Abstract
The etiology of dental pulp stones, one type of extraskeletal calcification disease, remains elusive to date. Calcifying nanoparticles (CNPs), formerly referred to as nanobacteria, were reported to be one etiological factor in a number of extraskeletal calcification diseases. We hypothesized that CNPs are involved in the calcification of the dental pulp tissue, and therefore investigated the link between CNPs and dental pulp stones. Sixty-five freshly collected dental pulp stones, each from a different patient, were analyzed. Thirteen of the pulp stones were examined for the existence of CNPs in situ by immunohistochemical staining (IHS), indirect immunofluorescence staining (IIFS), and transmission electron microscope (TEM). The remaining 52 pulp stones were used for isolation and cultivation of CNPs; the cultured CNPs were identified and confirmed via their shape and growth characteristics. Among the dental pulp stones examined in situ, 84.6% of the tissue samples staines positive for CNPs antigen by IHS; the corresponding rate by IIFS was 92.3 %. In 88.2% of the cultured samples, CNPs were isolated and cultivated successfully. The CNPs were visible under TEM as 200–400 nm diameter spherical particles surrounded by a compact crust. CNPs could be detected and isolated from a high percentage of dental pulp stones, suggesting that CNPs might play an important role in the calcification of dental pulp.
Introduction
Frequently found on bitewing and periapical radiographs, pulp stones are discrete calcified bodies that are either located in the pulp tissue or attached to or embedded into dentine. Pulp stones are an oral disease with high incidence. It was reported that in young Australian adults, the prevalence of pulp stones was 46.1% among dental patients who go to clinics and 10.1% of the teeth were affected. Occurrences were rare in premolars (0.4%), but significantly higher in molars (19.7%).Citation1 The first and second molars were the teeth most commonly affected.Citation2
Pulp stones may occur in one or all of the teeth in one person, even in unerupted or impacted teeth. Located in the pulp cavity or root canal, these stones often narrow or even obstruct the access to the apical point root canal. The calcification of pulp tissue might lead to the failure of root canal therapy and loss of the teeth.
The etiology of pulp stones has been controversial. Etiological factors implicated include aging,Citation3 biological factors,Citation4 physical factors,Citation5,Citation6 and chemical factors.Citation7,Citation8
The discovery of calcifying nanoparticles (CNPs), also referred to as nanobacteria, led to novel theories for pathological calcification. CNPs first appeared as self-propagating calcifying macromolecular complexes found in bovine and human blood and blood products. These nanoparticles could produce biogenic carbonate apatite on their cell envelope at all growth phases, which resulted in white biofilm and mineral aggregates closely resembling those found in tissue calcification in the human body.Citation9 CNPs are capable of producing nucleate hydroxyapatite; thus, they have been heralded as one potential etiological factor of pathological calcification, such as kidney stones or kidney cyst,Citation10–Citation18 gall stones,Citation18–Citation20 atherosclerosis,Citation21–Citation23 and dental calculus in periodontitis.Citation24 As the lacking of satisfactory 16S rRNA sequence, the controversy exists on the bacteria state on the so-called ‘nanobacteria’, so CNPs best describes the agent.Citation25
Pulp stones are the characteristic manifestation of physiological or pathological calcification of the human body reflected in the dental pulp tissue. It could be hypothesized that CNPs are involved in the calcification of the dental pulp tissue.
Materials and methods
Collection of pulp stones
Sixty-five randomly collected pulp stones, one from each patient, were scaled out by endodontists during root canal therapy (38 from maxillary molars and 27 from mandibular molars) in the Hospital of Stomatology, Sun Yat-sen University, Guangzhou, China. Ages of the patients ranged from 28 to 60 years. Sample information is listed in . Written informed consent was obtained from each participant prior to enrollment, with approval of the ethical committee of the Hospital of Stomatology, Sun Yat-sen University. The pulp stones were collected over a period of 1 year. All of the dental pulp stones were kept in sterile normal saline before further treatments.
Identification of pulp stones in situ
Thirteen of the dental pulp stones were immersed in 4% paraformaldehyde and nitric acid for demineralization for 24–72 h and neutralized with 5% sodium sulfate. After washing in water for 12 h, each pulp stone, considered as one sample, was paraffin embedded and cut into 5-μm sections. Three randomly selected tissue sections from each sample were prepared for immunohistochemical staining (IHS) and indirect immunofluorescence staining (IIFS). Each tissue section was deparaffinized, rehydrated, and demineralized and then processed as described earlier.Citation9 IgG1 class anti-CNP mAbs, 8D10 (Nanobac Oy, Kuopio, Finland) was used as primary antibody in IHS and IIFS. The samples were examined under a Microphot-FXA microscope (Nikon Corporation, Tokyo, Japan) with fluorescence and differential interference contrast optics. Negative control sections underwent the same staining process, except that the primary mAb step was replaced with phosphate-buffered saline (PBS) buffer. Those with positive results were further examined with a transmission electron microscope (TEM; Hitachi H-600; Hitachi, Tokyo, Japan) operated at 15 kV, and micrographs were recorded.
Culture of CNPs from pulp stones
The remaining 52 pulp stones were randomly classified into 17 samples, with 3–4 for each sample. Samples were immersed in 0.3 μg/mL tetracycline and then demineralized in 1 N HCl for 30 min, then powdered and neutralized with 0.5 M Tris (pH 10.5; Sigma, St Louis, MO). Suspensions were centrifuged at 14,000 g for 15 min in a Minispin® Centrifuge and sterile filtered through 0.22-μm millipore filters. The filtrate was cultured in flask containing Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Carlsbad, CA) with 10% fetal bovine serum (FBS; Sigma) as described in previous studies.Citation26 All FBS was γ-irradiated at a minimum dose of 30 kGy at room temperature for 24 h. DMEM incubated with FBS and Tris but without stone filtrate was used as negative controls. Subcultures were derived with a rubber scraper in serum-contained DMEM after 4 weeks of initial inoculation. All of the cultures were harvested by centrifugation at 20,000 g for 45 min at 4°C, washed with PBS (pH 7.2), and used for identification of CNPs.
Culture examination
The cultures were incubated for 8 weeks and evaluated microscopically for the growth of CNPs during and at the end of the incubation period. After incubation for 2, 4, or 8 weeks, the cultures were washed with PBS and photographed under inverted phase contrast microscope (Axiovert 40; Zeiss, Oberkochen, Germany). The cultures were examined by TEM to verify the detail on the morphology. For negative staining, samples were isolated by centrifugation at 20,000 g for 1 h directly from FBS and diluted 1:5 in PBS. A carbon-coated 400-mesh copper grid was placed on a drop of CNP suspension in PBS for 1 min, washed with water, and stained on a drop of 1% phosphotungstic acid for 90 sec. Photographs were taken using TEM (JEM-100CXII; JEM, Tokyo, Japan) for morphological identification.
Identification of CNPs from cultures
All culture samples were harvested by 20,000 g centrifugation for 30 min at 4°C after 8 weeks of incubation. The pellets were analyzed for IHS and IIFS using CNP surface-antigen-specific mAb 8D10 to verify the existence of CNPs. In both IHS and IIFS, culture samples were spread on coverslips, fixed with 4% paraformaldehyde for 1 h, incubated at 70°C for 10 min, and blocked in 3% H2O2 and 5% bovine serum albumin (BSA)-containing normal serum for 30 min, respectively. In IHS, the particles were incubated with mouse monoclonal antinanobacteria antibodies 8D10 (NanoBac Oy) at 4°C overnight; subsequently, the particles were sequentially incubated with biotinylated goat antimouse IgG antibody and streptavidin–biotin–peroxidase complex at 37°C for 30 min and visualized in diaminobenzidine solution for 10 min. In IIFS, the cells were incubated with tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat antimouse IgG antibody at 37°C for 30 min; slides were then washed, dehydrated, and mounted for observation under a fluorescent microscope. In IHS and IIHS, the primary antibody was omitted in negative controls. Furthermore, von Kossa staining was applied to confirm the presence of mineralization. For von Kossa staining, cultures were harvested and reseeded into wells of a 24-well plate containing medium; after 24-h incubation, von Kossa staining was performed as described previously.Citation27 Energy-dispersive X-ray analysis (EDX) were performed to show the topographic features by a scanning electron microscope (Quanta400; Philip FEI, Amsterdam, The Netherlands) equipped with EDX (INCA, Oxford, UK) as previously described.Citation9
Results
Identification of CNPs in pulp stones in situ
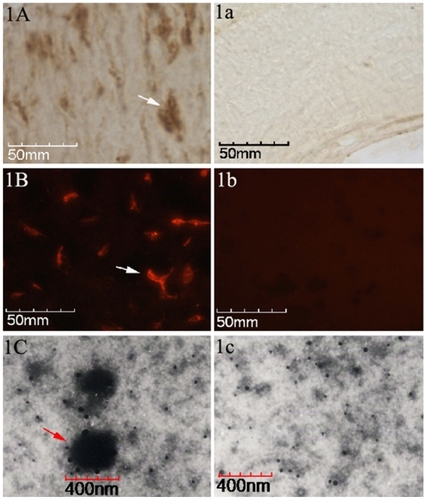
Eleven out of the 13 tissue samples (84.6%) stained positive for CNPs antigen for IHS, whereas by 12 positive samples were detected by IIFS (92.3%). Positive staining results for IHS, as a brown-colored precipitate at the antigen site, are shown in . To negative result is shown in . The red-fluorescence-labeled signals of positive results for IIFS are shown in , and the corresponding negative result is shown in . TEM identified a large number of spherical coccoid particles, from 200 to 400 nm, as shown in , and the negative control is shown in .
Figure 1 Identification of CNPs in paraffin-embedded pulp stone sections. A) The brown colored areas indicated by black arrows, by point indicate positive signals (existence of CNPs antigen) in the tissues by IHS. a) Negative control by IHS. B) Positive signals can be identified as red fluorescence by, IIFS. b) Negative control by IIFS. C) A TEM image at 35,000× zoom-in level shows the CNPs as spherical particles surrounded by a compact crust, 200–400 nm in diameter. c) Negative control in TEM image.
Morphology of CNPs in culture
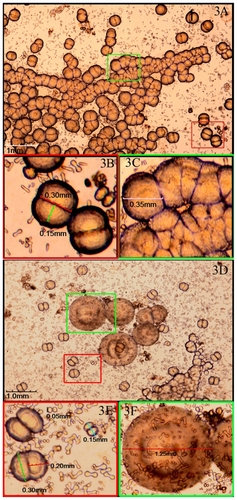
CNPs were successfully cultured from 15 of the 17 samples (see ‘Materials and methods’) after incubation for 2 weeks, demonstrating a positive rate of 88.2%. The CNPs were first seen as individually separated and shaped typically as coccoid, bacilliform, or dumbbell with a highly refractile crust after 2 weeks, culture (). Those particles were very tiny with a diameter of 0.125 to 0.05 mm and barely detectable (). In 4 weeks, particles grew bigger and the average diameter was about 0.10 mm (). After 6–8 weeks, larger carbonate particles were aggregated in clumps and easily observed under the microscope. They were shown as coccoid particles with a diameter of 0.30–0.40 mm and aggregated in crowds (). Some of them nearly fused together to form ‘concentric circles’ () with a diameter of nearly 1.25 mm. Surrounding the ‘concentric circles’, smaller size particles could be also seen () and aggregated around relatively larger particles like ‘satellites’.
Figure 2 Cultures of CNPs observed through inverted phase contrast microscopy at 2–4 weeks. A) Particles were individually separated and were typically in coccoid, bacilliform, or dumbbell shape with a highly refractile crust after 2-week culture. a) Enlarged picture of (A) showing the particles were very tiny with a diameter of 0.025–0.05 mm. B) Particles grew bigger after 4-week culture. b) Enlarged picture of (B) showing the average diameter was about 0.10 mm.
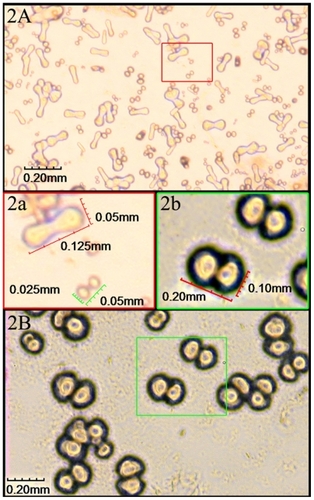
Figure 3 Viewing CNPs in particular culture stages at 6–8 weeks. A) Larger particles aggregated in clumps after 6–8 weeks of culture. B) and C) Enlarged pictures of (A) showing coccoid particles with a diameter of 0.30–0.40 mm and aggregated in crowds. D) ‘Concentric circles’ by the fused particles. E) and F) Enlarged pictures of (D) showing the ‘concentric circles’ with a diameter of nearly 1.25 mm and surrounded by smaller particles like ‘satellites’.
Culture examination
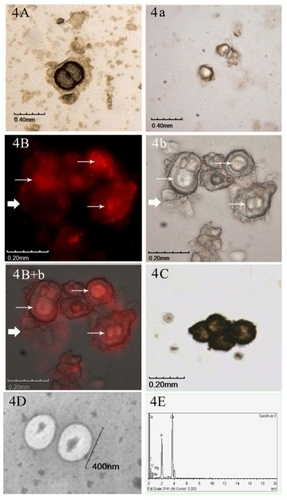
By IHS, the harvested cultured particles exhibited a clearly visible yellow or brown staining with the anti-CNPs antibody 8D10 (), and no visible positive signal was detected in the negative controls (). Similarly, strong red fluorescent signals were detected by IIFS (). The same view under the light microscope is shown in . In the merged image under fluorescence and light microscope (), the thin arrow represents positive signals, and those represented by the thick arrow show the negative control. After von Kossa staining, the particles were colored black or brown, and their surrounding calcifying crust could be distinguished (). TEM image of the CNPs at 20,000× magnification, showing as 200–400 nm spherical particles (), and the topographic features on chemical composition of CNPs, were identified with calcium and phosphate peaks by EDX ().
Figure 4 Identification of CNPs in cultures. A) IHS positive result using anti-CNPs monoclonal antibody. a) Negative control by IHS. B) IIFS positive results of cultured CNPs using anti-CNPs monoclonal antibody. b) The same view under the light microscope by IIFS. B + b) Merged picture of image under fluorescence and light microscope. Thin arrow represents positive signals, and those represented by thick arrows show the negative control by IIFS. C) von Kossa staining of the CNPs. D) TEM image of the CNPs at 20,000× magnification, showing 200–400 nm spherical particles. E) Chemical analysis of CNPs by EDX showed calcium and phosphate peaks.
Discussion
CNPs have spurred a major controversy in modern microbiology. Ambiguity of bacteriologic evidence for CNPs has led to various hypotheses of the nature and origin of CNPs, such as those of primordial life formsCitation28–Citation30 and those of inert mineral fetuin complexes.Citation16,Citation31,Citation32 However, evidence for a significant role of CNPs in human biology has been accumulating.
CNPs were first identified in human kidney stones.Citation33 Subsequently, a rat model of CNPs-induced kidney stones was successfully established.Citation11 Since then, the significance of CNPs had extended substantially beyond kidney stones. Now, they are believed to be implicated in a variety of pathological calcifications in the human body.
Ciftcioglu et alCitation34 suggested observation of mineralized fibers and numerous small lobular on the teeth with pulp stones, and if being cultured with nanobacterias (CNPs), revealed voluminous mineral formation, resembling dental pulp stones. Based on Ciftcioglu’s morphological observation, it could be hypothesized that CNPs might be implicated in the calcification of dental pulp stones. In the present study, in order to test this hypothesis, first, CNPs were identified in the dental pulp stones in situ. Furthermore, CNPs were isolated and characterized from the stone samples derived from cultures. Among the dental pulp stones examined in situ, the CNP-positive rates by IHS and IIFS analysis were 84.6% and 92.3%, respectively. In 88.2% of the cultured samples, CNPs were isolated and cultivated successfully. Therefore, the results suggested a potential link between the CNPs and human dental pulp stones.
Efforts were made in the present study to rule out of possibility of false positives of CNPs cultured from pulp stones. First, CNPs were reportedly found in 80% of the commercial FBS products.Citation9 In this study, high dose of γ-ray (30 kGy) radiation was applied for FBS to destroy the FBS-contained CNPs and thus to avoid false positive results.Citation35,Citation36 Second, FBS contained inhibitors on apatite crystal formation, such as osteopontin, osteocalcin, and fetuin.Citation35,Citation37 These inhibitors might interfere with the mineralization and, therefore, the visualization of CNPs. In this study, heat-inactivated FBS was used to remove the inhibitors of apatite crystal formation and avoid false negative results. Third, it was of great importance to ensure the CNPs isolated in this study were from pulp stone samples, not from other contaminating sources. Three anticontamination measures were taken. 1) All dental pulp stones used for the isolation of the CNPs were initially immersed in tetracycline for 30 min. 2) All reagents and instruments were sterilized, and rubber dam were utilized in clinical treatment. 3) The supernatants of CNPs cultures were filtered through a 0.22-μm minipore filter, a generally accepted method to remove the common bacteria, fungi, and mycoplasma. Fourth, no other cellular entities were present as proved by microscopy and culture tests, and no cloudiness of color or pH changes were observed in medium. Therefore, it could be concluded that CNPs were, for the first time, isolated and identified from dental pulp stones.
Zhang et alCitation24 described the detection of CNPs in gingival crevicular fluid and dental calculus as refractive aggregates in clumps after 6-week culture and the formation of a white-colored layer visible to the naked eye after 2 months. Our study provided more detailed information on the culture of CNPs in different growing states. CNPs isolated in the present study were coccoid with a highly refractive shell, similar in size and morphology to the CNPs reported in other pathological calcification tissues in the human body.Citation22,Citation38,Citation39 The morphological changes could be observed during the culture period. From the barely detectable tiny coccoid or dumbbell particles with a diameter of 0.025–0.05 mm after 2-week culture, these particles grew gradually to an average diameter of about 0.10 mm after 1 month. Furthermore, clumps aggregated in crowds were generated with average diameter of 0.30–0.40 mm in 6–8 weeks, and particles of all sizes could be observed. These observations indicated that CNPs were in a growing state and could bud off new particles unceasingly. In addition, two interesting phenomena in the cultures of CNPs from pulp stone were indicated.
First, ‘concentric circles’ formed after about 6–8 weeks of culture; this morphology has not been reported before for CNPs. It could be speculated that the individually separated CNPs not only aggregated into clusters, but also merged and exhibited this specific appearance. Coincidentally, one of the characteristic features of microcosmic structures observed in the dental pulp stone sections under scanning electron microscopy was also concentric circles.Citation40,Citation41 Thus, it is possible that the miniature form of concentric circles could be the very onset of dental pulp stone formation, and the gradual accumulation of calcium and phosphate around CNPs might lead to larger mineralization such as pulp stones.
Second, minute particles like ‘satellites’ aggregated around relatively larger particles, which could be observed in the subculture flasks. Interestingly, all these particles always appeared ~24 h later in subcultures than those in primary cultures. It could be hypothesized that the subculturing procedure of filtering through 0.22-μm minipore prevented the passage of larger particles or stripped them off the outer apatite crusts. As a result, CNPs in the subcultures were smaller and needed time to accumulate calcium and phosphate around their outer walls to become visible again.
In conclusion, this study has revealed the prevalence of CNPs in human dental pulp stones. The findings raised the possibility that CNPs might be implicated in the formation or development of dental pulp stones. Further studies are required to test whether CNPs are the causative agents of pulp stones or are simply a marker of the pathologic state. These investigations might lead to novel approaches for preventing or treating pulp stones and consequentially facilitating successful root canal therapy.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (No. 30471884).
Supplementary material
Table S1 Sample information on the selected teeth for pulp stones collection
Disclosure
The authors report no conflicts of interest in this work.
References
- RanjitkarSTaylorJATownsendGCA radiographic assessment of the prevalence of pulp stones in AustraliansAust Dent J2002471364012035956
- al-Hadi HamashaADarwazehAPrevalence of pulp stones in Jordanian adultsOral Surg Oral Med Oral Pathol Oral Radiol Endod19988667307329868733
- HillmannGGeurtsenWLight-microscopical investigation of the distribution of extracellular matrix molecules and calcifications in human dental pulps of various agesCell Tissue Res199728911451549182609
- YamazoeTAokiKSimokawaHOhyaKTakagiYGene expression of bone matrix proteins in a calcified tissue appeared in subcutaneously transplanted rat dental pulpJ Med Dent Sci2002491576612160227
- RobertsonALundgrenTAndreasenJODietzWHoyerINorénJGPulp calcifications in traumatized primary incisors. A morphological and inductive analysis studyEur J Oral Sci199710531962069249185
- SübayRKKayaHTarimBSübayACoxCFResponse of human pulpal tissue to orthodontic extrusive applicationsJ Endod200127850851111501587
- HoltgraveEAHopfenmullerWAmmarSTablet fluoridation influences the calcification of primary tooth pulpJ Orofac Orthop2001621223511227204
- HoltgraveEAHopfenmullerWAmmarSAbnormal pulp calcification in primary molars after fluoride supplementationASDC J Dent Child2002692126201206
- KajanderEOCiftciogluNNanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formationProc Natl Acad Sci U S A19989514827482799653177
- JonesJACiftciogluNSchmidJFBarrYRGriffithDCalcifying nanoparticles (nanobacteria): an additional potential factor for urolithiasis in space flight crewsUrology2009731210.e11e1318718644
- García CuerpoEOlavi KajanderECiftçiogluNNanobacteria. An experimental neo-lithogenesis modelArch Esp Urol200053429130310900759
- Garcia CuerpoENanobacteria and urinary system lithiasisArch Esp Urol200356219119212731450
- JelicTMRoqueRYasarUCalcifying nanoparticles associated encrusted urinary bladder cystitisInt J Nanomedicine20083338539018990947
- KajanderEOCiftciogluNMiller-HjelleMAHjelleJTNanobacteria: controversial pathogens in nephrolithiasis and polycystic kidney diseaseCurr Opin Nephrol Hypertens200110344545211342811
- Lopez-BreaMSelgasRNanobacteria as a cause of renal diseases and vascular calcifying pathology in renal patients (“endovascular lithiasis”)Enferm Infecc Microbiol Clin2000181049149211197997
- DrancourtMJacomoVLépidiHAttempted isolation of nanobacterium sp. microorganisms from upper urinary tract stonesJ Clin Microbiol200341136837212517874
- ShiekhFAKhullarMSinghSKLithogenesis: induction of renal calcifications by nanobacteriaUrol Res2006341535716425019
- LiYWenYYangZCulture and identification of nanobacteria in bileZhonghua Yi Xue Za Zhi200282221557156012609067
- WenYLiYGYangZLDetection of nanobacteria in serum, bile and gallbladder mucosa of patients with cholecystolithiasisChin Med J (Engl)2005118542142415780215
- WenYLiYGYangZLNanobacteria in serum, bile and gallbladder mucosa of cholecystolithiasis patientsZhonghua Wai Ke Za Zhi200341426727012882669
- PiperCDreierJKleesiekKKörferRHorstkotteDNanobacteria to promote degenerative aortic valve stenosis?J Heart Valve Dis200615449950116901042
- PuskásLGTiszlaviczLRázgaZTordayLLKrenácsTPappJGDetection of nanobacteria-like particles in human atherosclerotic plaquesActa Biol Hung20055634233245
- Bratos-PérezMASánchezPLGarcía de CruzSAssociation between self-replicating calcifying nanoparticles and aortic stenosis: a possible link to valve calcificationEur Heart J200829337137618192703
- ZhangSMTianFJiangXQEvidence for calcifying nanoparticles in gingival crevicular fluid and dental calculus in periodontitisJ Periodontol20098091462147019722797
- KajanderEONanobacteria – propagating calcifying nanoparticlesLett Appl Microbiol200642654955216706890
- CiftçiogluNBjörklundMKuorikoskiKBergströmKKajanderEONanobacteria: an infectious cause for kidney stone formationKidney Int19995651893189810571799
- UenoAKitaseYMoriyamaKInoueHMC3T3-E1-conditioned medium-induced mineralization by clonal rat dental pulp cellsMatrix Biol20012056347355
- SommerAPMcKayDSCiftciogluNOronUMesterARKajanderEOLiving nanovesicles – chemical and physical survival strategies of primordial biosystemsJ Proteome Res20032444144312938934
- SommerAPMiyakeNWickramasingheNCNarlikarJVAl-MuftiSFunctions and possible provenance of primordial proteinsJ Proteome Res2004361296129915595742
- SommerAPPavlathAESealing porous nanovesicles – solutions inspired by primordial biosystemsJ Proteome Res20032555856014582654
- RaoultDDrancourtMAzzaSNanobacteria are mineralo fetuin complexesPLoS Pathog200842e4118282102
- YoungJDMartelJYoungLWuCYYoungAYoungDPutative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasisPLoS One200942e441719198665
- DorrellSNanobacteria linked to kidney diseaseMol Med Today19995937310462747
- CiftciogluNCiftciogluVValiHTurcottEKajanderEOSedimentary rocks in our mouth: dental pulp stones made by nanobacteriaProc SPIE Int Soc Opt Eng199834417130135
- CiftciogluNPeltarriAKajanderEOExtraordinary growth phases of nanobacteria isolated from mammalian bloodProc SPIE Soc Int Opt Eng19973111429435
- KajanderEOBjorklundMCiftciogluNMineralization by nanobacteriaProc SPIE Soc Int Opt Eng199834418693
- KajanderEOKuronenIAkermanKKPelttariACiftciogluNNanobacteria from blood: the smallest culturable autonomously replicating agent on EarthProc SPIE Int Soc Int Opt Eng19973111420428
- KhullarMSharmaSKSinghSKMorphological and immunological characteristics of nanobacteria from human renal stones of a north Indian populationUrol Res200432319019515205851
- AltundagKAltundagOAkyurekSAtikMAPossible association between nanobacteria and malignant microcalcifications in breast cancerBreast J200612328716684338
- Le MayOKaquelerJCScanning electron microscopic study of pulp stones in human permanent teethScanning Microsc1991512572672052927
- Le MayOKaquelerJCElectron probe micro-analysis of human dental pulp stonesScanning Microsc1993712672718316798