Abstract
By using triangular silver (Ag) nanoparticle array, a localized surface plasmon resonance (LSPR) nanosensor was fabricated and shown to sense serum p53 protein in vitro, which is involved in head and neck squamous cell carcinoma (HNSCC). The nanosensor consists of a triangular Ag nanoparticles array with single particle dimension of 120 nm in-plane width and 45 nm out-of-plane height. When examined using LSPR nanobiosensor, the results indicated significant difference in LSPR shifts (Δλmax) between HNSCC patient and control. Although there is need for precise quantification and large-scale prospective, this report shows that the LSPR nanobiosensor provides a promising platform with attractive advantages for serological diagnosis or molecular diagnosis in tumor, such as HNSCC. This is the first clinical application of the LSPR nanosensor in HNSCC.
Introduction
p53 protein is a 53-kDa nuclear tumor suppressor protein present in humans and is encoded by the TP53 gene. When tumors develop, point mutations at the TP53 gene can lead to overexpression of p53 proteins, which contribute to continuous cell division and canceration. Overexpression of p53 has been reported in 60% of laryngeal carcinomas, 37% of hypopharyngeal carcinomas, and 52% of tongue carcinomas.Citation1–Citation3 With the mortality and disintegration of tumor cells, p53 protein released from cancer cells will enter into the circulation. The serum p53 protein level in carcinoma of colon, lung, and pancreas was significantly increased compared with normal controls.Citation4–Citation6 It was reported that 68 out of 75 patients (91%) with head and neck squamous cell carcinoma (HNSCC) had detectable serum p53 protein in the preoperative blood.Citation7 The detection of serum p53 protein may play an important role in serological diagnosis of tumor, including HNSCC. The serum p53 protein level can be examined mainly by enzyme-linked immunosorbent assay (ELISA). ELISA has advantages of sensitivity, reproducibility, and stability. Its applications are constrained for high-titer anti-p53 antibody interference of some noncancer patients and it is a time-consuming, complicated and inconvenient procedure. To address these considerations, sensitive, easy to use, and cost-effective portable biosensors that are capable of providing continuous monitoring and rapid detection of serum p53 protein need to be developed.
The localized surface plasmon resonance (LSPR)-based nanobiosensor is a new type of optical biosensor technique that combines nanotechnology with optical biosensor technology. LSPR is one of the specific characteristics of metallic or metalized nanostructured materials, such as noble metal nanoparticles. It can be excited when the incident photon frequency is resonant with the collective oscillation of the conduction electrons.Citation8 Transmission peaks of LSPR-related spectra are sensitive to the electric medium on the surface of noble metals. It demonstrates that the applicability of this biosensor can be more defined in a wide range of fields, such as medical, food safety, environmental monitoring, and drug screening.Citation9–Citation11
The aim of the present study is to apply the developed LSPR biosensor based on the triangular silver (Ag) nanoparticles for the detection of p53 protein levels in samples from HNSCC patients. This is the first case report of the LSPR sensor that responds to serum p53 protein of a cancer patient and a healthy control.
Materials and methods
Materials
11-Mercaptoundecanoic acid (11-MUA) and 1-octanethiol (1-OT) were purchased from Sigma Aldrich (St. Louis, MO). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and sulfo-N-hydroxysuccinimide (S-NHS) were purchased from Aladdin (Shanghai, China). A mouse monoclonal p53 antibody raised against full-length p53 of human origin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Absolute ethanol and phosphate-buffered saline (PBS; 10 mM, pH 7.4) were purchased from Beijing Biosynthesis Biotechnology Company (Beijing, China). The buffer used in the experiments was prepared using double-distilled water.
Patients and sample
Two serum samples from one HNSCC patient and one healthy control were collected from West China Hospital of Stomatology, Sichuan University, Chengdu, People’s Republic of China, in 2009. Informed consent documents were signed prior to the study.
The HNSCC patient was diagnosed by biopsy preoperatively and had no prior radiotherapy or chemotherapy. One venous blood sample was obtained from the patient before operation and another from a healthy volunteer as healthy control.
Once blood was collected, it was allowed to stand at 37°C, for 2 h, and then was centrifuged at 800 g for 10 min. The serum was collected and stored at −80°C until analysis.
Preparation and functionalization of the LSPR-based nanobiosensor
The sensor chip in this work was produced using nanosphere lithography (NSL) technology. K9 glass substrates (Juke Co., Chengdu, China) were cleaned by piranha solution (1:3 30% H2O2/H2SO4) at 80°C for 30 min. Then they were rinsed with copious amounts of second distilled water and sonicated for 60 min in 5:1:1 H2O/NH4OH/30% H2O2. After 7 μL of nanosphere solution (Duke Scientific, Palo Alto, CA) was spin coated onto the substrate, 50 nm Ag was deposited by a thermal evaporation system (C-Vac400-I; C-Vac Inc., Chengdu, China). Finally, the Ag nanostructures on the substrate for the experiments were formed after the nanospheres were removed by ultrasonic in ethanol.
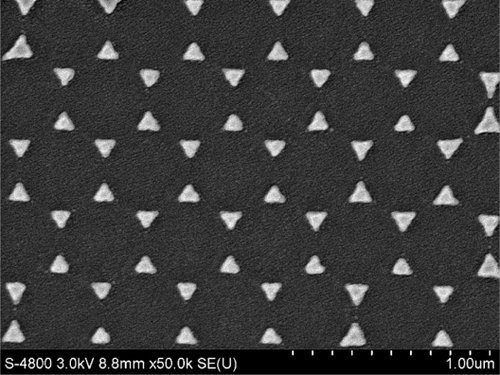
We measured the surface modality of the samples by using a scanning electron microscope (S-4800; Hitachi, Tokyo, Japan). The triangular Ag nanoparticles have dimensions of 120 nm in-plane widths, 400 nm period of the nanoparticles array as measured by SEM, and 45 nm out-of-plane heights as measured by a sidestep apparatus. shows the SEM micrograph of the nanoparticles.
Figure 1 SEM image of topography of the triangular Ag nanoparticles fabricated by nanosphere lithography (×50,000).
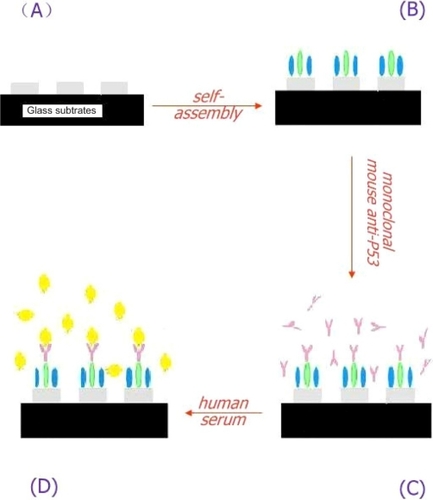
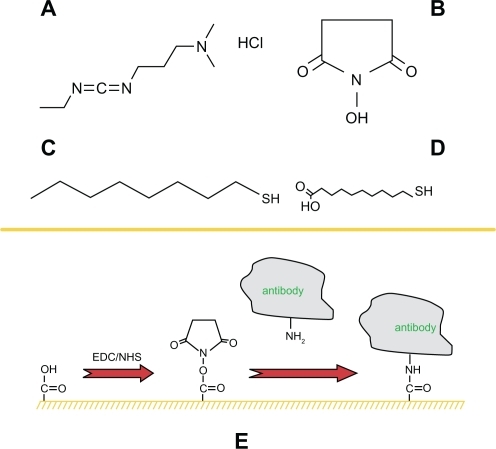
Functionalization of the sensor chip is a multistep process that provides the surface for specific detection applications. First, a self-assembled monolayer of 2 mM 1-OT/1 mM 11-MUA acid was formed onto the surface by incubation in an ethanoic solution for 24 h. After thoroughly rinsing and drying, the biochip was then incubated in 20 mM EDC/10 mM S-NHS/2 μg/mL monoclonal mouse anti-p53 solution for 12 h in the dark to link the antibody to the surface of nanoparticles. EDC, a zero-length coupling agent, can couple the amine groups on the antibody to the carboxyl groups on 11-MUA. This process is facilitated by the presence of S-NHS. The samples were thoroughly rinsed with 0.01 M PBS buffer and then dried by blowing N2 with high pressure. Finally, an Ag p53 nanobiosensor was formed. A schematic illustration of the prefunction for the nanoparticles surface is shown in .
Figure 2 A schematic illustration of the prefunction for the nanoparticles surface. Four chemical structures: A) EDC·HCl; B) S-NHS; C) 1-OT; D) 11-MUA. E) A mixed monolayer of 1-OT and 11-MUA is formed on the exposed surfaces of the Ag nanoparticles. EDC is a zero length coupling agent and can couple the amine groups on the antibody to the carboxyl groups on 11-MUA.
The serum samples were then rinsed using 0.01 M PBS buffer, and 40 μL of diluted serums from human serum were spotted onto the surface of this functionalized biochip for 150 min ().
Spectroscopy and observation
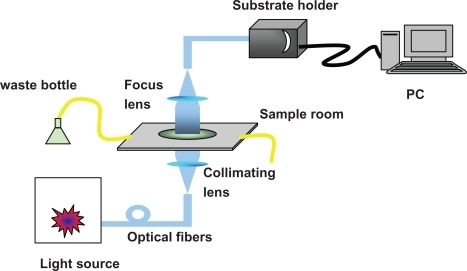
The integrated LSPR sensor used in this study is our home-built system (). The peak wavelength of LSPR extinction spectrum (λmax) excited by the Ag triangular nanoparticles array was measured and recorded using a UV-visible spectroscope (Sciencetech 9005; Sciencetech, Ottawa, Canada). Practical measurements of the UV-visible extinction spectra were carried out using a fiber-coupled spectrometer (USB4000; Ocean Optics, Dunedin, FL) and a CCD detector (Koan Electro-Optics Co., Shanghai, China).
Figure 4 Schematic representation of integrate LSPR nanobiosensor. The sensor chip is loaded by substrate holder (on the top of the system).
The measuring procedure was divided into three steps: 1) collecting the reference light without sensor chip, 2) collecting the background noise to increase the accuracy, and 3) measuring the transmission from the sensor chip without any analysis. The sensor’s extinction spectra can be caculated by software. With these processes, the sensor’s extinction spectra can be easily obtained by in computer, and we can record and compare the data of different sensor chips. The shift toward longer wavelengths is referred to as a redshift and is denoted as (+), whereas the shift toward shorter wavelengths is referred to as a blueshift and is denoted as (−). The system resolution of this biosensor is about 5 nm. When the wavelength shift of the absorbance peak is less than + 5 nm, it indicates that the biosensor cannot sense the medium at the nanoparticles–solution interface.
Results
LSPR spectra of the Ag nanosensors in each processing step are shown in and , with the incidence wavelength ranging from 400 to 700 nm. The resulting extinction spectrum of the bare Ag nanoparticles is depicted in , where the LSPR λmax was measured to be 588.2 nm (). The LSPR λmax of Ag nanoparticles after modification with 11-MUA/1-OT was 623.5 nm (). The LSPR λmax shift corresponding to this step was a 35.3-nm redshift. After p53 antibody attachment, the λmax of modified Ag nanoparticles chip was 673.84 nm (). There is an additional 50.34-nm redshift from the second peak. The LSPR shifts from 673.84 to 676.08 nm during the serum incubation for the control serum, and then Δλmax was a 2.24-nm shift (). Redshift volume of 2.24 nm is less than 5 nm, which demonstrates that LSPR sensor chip shows no response to control serum.
Figure 5 Design of the LSPR biosensor for serum p53 protein detection in control. A) Ag nanoparticles before chemical modification,λmax = 588.2 nm; B) Ag nanoparticles after modification with 11-MUA/1-OT, λmax = 623.5 nm; C) The chip with 2 μg/mL mouse monoclonal p53 antibody, λmax = 673.84 nm; D) The biochip after incubation with control serum sample, λmax = 676.08 nm. All extinction measurements were collected at room temperature.
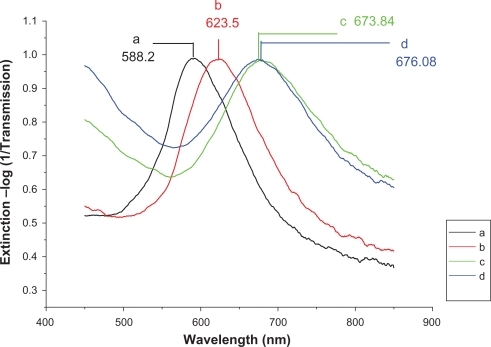
Figure 6 Design of the LSPR biosensor for serum p53 protein detection in HNSCC. A) Ag nanoparticles before chemical modification, λmax = 584.13 nm; B) Ag nanoparticles after modification with 11-MUA/1-OT, λmax = 610.9 nm; C) The chip with 2 μg/mL mouse monoclonal p53 antibody, λmax = 671.41 nm; D) The biochip after incubation with HNSCC serum sample, λmax = 689.08 nm. All extinction measurements were collected at room temperature.
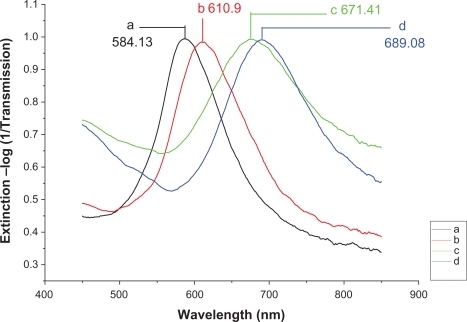
The measured extinction spectrum of serum from the HNSCC patient is shown in . The λmax of the biochip before and after incubation with HNSCC serum sample was 671.41 and 689.08 nm, respectively (). There was a 17.67-nm shift, which is a significant shift to demonstrate the p53 level change in the sample. The peak shift for the HNSCC patient is more than 5 nm, which is larger than that of the control. These results suggest that the LSPR nanobiosensor can respond to p53 protein in HNSCC patient’s serum and illustrate the difference of serum p53 level of the HNSCC patient and healthy control.
Discussion
The optical characteristics of the nanochip were indexed by the wavelength shift of the absorbance peak, namely Δλmax.Citation8 A significant change was observed in the wavelength shift using our LSPR-based nanochip. We found that the extinction spectroscopy varies with each step of surface functionalization for the Ag nanoparticles. In addition, the results showed a significant difference in LSPR shifts (Δλmax) between the HNSCC patient and control. LSPR sensor chip showed no response to control serum, whereas it showed obvious response to HNSCC serum. This suggested a different concentration of p53 protein level between two groups. Chow et al demonstrated that the mean serum p53 protein concentration of the HNSCC patients was 59.45 pg/mL, which was significantly higher than that of the healthy donors (16.4 pg/mL) when examined using ELISA.Citation7 This result is similar to our experimental results. Moreover, our LSPR method provides free-labeling process, which cannot be obtained using ELISA.
These results indicate the future prospects of LSPR sensor chip application in tumor serological diagnosis. However, further investigation is necessary to evaluate the feasibility of clinical application of this LSPR biosensor system. A large-sample clinical random case-control study with a long follow-up period is in preparation.
This experiment is the first case report of the LSPR sensor, which responded to serum p53 protein of a HNSCC patient and a healthy control. The triangular Ag nanoparticles have dimensions of 120 nm in-plane width, 45 nm out-of-plane height, and 400-nm period of the nanoparticles array. The size of Ag triangle is different from that used in published research. Ag nanoparticles can produce stronger localized surface plasmon than gold nanoparticles, although they are more easily oxidized. In addition, the LSPR peak of Ag particles is also easier to detect.Citation12 The chip can be preserved in a vacuum plastic bag as long as 1 month. In a normal environment at room temperature, the storage period is 1 week. Meanwhile, the regeneration can be achieved by removing the Ag nanoparticles on the substrate, which is advantageous because the substrate can be thoroughly cleaned so as to improve the fabrication quality of the chips for future experimental use.
All the extinction measurements were carried out at room temperature, and all the samples were exposed in ordinary room conditions with dust and vapor, which illustrates that our Ag particle-based sensor can be used in an open space without any sterilization process. The real-time LSPR-shift assay had a similar response to a commercial surface plasmon resonance instrument, but it demonstrated less interference from bulk refractive index. In addition, LSPR possesses greater spatial resolution, both lateral and normal, when compared with SPR.
Conclusion
For the first time, we have proposed a LSPR-based nanobiosensor for the detection of p53 protein from an HNSCC patient and a control. We discovered dramatic differences in LSPR shifts (Δλmax) between the HNSCC patient and control. Further large-scale prospective study is worthwhile to evaluate the value of our nanobiosensor for serological diagnosis or molecular diagnosis in tumors, such as HNSCC. However, we anticipate that this technology will be a promising candidate for point-of-care diagnosis and therefore promote the development of personalized medicine in a simple and rapid format.
Acknowledgements
This work has been supported by the Natural Science Foundation of China (60736037), the 973 Program of China (No. 2006-CB302900), and the Key Technology R & D of Sichuan (NO. 2009SZ0202). The authors also thank Ping Gao and Fei Li for their kind contribution to this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- MaestroRDolcettiRGasparottoDHigh frequency of p53 gene alterations associated with protein overexpression in human squamous cell carcinoma of larynxOncogene199276115911661594246
- FrankJLBurMEGarbJLP53 tumor suppressor oncogene expression in squamous cell carcinoma of hypopharynxCancer19947311811868275422
- LeedyDATruneDRKronzJDTumor angiogenesis, the p53 antigen, and cervical metastasis in squamous cell carcinoma of the tongueOtolaryngol Head Neck Surg199411144174227524005
- LuoJCNeugutAIGarbowskiGLevels of p53 antigen in the plasma of patients with adenomas and carcinomas of the colonCancer Lett19959122352407767914
- LuoJCZehabRAnttilaSDetection of serum p53 protein in lung cancer patientsJ Occup Med19943621551608176513
- SuwaHOhshioGOkadaNClinical significance of serum p53 antigen in patients with pancreatic carcinomaGut19974056476539203945
- ChowVWingAPLamKYHoWKWeiWIPrognostic significance of serum p53 protein and p53 antibody in patients with surgical treatment for head and neck squamous cell carcinomaHead Neck200123428629111400229
- ZhaoJZhangXYonzonCRHaesAJvan DuyneRPLocalized surface plasmon resonance biosensorsNanomedicine20061221922817716111
- HaesAJHallWPChangLKleinWLvan DuyneRPA localized surface plasmon resonance biosensor: first steps toward an assay for Alzheimer’s diseaseNano Lett20044610291034
- ZhuSDuaCFuYLocalized surface plasmon resonance-based hybrid Au–Ag nanoparticles for detection of Staphylococcus aureus enterotoxin BOpt Mater2009311116081613
- KreuzerMPQuidantRSalvadorJ-PMarcoMPBadenesGColloidal-based localized surface plasmon resonance (LSPR) biosensor for the quantitative determination of stanozololAnal Bioanal Chem20083911813182018373230
- HaoESchatzGCElectromagnetic fields around silver nanoparticles and dimersJ Chem Phys2004120135736615267296