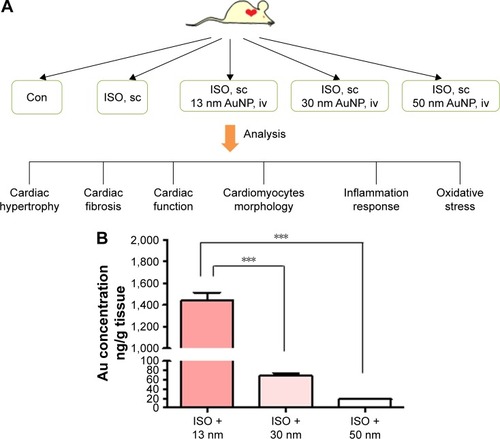
To investigate the effect of AuNPs on β-AR-mediated cardiac remodeling, experiments were designed as shown in the working flow chart, . Firstly, the accumulation of AuNPs was determined by ICP-MS in mouse heart. As shown in , accumulation of AuNPs in the heart was size-dependent. The 13 nm AuNPs showed the highest accumulation in heart (1,438+236.9 ng/g) which was extremely high in comparison with that of 30 nm (68.76+17.33 ng/g) and 50 nm (18.21+4.052 ng/g).
Figure 1 Experimental flow chart and Au accumulation in heart.
Notes: (A) The experimental flow chart of the present study. (B) The Au content in mouse heart was determined with inductively coupled plasma mass spectrometry. ***P<0.001. Data represent mean ± SEM.
Abbreviations: AuNPs, gold nanoparticles; ISO, isoproterenol; sc, subcutaneous; iv, intravenous; Con, control.
Effects of the AuNPs on cardiac hypertrophy
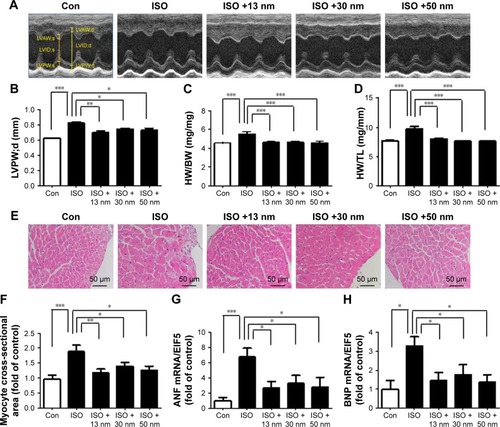
The ratio of heart weight to tibia length (HW/TL), the ratio of heart weight to body weight (HW/BW), LVPW;d, myocyte cross-sectional area, ANF, and BNP are all major indicators of cardiac hypertrophy. As shown in , ISO markedly increased LVPW;d and AuNPs (13, 30, and 50 nm) reversed this process. Similarly, AuNPs (13, 30, and 50 nm) attenuated HW/BW () and HW/TL () increased significantly by ISO. Moreover, AuNPs (13, 30, and 50 nm) reduced myocyte cross-sectional area () increased by ISO. AuNPs (13, 30, and 50 nm) also decreased the mRNA expression of ANF () and BNP ().
Figure 2 The effects of AuNPs on cardiac hypertrophy.
Notes: *P<0.05, **P<0.01, ***P<0.001. (A) Representative M-mode echocardiography images were taken to show left ventricular wall thickness. (B) Quantitative analysis of diastolic left ventricular posterior wall thickness. (C) Quantitative analysis of HW/BW ratio. (D) Quantitative analysis of HW/TL ratio. Data represent mean ± SEM. (E) Representative micrographs of myocyte cross-sectional area. (F) Quantitative analysis of myocyte cross-sectional area. (G) Quantitative analysis of ANF mRNA expression. (H) Quantitative analysis of BNP mRNA expression.
Abbreviations: AuNPs, gold nanoparticles; HW/BW, ratio of heart weight to body weight; HW/TL, ratio of heart weight to tibia length; ISO, isoproterenol; Con, control; LVAW;s, systolic left ventricular anterior wall thickness; LVID;s, left ventricular end-systolic inner-diameter; LVPW;s, systolic left ventricular posterior wall thickness; LVAW;d, diastolic left ventricular anterior wall thickness; LVID;d, left ventricular end-diastolic inner-diameter; LVPW;d, diastolic left ventricular posterior wall thickness.
Effects of the AuNPs on cardiac fibrosis
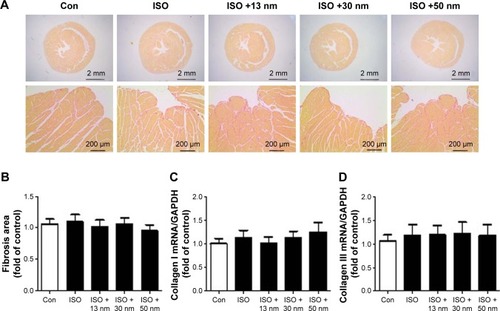
Chronic long-term ISO stimulation could cause fibrosis.Citation21 Our results indicated that acute short-term ISO stimulation did not induce obvious fibrosis. Furthermore, AuNPs did not affect cardiac fibrosis either (). Collagen I and Collagen III are also fibrosis markers. The mRNA expressions of Collagen I () and Collagen III () were consistent with the previously mentioned conclusion.
Figure 3 The effects of AuNPs on cardiac fibrosis.
Notes: (A) Representative micrographs of picrosirius red-stained sections of the ventricle. Red parts represent collagen. (B) Quantification of cardiac interstitial collagen content from picrosirius red-stained sections with results expressed as the ratio of collagen area to heart area. There is no significance among all the groups. (C) The mRNA expression of Collagen I in the heart tissue. (D) The mRNA expression of Collagen III in the heart tissue.
Abbreviations: AuNPs, gold nanoparticles; ISO, isoproterenol; Con, control.
Effects of the AuNPs on cardiac functions
Assessment of cardiac function is important in cardiac diseases. The left ventricular EF and FS were the most important indicators to evaluate the cardiac contraction function. Results showed that the EF and FS were still normal after exposure to ISO. AuNPs (13, 30, and 50 nm) did not affect EF or FS (). E/A, E′/A′ and E/E′ were used to evaluate cardiac diastolic function. Similar to cardiac contraction function, ISO did not affect cardiac diastolic function obviously in this model. AuNPs (13, 30, and 50 nm) did not affect cardiac diastolic function either ().
Figure 4 The effects of AuNPs on cardiac function.
Notes: (A) Left ventricular EF and (B) FS were measured to reflect cardiac contraction function. (C) Representative Doppler echocardiographic images. (D) E/A, (E) E′/A′ (F) and E/E′ ratios were used to reflect cardiac diastolic function. No significance was found.
Abbreviations: EF, ejection fraction; FS, fractional shortening; AuNPs, gold nanoparticles; ISO, isoproterenol; Con, control.
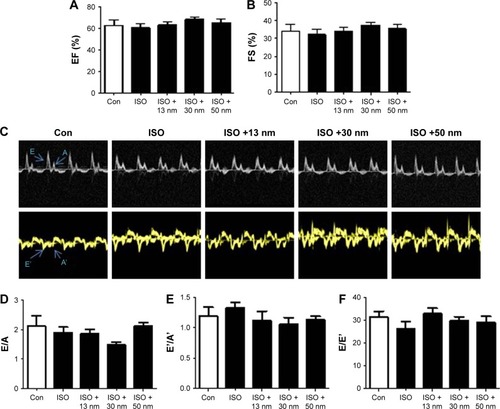
Effects of the AuNPs on β-AR-mediated IL-6 mRNA expression
First, H&E staining showed that ISO induced inflammatory cell infiltration. However, the additional accumulation of AuNPs in the heart did not aggravate ISO-induced cardiac inflammation (). Furthermore, inflammatory cytokines TNF-α, IL-1β, and IL-6 were detected with real-time PCR. Consistent with cardiac hypertrophy, AuNPs (13, 30, and 50 nm) significantly inhibited IL-6 production increased by ISO (). In contrast to IL-6, ISO did not increase the production of TNF-α () and IL-1β ().
Figure 5 The effects of AuNPs on inflammation in the heart.
Notes: (A) Representative images showing H&E staining of heart sections. The arrows refer to the infiltrated inflammatory cells. The scale bars of 12.5× images are 2 mm, of 250× images are 100 μm, and those of 500× images are 50 μm. (B) The mRNA expression of IL-6 in the heart tissue. (C) The mRNA expression of TNF-α in the heart tissue. (D) The mRNA expression of IL-1β in the heart tissue. *P<0.05, **P<0.01. Data represent mean ± SEM.
Abbreviations: AuNPs, gold nanoparticles; H&E, hematoxylin and eosin; ISO, isoproterenol; Con, control; ns, no significance.
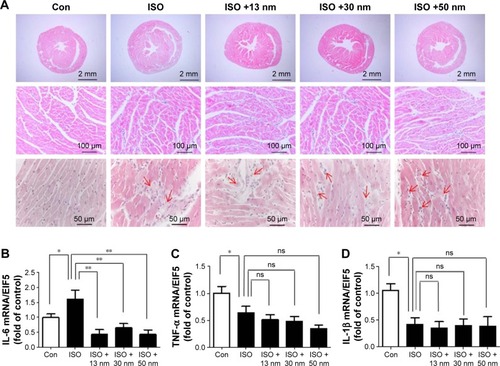
Effects of the AuNPs on β-AR mRNA expression
It has been reported that chronic ISO stimulation downregulated the expression of both β1-AR and β2-AR.Citation22 In this study, the result showed that acute ISO stimulation upregulated β1-AR but not β2-AR mRNA expression (). AuNPs (13, 30, and 50 nm) inhibited the upregulation of β1-AR mRNA expression significantly () but had no influence on β2-AR mRNA expression ().
Figure 6 The effects of AuNPs on β-adrenergic receptor (β-AR) mRNA expression in the heart.
Notes: (A) The mRNA expression of β1-AR in the heart tissue. (B) The mRNA expression of β2-AR in the heart tissue. *P<0.05, **P<0.01. Data represent mean ± SEM.
Abbreviations: AuNPs, gold nanoparticles; ISO, isoproterenol; Con, control; ns, no significance.
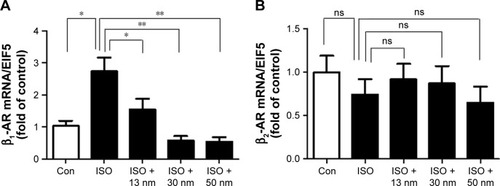
Effects of the AuNPs on β-AR-mediated ERK1/2 pathway activation
It is well known that ERK1/2 MAPK pathway plays an important role in β-AR-mediated cardiac hypertrophy.Citation23,Citation24 The results indicated that AuNPs (13, 30, and 50 nm) decreased the phosphorylation of ERK1/2 induced by ISO obviously (), which is consistent with the function of AuNPs in inhibiting β-AR-mediated cardiac hypertrophy.
Figure 7 The effects of AuNPs on the phosphorylation of ERK1/2.
Notes: (A) The protein expression of P-ERK1/2 (phosphorylated ERK1/2) and T-ERK1/2 (total ERK1/2) of all the groups. (B) Quantitative analysis of the level of P-ERK1/2 of all the groups. *P<0.05, **P<0.01.
Abbreviations: AuNPs, gold nanoparticles; ISO, isoproterenol; Con, control.
Discussion
In this study, we investigated the safety of AuNPs in the acute cardiac hypertrophy model induced by large dose of ISO. The result suggested that AuNPs could inhibit cardiac hypertrophy in this model. The mechanism may be related to the inhibition of β1-AR expression and its downstream signaling pathway, such as inflammation and the phosphorylation of ERK1/2.
Our study indicated that AuNPs (13, 30, and 50 nm) inhibited the increase of β1-AR mRNA expression induced by ISO but not β2-AR, which is consistent with the result showing that β-AR-mediated cardiac hypertrophy is primarily due to the activation of β1-AR.Citation25,Citation26 Recent studies have proven that AuNPs could affect the synthesis and function of biological macromolecules. For example, AuNPs could interfere with the synthesis of ribosomal protein.Citation27 Further, AuNPs could conjugate to G-protein and affect the activity of G-protein.Citation28 These studies suggest that AuNPs may regulate the expression of β1-AR and its downstream signaling pathway which reflects the activity of β1-AR.
Many studies have suggested that inflammation is involved in cardiac hypertrophy. Pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α promote cardiac hypertrophy.Citation29,Citation30 In this study, the mRNA expression of IL-1β and TNF-α did not increase, but the mRNA expression of IL-6 increased after acute ISO treatment. Some studies have indicated that ISO could increase the expression of IL-6, IL-1β, and TNF-α.Citation31,Citation32 However, it has also been reported that ISO treatment downregulated TNF-α production and caused no change in IL-6 production.Citation33 This suggested that inflammation is a complicated process, and different inflammatory cytokines may have different reactions after β-receptor activation. In addition, the dose of ISO and the time course of administration may also have an influence on the inflammatory cytokines’ production. The specific mechanisms still need to be researched more. It has also been reported that AuNPs of 21 nm could reduce IL-6 mRNA level in the fat.Citation34 Consistent with the previous study, in our study, AuNPs (13, 30, and 50 nm) inhibited ISO-increased IL-6 mRNA expression which contributed to the β-AR-mediated cardiac hypertrophy.
ERK1/2 signaling pathways are important regulators of β1-AR-mediated cardiac hypertrophy.Citation35–Citation37 In the present study, we found that AuNPs inhibited the phosphorylation of ERK1/2 induced by ISO. In addition, recent work has suggested that ERK1/2 signaling is regulated, at least in part, by oxidative stressCitation38,Citation39 and that antioxidants can function to block the activation of ERK1/2 both in vitro and in vivo.Citation40,Citation41 While some other studies have shown that AuNPs could suppress oxidative stress and elevate the antioxidant defense system in vivo.Citation42–Citation45 So AuNPs may inhibit the phosphorylation of ERK1/2 partly through the inhibition of oxidative stress.
AuNPs (13, 30, 50 nm) inhibited ISO-induced cardiac hypertrophy in the present study, but AuNPs have no effect on either the systolic or diastolic functions. It is noteworthy that both the systolic and diastolic functions did not change after ISO treatment. The possible reason is that cardiac remodeling is in a compensation stage, so both the systolic and diastolic functions are not damaged in this stage.
There are some differences between the results of all the studies about the toxicity of AuNPs. It is likely due to their modifications, functional attachment of their surfaces, shapes, and sizes.Citation46,Citation47 For example, known as a modifying polymer, PEG decreases immunogenicity and increases stability of drugs in the circulatory system. Therefore, the PEG coating reduces the chance of heart toxicity induced by AuNPs.Citation48–Citation50
Taken together, our results showed that AuNPs inhibited cardiac hypertrophy mediated by β-AR, and this effect depends on a complex mechanism involving inhibition of β1-AR expression and its downstream effectors IL-6 and ERK1/2 (). These results raise the hope that AuNPs might be used as multi-functional materials (drug carrier systems and anti-cardiac hypertrophy agents) for cardiac diseases’ treatment.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (grant no 2014CBA02000), the National Natural Science Foundation of China (81471893, 81270157, 91539123, 81070078), and Beijing Municipal Natural Science Foundation (7172235).
Disclosure
The authors report no conflicts of interest in this work.
References
- YangLiZhangYYanBNanotoxicity overview: nano-threat to susceptible populationsInt J Mol Sci20141533671369724590128
- ToyRBauerLHoimesCGhaghadaKBKarathanasisETargeted nanotechnology for cancer imagingAdv Drug Deliv Rev201476799725116445
- SpivakMYBubnovRVYemetsIMLazarenkoLMTymoshokNOUlbergZRGold nanoparticles – the theranostic challenge for PPPM: nanocardiology applicationEPMA J2013411823800174
- HousniAZhaoYGold nanoparticles functionalized with block copolymers displaying either LCST or UCST thermosensitivity in aqueous solutionLangmuir20102615129331293920604503
- LiuAYeBApplication of gold nanoparticles in biomedical researches and diagnosisClin Lab2013591–2233623505903
- MieszawskaAJMulderWJFayadZACormodeDPMultifunctional gold nanoparticles for diagnosis and therapy of diseaseMol Pharm201310383184723360440
- FergusonJFAllayeeHGersztenRENutrigenomics, the microbiome, and gene-environment interactions: new directions in cardiovascular disease research, prevention, and treatment: a scientific statement from the American Heart AssociationCirc Cardiovasc Genet20169329131327095829
- DavisMEHsiehPCTakahashiTLocal myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarctionProc Natl Acad Sci USA2006103218155816016698918
- NagaokaKMatobaTMaoYA new therapeutic modality for acute myocardial infarction: nanoparticle mediated delivery of pitavastatin induces cardioprotection from ischemia-reperfusion injury via activation of PI3K/Akt pathway and anti-inflammation in a rat modelPLoS One2015107e013245126167913
- SpivakMYBubnovRVYemetsIMLazarenkoLMTymoshokNOUlbergZRDevelopment and testing of gold nanoparticles for drug delivery and treatment of heart failure: a theranostic potential for PPP cardiologyEPMA J2013412023889805
- BietenbeckMFlorianASechtemUYilmazAThe diagnostic value of iron oxide nanoparticles for imaging of myocardial inflammation – quo vadis?J Cardiovasc Magn Reson2015175426152269
- YangCTianALiZReversible cardiac hypertrophy induced by PEG-coated gold nanoparticles in miceSci Rep201662020326830764
- OkinPMDevereuxRBNieminenMSElectrocardiographic strain pattern and prediction of new-onset congestive heart failure in hypertensive patients: the losartan intervention for endpoint reduction in hypertension (LIFE) studyCirculation20061131677316365195
- ShimizuIMinaminoTPhysiological and pathological cardiac hypertrophyJ Mol Cell Cardiol20169724526227262674
- LiXMattaSMSullivanRDBahouthSWCarvedilol reverses cardiac insufficiency in AKAP5 knockout mice by normalizing the activities of calcineurin and CaMKIICardiovasc Res2014104227027925225170
- NichtovaZNovotovaMKralovaEStankovicovaTMorphological and functional characteristics of models of experimental myocardial injury induced by isoproterenolGen Physiol Biophys201231214115122781817
- KuokQYYehCYSuBCThe triterpenoids of Ganoderma tsugae prevent stress-induced myocardial injury in miceMol Nutr Food Res201357101892189623610080
- RizziEGuimaraesDACeronCSβ1-Adrenergic blockers exert antioxidant effects, reduce matrix metalloproteinase activity, and improve renovascular hypertension-induced cardiac hypertrophyFree Radic Biol Med20147330831724933619
- WallnerMDuranJMMohsinSAcute Catecholamine exposure causes reversible myocyte injury without cardiac regenerationCirc Res2016119786587927461939
- YangCYangHWuJNo overt structural or functional changes associated with PEG-coated gold nanoparticles accumulation with acute exposure in the mouse heartToxicol Lett2013222219720323906719
- YangLJiaZYangKExercise protects against chronic β-adrenergic remodeling of the heart by activation of endothelial nitric oxide synthasePLoS One201495e9689224809512
- YinQYangCWuJDownregulation of β-adrenoceptors in isoproterenol-induced cardiac remodeling through hurPLoS One2016114e015200527035432
- ChowdhuryIThompsonWEThomasKProhibitins role in cellular survival through Ras-Raf-MEK-ERK pathwayJ Cell Physiol20142298998100424347342
- VidalMWielandTLohseMJLorenzKβ-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gβγ/Erk-dependent pathwayCardiovasc Res201296225526422843704
- MoriscoCMarroneCGaleottiJEndocytosis machinery is required for β1-adrenergic receptor-induced hypertrophy in neonatal rat cardiac myocytesCardiovasc Res2008781364418194989
- MoriscoCZebrowskiDCVatnerDEVatnerSFSadoshimaJβ-Adrenergic cardiac hypertrophy is mediated primarily by the β1-subtype in the rat heartJ Mol Cell Cardiol200133356157311181023
- CuiYZhaoYTianYZhangWLüXJiangXThe molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coliBiomaterials20123372327233322182745
- SinghVNairSPAradhyamGKChemistry of conjugation to gold nanoparticles affects G-protein activity differentlyJ Nanobiotechnology201311723510390
- van VurenEJMalanLvon KänelRCockeranMMalanNTHyperpulsatile pressure, systemic inflammation and cardiac stress are associated with cardiac wall remodeling in an African male cohort: the SABPA studyHypertens Res201639964865327169396
- ZhaoLChengGJinRDeletion of interleukin-6 attenuates pressure overload-induced left ventricular hypertrophy and dysfunctionCirc Res2016118211918192927126808
- KumarSSethSJaiswalAChronic beta-adrenergic activation-induced left ventricular systolic dysfunction is associated with systemic release of TNF-alpha and IL-1-beta in ratsPharmacol Rep200961587087619904010
- Nagoor MeeranMFJagadeeshGSSelvarajPThymol attenuates inflammation in isoproterenol induced myocardial infarcted rats by inhibiting the release of lysosomal enzymes and downregulating the expressions of proinflammatory cytokinesEur J Pharmacol201575415316125724787
- GoebelMUMillsPJIrwinMRZieglerMGInterleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathwaysPsychosom Med200062459159810949106
- ChenHDorriganASaadSHareDJCortieMBValenzuelaSMIn vivo study of spherical gold nanoparticles: inflammatory effects and distribution in micePloS One201382e5820823469154
- GutkindGSOffermannsSA new G(q)-initiated MAPK signaling pathway in the heartDev Cell200916216316419217418
- BuenoOFDe WindtLJTymitzKMThe MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic miceEMBO J200019236341635011101507
- FuYXiaoHZhangYBeta-adrenoceptor signaling pathways mediate cardiac pathological remodelingFront Biosci (Elite Ed)201241625163722201979
- SonYCheongYKKimNHChungHTKangDGPaeHOMitogen-activated protein kinases species: how can ROS activate MAPK pathways?J Signal Transduct2011201179263921637379
- AradMSeidmanCESeidmanJGAMP-activated protein kinase in the heart: role during health and diseaseCirc Res2007100447448817332438
- McCubreyJALahairMMFranklinRAReactive oxygen species-induced activation of the MAP kinase signaling pathwaysAntioxid Redox Signal200689–101775178916987031
- DyckJRLopaschukGDAMPK alterations in cardiac physiology and pathology: enemy or ally?J Physiol2006574Pt 19511216690706
- ShaheenTIEl-NaggarMEHusseinJSAntidiabetic assessment; in vivo study of gold and core-shell silver-gold nanoparticles on streptozotocin-induced diabetic ratsBiomed Pharmacother20168386587527505864
- DkhilMABauomyAADiabMSAl-QuraishySAntioxidant and hepatoprotective role of gold nanoparticles against murine hepatic schistosomiasisInt J Nanomedicine2015107467747526719689
- NegahdaryMChelongarRZadehSKAjdaryMThe antioxidant effects of silver, gold, and zinc oxide nanoparticles on male mice in in vivo conditionAdv Biomed Res201546925878994
- SelimMEAbd-ElhakimYMAl-AyadhiLYPancreatic response to gold nanoparticles includes decrease of oxidative stress and inflammation in autistic diabetic modelCell Physiol Biochem201535258660025612738
- TakahashiHNiidomeYNiidomeTKanekoKKawasakiHYamadaSModification of gold nanorods using phosphatidylcholine to reduce cytotoxicityLangmuir20062212516378388
- PanYNeussSLeifertASize-dependent cytotoxicity of gold nanoparticlesSmall20073111941194917963284
- VeroneseFMPasutGPEGylation, successful approach to drug deliveryDrug Discov Today200510211451145816243265
- AlkilanyAMMurphyCJToxicity and cellular uptake of gold nanoparticles: what we have learned so far?J Nanopart Res20101272313233321170131
- FadeelBGarcia-BennettAEBetter safe than sorry: understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applicationsAdv Drug Deliv Rev201062336237419900497