 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Drug molecules transformed into nanoparticles or endowed with nanostructures with or without the aid of carrier materials are referred to as “nanomedicines” and can overcome some inherent drawbacks of free drugs, such as poor water solubility, high drug dosage, and short drug half-life in vivo. However, most of the existing nanomedicines possess the drawback of low drug-loading (generally less than 10%) associated with more carrier materials. For intravenous administration, the extensive use of carrier materials might cause systemic toxicity and impose an extra burden of degradation, metabolism, and excretion of the materials for patients. Therefore, on the premise of guaranteeing therapeutic effect and function, reducing or avoiding the use of carrier materials is a promising alternative approach to solve these problems. Recently, high drug-loading nanomedicines, which have a drug-loading content higher than 10%, are attracting increasing interest. According to the fabrication strategies of nanomedicines, high drug-loading nanomedicines are divided into four main classes: nanomedicines with inert porous material as carrier, nanomedicines with drug as part of carrier, carrier-free nanomedicines, and nanomedicines following niche and complex strategies. To date, most of the existing high drug-loading nanomedicines belong to the first class, and few research studies have focused on other classes. In this review, we investigate the research status of high drug-loading nanomedicines and discuss the features of their fabrication strategies and optimum proposal in detail. We also point out deficiencies and developing direction of high drug-loading nanomedicines. We envision that high drug-loading nanomedicines will occupy an important position in the field of drug-delivery systems, and hope that novel perspectives will be proposed for the development of high drug-loading nanomedicines.
Introduction
Conventional free-drug-treatment methods mainly involve oral, subcutaneous, and intravenous administrations. A drug spreads all over the body via blood circulation, and accumulates to a certain concentration in the target location to achieve a therapeutic effect. However, the desirable effects of drug treatment often decrease, due to the disadvantages of free drugs, such as the short half-life in the body, high dosage, poor selectivity, side effects on normal tissues, and multidrug resistance. Additionally, considerable amounts of marketed drugs and many new chemical drug candidates (as much as 70%)Citation1 are neither absorbed through the intestines nor circulated by intravenous injection because of low water solubility. For instance, paclitaxel (Ptx), an effective anticancer drug, has a solubility of ~1 μg/mL in water,Citation2 docetaxel, acknowledged as a drug with good solubility, has slightly higher solubility of 6–7 μg/mL in water.Citation3 Therefore, solubilizing agents, such as dimethyl sulfoxide or surfactants, are typically added for systemic administration.Citation4 However, such agents can still lead to neurotoxicity or other toxicities, even at very low dosages, thereby restricting the applications of free drugs.Citation5 The application of nanomedicines, such as inert carrier-based nanomedicines, polymer–drug conjugates (PDCs), coordination polymer nanoparticles (NPs), and carrier-free nano-medicines, can partly overcome the potential problems of free drugs in therapy. Excellent properties of nanomedicines are simultaneously or sequentially obtainable as follows: 1) expanded application ranges of hydrophobic drugs as a result of enhanced water solubility; 2) long circulation times of drugs when dosed into the circulatory system; 3) targeted delivery of drugs at the sites of the diseases through nonspecific targeting, such as the enhanced permeability and retention (EPR) effect or specific NP–cell surface interactions; 4) responses to local stimulation of the target location, such as abnormal pH, temperature, and ionic strength; 5) synergistic therapy via codelivery of multiple drugs at the same time and location; and 6) real-time monitoring of carrier (and drug) biodistribution and targeted accumulation.Citation6,Citation7 Since the first US Food and Drug Administration (FDA) approval of nanomedicine (liposomal amphotericin B in 1990), more than 24 nanomedicines have been approved for clinical use, with total sales exceeding US$5.4 billion.Citation8,Citation9 The average cost and time required to develop a new nanomedicine (approximately $20–$50 million and 3–4 years) are significantly lower than for a new chemical drug (approximately $500 million and over 10 years).Citation10 Therefore, the design and development of novel nanomedicines have higher market value and application prospects than that for conventional chemical drugs.
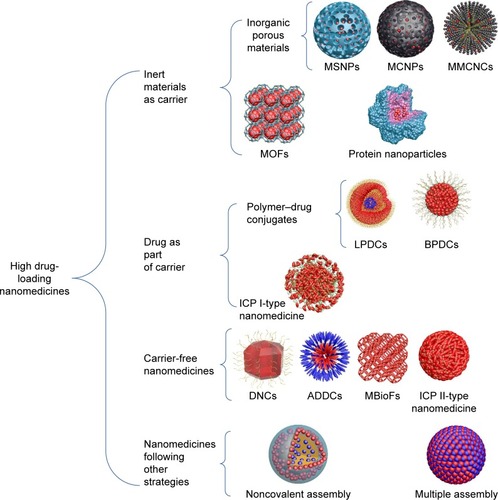
According to the fabrication strategies of nanomedicines, high drug-loading nanomedicines can be divided into four main classes (): 1) nanomedicines with inert materials as carriers, including inorganic porous carrier-based nanomedicines and absorption/desorption-type metal–organic framework (MOF)-based nanomedicines; 2) nanomedicines with drugs as part of the carrier, including linear and branched PDCs and infinite coordination polymer (ICP) I-type nano-medicines; 3) carrier-free nanomedicines, including drug nanocrystals (DNCs), amphiphilic drug–DCs (ADDCs), metal–biomolecule frameworks (MBioFs), and ICP II-type nanomedicines; and 4) nanomedicines following niche and complex strategies, such as aqueous noncovalent assembly and multiple assembly of drugs. For a visual comparison, the drug-loading content and drug-loading efficiency cited in this review are expressed according to the following equations:
Drug-loading content and drug-loading efficiency are two important parameters of nanomedicines. Drug-loading content reflect the mass ratio of drugs to nanomedicines, and drug-loading efficiency reflects the utilization of drugs in feed during the nanomedicine-preparation process. In essence, drug-loading content is determined by the structure and physical and chemical properties of the carrier material; drug-loading efficiency is determined by the drug-loading mechanism, mass of the drugs in feed, and other experimental conditions. It is more difficult to obtain high drug-loading content than high drug-loading efficiency for most nanomedicines. Through physical and electrostatic adsorption, the drug-loading process often results in low drug-loading efficiency, while through crystallization and covalent and coordinate bonds it often results in high drug-loading efficiency. Nano-medicines with high drug-loading content are usually prepared using processes with high drug-loading efficiency.Citation11–Citation14 If the space capacity of the carrier material is low, it is difficult to get high drug-loading content, even if the preparation process of nanomedicines shows high drug-loading efficiency.Citation15–Citation17 Taking into account most of the concepts investigated, high drug-loading efficiency may be necessary but not sufficient for a gain in high drug-loading content. In this paper, we focus mainly on drug-loading content, because this parameter is closely related to drug metabolism, side effects, extra burden, and therapeutic effect of the nanomedicines in vivo.
In most existing nanomedicines, inert carrier materials are mainly responsible for low drug-loading content (generally less than 10%).Citation18–Citation21 To administer a required drug dose in a target location, copious levels of carrier materials have to be used. For intravenous administration, the extensive use of these carrier materials may cause systemic toxicity, such as lipotoxicity, and impose an extra burden for patients to degrade, metabolize, and excrete these carriers. As such, FDA approval for most carrier-based nanomedicines is challenging to obtain, thereby limiting their clinical applications. On the premise of guaranteeing therapeutic effect and function, reducing or avoiding the use of carrier materials is a direct way of solving this problem. Moreover, it is easier for high drug-loading nanomedicines to achieve multidrug codelivery and combination therapy, which benefit from high drug loading. Given the advantages of combination therapy, including maximum therapeutic effect, minimum delay in induction of drug, and potential overcoming of multidrug-resistance mechanisms of tumors,Citation22–Citation24 multidrug-loaded high drug-loading nanomedicines will become a new hot spot in the field of drug-delivery systems. Although considerable efforts have been devoted to the fabrication of various high drug-loaded nanomedicines, the therapeutic effects are not always satisfactory and many theoretical and actual problems remain unsolved. In addition, not enough attention is paid to the study of high drug-loading nanomedicines, and innovative strategies are seldom reported. Therefore, the research status of high drug-loading nanomedicines must be reviewed and summarized, and their prospects should be discussed. In this review, the current research development of high drug-loading nanomedicines is investigated. The features of each fabrication strategy and proposals for possible optimization are discussed in depth. The inevitable role of high drug-loading nanomedicines in future drug-delivery systems is highlighted. We also hope that this study will provide us with a new perspective that will lead us to design and develop novel high drug-loading nanomedicines.
Nanomedicines with porous material as carrier
In this section, nanomedicines with porous materials, including inorganic porous materials, MOFs and protein NPs as carriers, are investigated. All these carrier materials provide high surface areas, large pore volumes, and feasible drug-loading methods, which contribute to high drug-loading capacity. The advantages and disadvantages of each material, as well as optimization options, are described.
Inorganic porous materials as carriers
To date, different kinds of inorganic porous materials, including mesoporous silica-based NPs (MSNPs), mesoporous carbon NPs (MCNPs), magnetic colloidal NCs (MCNCs), mesoporous TiO2 NPs,Citation25 and other inorganic porous materials, have been used to fabricate high drug-loading nanomedicines based on their intrinsic properties, such as large hollow interior, porous surface, and high surface:volume ratio. As summarized in , nanomedicines with inert material carriers can deliver different kinds of guest molecules.Citation26 In general, drug molecules are loaded into nanocarriers with high loading content through noncovalent electrostatic, π–π stacking, hydrogen bond, and hydrophobic interactions with carrier materials. In recent studies, drug molecules have been conjugated with the porous surface of carrier materials to acquire further increased drug-loading content and additional stimuli-responsive ability.
Table 1 Overview of two strategies of fabricating high drug-loading nanomedicines with porous materials as carriers
Mesoporous silica-based materials as carriers
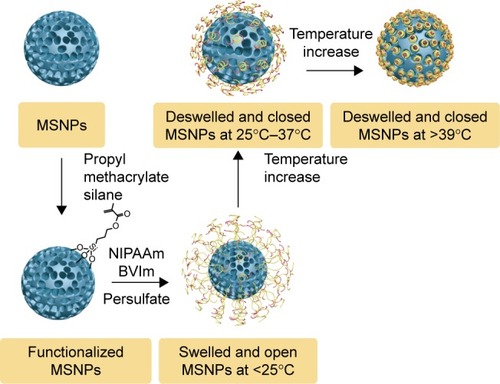
Since Vallet-Regi et al proposed MCM41 as a drug-delivery system in 2001,Citation27 MS-based materials, including MS, mesoporous calcium silicate, and mesoporous magnesium silicate, have been widely used in the fabrication of high drug-loading nanomedicines because of their intrinsic structure properties. The surface area and pore-volume ratio of MSNPs often exceeds 1,000 m2/g and 0.5–1 cm3/g, respectively, which facilitates the loading of large amounts of drug molecules.Citation28 Li et al showed that MSNPs could effectively load ibuprofen, a typical anti-inflammatory drug, with high drug-loading content of up to 21.6%.Citation29 Moreover, MSNPs present a site-selective controlled-release pattern by surface functionalization via any of the following three methods: cocondensation (one-pot synthesis), grafting (postsynthesis modification), and imprint coating.Citation30–Citation32 For example, MSNPs functionalized with chitosan have a pH-triggered release characteristic, due to the swelling effect of chitosan in acidic environments.Citation33 This nanomedicine was used to deliver an oral sustained drug, carvedilol, with loading content and efficiency reaching 32.49%±1.57% and 96.25%±3.12%, respectively. Coincidentally, MSNPs exhibit temperature-responsive properties through a pore-capping copolymer poly-[N-isopropylacrylamide-co-1-butyl-3- vinyl imidazolium bromide].Citation34 When the temperature changes between 36°C and 40°C, the pore copolymers of MSNPs swelled or shrank to load or release the encapsulated cytochrome C. The drug-loading content of cytochrome C in this nanomedicine was 26.3% ().
Figure 1 Preparation and temperature-dependent working mechanism of the cationic, thermosensitive, copolymer-capped MSNPs.
Abbreviations: MSNPs, mesoporous silica nanoparticles; NIPAAm, N-isopropylacrylamide; BVIm, butyl vinylimidazolium.
To enhance drug-loading content further, MSNPs with enlarged nanopores (E-MSNPs) and hollow MSNPs (HMSNPs) can be selected as carriers. Compared with conventional MSNPs, E-MSNPs and HMSNPs have larger pore volume, which can accommodate more drug molecules.Citation35 Eltohamy et al proved that E-MSNPs had higher drug-loading content for cytochrome C than normal MSNPs (50 wt% in E-MSNPs and 35 wt% in normal MSNPs, respectively).Citation36 Similarly, Zhu et alCitation37 proved that HMSNPs had significantly higher drug-loading content for aspirin than conventional MCM48 and MCM41, which were two nanocarriers discovered by Mobil researchers in 1992.Citation38 The drug-loading contents of HMSNPs, MCM48, and MCM41 were 25.1 wt%, 19.2 wt%, and 16.6 wt%, respectively, under the same experimental conditions. In following study, they added polyelectrolyte multilayers to the surface of HMSNPs to fabricate a stimuli-responsive controlled-release system. The polyelectrolyte multilayers of sodium polystyrene sulfonate and polycationic poly(allylaminium hydrochloride), prepared via a layer-by-layer method, yielded a drug-loading content of up to 41.64% for ibuprofen.Citation39 Geng et al recently synthesized HMSNPs using hard template phenolic resin NPs to encapsulate two insoluble model drugs, namely carvedilol and fenofibrate.Citation40 The resulting drug-loading contents for carvedilol and fenofibrate were 26.2%±2.1% and 31.2%±2.7% in normal MSNPs and 37.5%±2.8% and 43.7%±3.2% in HMSNPs, respectively. These findings indicate that HMSNPs have higher drug-loading content than normal MSNPs under the same conditions.
Another way to improve the drug-loading content of MSNPs is to conjugate drug molecules on the porous surface by covalent grafting or coordination bonds. Ebabe et al grafted two antioxidant molecules, caffeic acid and rutin, on to the surface of MSNP-NH2 through amino groups on MSNPs and carboxylic groups in antioxidant molecules.Citation41 They reported that caffeic acid had drug-loading content of 44.2%. Coordination is another feasible method. Farsangi et al prepared HMSNP-COOH carriers and loaded cisplatin in them via coordination bonds between carboxylic groups and Pt atoms in cisplatin molecules.Citation42 These nanomedicines had a pH-dependent release pattern with drug-loading content as high as 48 wt%.
Mesoporous silicate-based NPs also have high drug-loading capacity. In the early research, calcium silicate is regarded as a candidate for bone replacement biomaterial due to its good bioactivity.Citation43–Citation46 Then, its mesoporous form is applicable in protein/drug delivery for bone regeneration because of its high surface area and large pore volume and good biocompatibility.Citation47 Wu et al prepared ibuprofen-loaded mesoporous calcium silicate NPs with fine hierarchically nanostructure via a low-cost, surfactant-free sonochemical method.Citation11 The drug-loading efficiency and drug-loading content of ibuprofen were as high as 86 wt% and 69.3 wt%, respectively. When the ibuprofen-loaded nanomedicine released the absorbed load, the calcium silicate material gradually transformed to hydroxyapatite, due to its good bioactivity. Similarly, Wang et al synthesized hollow mesoporous magnesium silicate NPs via a facile template-etching route.Citation48 These well-prepared NPs exhibited a significantly high drug-loading content of 68.15 wt% and held a sustained-release property, showing great potential for cancer therapy.
Despite the encouraging progress made in the research of MS-based high drug-loading nanomedicines, several major challenges still need to be addressed, such as the in vivo toxicity caused by MS-based carrier materials. Although silica is considered generally recognized as safe and has secured FDA approval for Phase I human clinical trials,Citation49 in vivo studies have described dose-dependent toxicity,Citation50 such as inflammatory response,Citation51 liver injury,Citation52 neurotoxicity, and pregnancy complications.Citation53 Studies have shown that particle size, surface charge,Citation54 and surface functional groups also play significant roles in the cytotoxicity of MS-based carrier materials.Citation55 Therefore, more careful and sufficient in vitro and in vivo studies are necessary to find the proper dose and type of MSNPs before clinical trials.
Mesoporous carbon as carrier
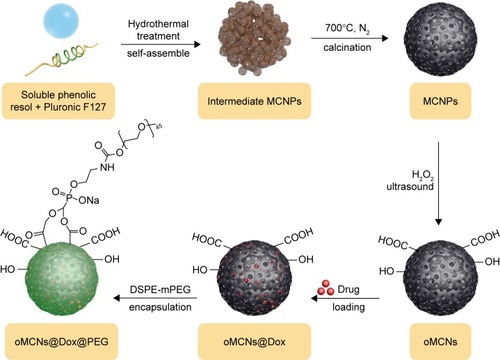
In contrast to the MSNPs already mentioned, MCNPs have even higher surface areas (as high as 2,000 m2/g) and volume:mass ratios (>1 cm3/g), which facilitate high levels of drug loading.Citation56–Citation58 In early reports, MC microparticles were used to improve the oral absorption of water-insoluble drugs with drug-loading content higher than 50%.Citation57,Citation59–Citation64 Applications of MCNP-based nanomedicines via intravenous administration have been quite limited, because of the following. First, ordered mesoporous carbons produced by carbonization of different chemical hydrocarbons usually have an undesirable size (>1 μm)Citation65,Citation66 for drug delivery (<200 nm).Citation67 Second, MCNPs exhibit poor dispersion in water under physiological conditions, due to their inherent hydrophobicity, which makes them impossible to use as carriers for intravenous drug administration. Finally, although some reports have shown that MCNPs have good biocompatibility,Citation56,Citation68 irregularly shaped MCNPs, such as carbon nanotubes or graphenes, can activate the complement system or induce immunotoxicity to some extent.Citation69,Citation70 With this limitation, MCNPs require further optimization to expand their application scope in drug-delivery systems. More recently, some MCNPs of <100 nm diameter were obtained via template or hydrothermal method. Moreover, the hydrophilicity of MCNPs was improved by adding a hydrophilic cover, such as hyaluronic acid (HA) and polyethylene glycol (PEG), or introducing large amounts of oxygen-containing groups, such as hydroxyl, carboxyl, and sulfonic groups onto the surface. In one study, Liu et al successfully synthesized MCNPs with spherical morphology and uniform size (71 nm) via hydrothermal reaction of bacterial cellulose nanofibers (30–50 nm).Citation12 These MCNPs disperse well in aqueous solutions, because of the introduction of a large amount of hydrophilic functional groups, such as –SO3H, phenolic –OH, and –COOH groups, onto the surfaces. The drug-loading efficiency and drug-loading content of this nanomedicine for doxorubicin (Dox) were 93.4 wt% and 52.3 wt%, respectively. In another work, spherical oxidized MCNPs with an average diameter of 90 nm were prepared through low-concentration hydrothermal treatment and mild oxidization. Then, PEGylated phospholipid 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)-methoxy-PEG2,000 was used as a protective layer added to the outer surface after Dox loading. The PEG layer on the surface prevented the premature and burst release of drugs in the circulation system, as well as helping to avoid recognition of the reticuloendothelial system.Citation71,Citation72 The drug-loading content of this nanomedicine for Dox was as high as 59.7%±2.6% under optimum conditions ().Citation73
Figure 2 Synthesis, drug loading, and surface modification of MCNPs.
Abbreviations: MCNPs, mesoporous carbon nanoparticles; DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; mPEG, methoxy polyethylene glycol; oMCNs, oxidized mesoporous carbon nanospheres; Dox, doxorubicin; PEG, polyethylene glycol.
Mesoporous carbon-based nanomedicines have two inherent advantages, due to their unique mesoporous structure and carbonaceous composition. First, mesoporous carbon-based high drug-loading nanomedicines often exhibit good pH sensitivity in drug-delivery systems. This feature is due to the fact that the adsorption of most anticancer drugs with aromatic structures, such as Dox, by carbonaceous structures via π–π stacking and hydrophobic interactions is pH-dependent. Second, MCNPs serve as efficient photosensitizers with high absorption in the near-infrared region.Citation74–Citation77 Therefore, they function as promising nanocarriers in photothermal and chemical combination therapy. Zhou et alCitation78 used these advantages to develop an effective dual-triggered (Hyal1/redox) chemo-photothermal synergistic therapy nanomedicine, which presented efficient heat transformation for photothermal therapy. Research on MCNPs in drug-delivery systems is still in its infancy, and MCNPs face the following unsolved problems: 1) scalable synthetic strategies and standard methodologies to obtain MCNPs with suitable size and hydrophilic surfaces for intravenous administration; 2) unsuitability of traditional oxidation methods in the surface modification of MCNPs, because such techniques partially destroy the carbonaceous framework and the mesostructure of MCNPs; and 3) the limited application scope of MCNPs in nanomedicines and absence of applications of MCNPs in the field of gene and protein drug delivery, as opposed to MSNPs.
Mesoporous magnetic colloidal nanocrystal clusters
Mesoporous/hollow MCNCs (MMCNCs/HMMCNCs) have drawn high attention in targeting drug-delivery systems during the past decade. HMMCNCs and MMCNCs are promising candidates for high drug-loading nanomedicines, because of their high magnetization, large surface area and pore volume, and excellent colloidal stability.Citation79–Citation81 Compared with other inorganic porous materials, HMMCNCs and MMCNCs are known as the “magnetic motor” for site-specific drug-delivery applications.Citation82–Citation84
The most common fabrication method of MMCNC-based nanomedicines begins with a combination of solvothermal reaction and electrostatic stabilization,Citation85–Citation89 and follows with drug loading via nanoprecipitation.Citation80,Citation88,Citation90 Luo et al synthesized Ptx-loaded Fe3O4 MMCNCs using poly(γ-glutamic acid) as an electrostatic stabilization agent.Citation88 The resulting MMCNC-based nanomedicine possessed high magnetization, large surface area (136 m2/g) and pore volume (0.57 cm3/g), excellent colloidal stability, prominent biocompatibility, acid degradability, and high drug-loading content of 35 wt% for Ptx.
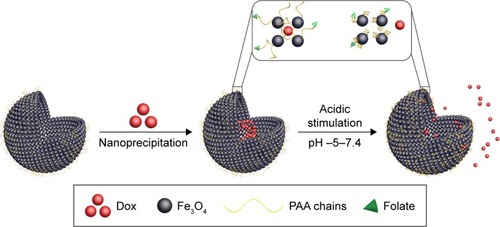
The fabrication of HMMCNCs involves both template-basedCitation81,Citation91,Citation92 and template-free solvothermal reaction methods.Citation80,Citation92–Citation94 Li et al synthesized an HMCNC-based Dox-loaded nanomedicine with porous shell and tunable hollow chamber by a one-pot solvothermal process.Citation81 This HMCNC carrier had a high surface area of 41.2 m2/g and pore volume of 0.36 cm3/g, which contributed to high drug-loading capacity. Furthermore, receptor-specific pH-sensitive folate-modified polyacrylic acid (PAA-FA) was grafted on the surface of HMCNCs via a versatile ligand-exchange method, because folate molecules can target cancer cells that overexpress folate receptors and enhance the endocytosis capability of such cancer cells. This nanomedicine is stable under high pH and quickly releases Dox under low pH. This site-specific targeting delivery was achieved by protonation and collapse of the PAA layers after cell internalization.Citation95 The NPs presented drug-loading content of 24 wt% for Dox ().
Figure 3 Dox encapsulation in HMCNCs and pH-stimulated release of Dox from folate–HMCNC-Dox.
Abbreviations: Dox, doxorubicin; HMCNC, hollow magnetic colloidal nanocrystal; PAA, polyacrylic acid.
As described, novel inert magnetic NCs are promising nanocarriers, and their drug-delivery application is potentially feasible. Exciting progress has been made in MMCNC cluster-based nanomedicines, because of their high magnetization and large surface area. However, the fabrication of magnetic nanocarriers that demonstrate enhanced biocompatibility and excellent colloidal stability remains a challenge, which is critical for the application of magnetically driven drug delivery. Currently, in vivo toxicity studies and pharmacological experiments are scarce, and further studies are necessary.
Absorption/desorption-type MOFs as carriers
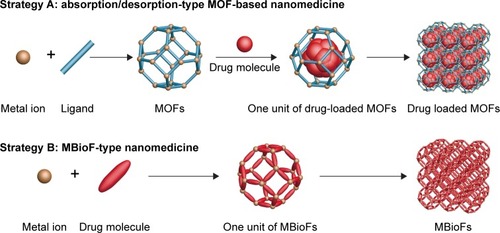
MOFs, a class of coordination polymers, are another type of porous material (the other class of coordination polymer is an ICP particle that is covered in later sections).Citation96 MOFs consist of transition metal ions/clusters and multidentate organic ligands, and are typically synthesized via coordination- directed self-assembly processes under mild conditions ().Citation97 Over the past two decades, MOFs have been applied in photonics,Citation98 catalysis,Citation99–Citation101 biosensors,Citation102,Citation103 gas storage,Citation104–Citation107 and drug delivery.Citation108–Citation111 For drug delivery, the high drug-loading capacity of MOFs relies largely on three factors: 1) high surface area (3,100–5,900 m2/g)Citation108 and large, regular, accessible cages and tunnels; 2) good physicochemical stability in circulation and quick stimuli response, especially pH response; and 3) selectable metal ions with low toxicity (ie, Fe or Zn) or metal ions associated with the mechanism of drug action (ie, Cu with gossypol).Citation112 Two approaches can be considered in fabricating high MOF-based drug-loading nanomedicines. For absorption/desorption-type MOF-based high drug-loading nanomedicines, drug molecules are absorbed in cages and tunnels of MOFs. For MBioFs, the bioactive drug molecules function as ligands that coordinate with the central metal atom. In this section, absorption/desorption-type MOF-based nanomedicines are summarized, whereas the latter are outlined in a following section.
Figure 4 Formation of absorption/desorption-type MOF-based nanomedicine and MBioF-type nanomedicine by coordination-directed self-assembly processes.
Abbreviations: MOF, metal–organic framework; MBioF, metal–biomolecule framework.
Férey et al first reported absorption/desorption-type MOF-based nanomedicines in 2004.Citation109 A model drug, ibuprofen, was absorbed by two dehydrated MOFs – chromium-based Material Institut Lavoisier (MIL)-100 and MIL101Citation109,Citation113 –effectively in hexane solution. X-ray powder diffraction and N2-adsorption experiments showed that both MOFs retained their excellent crystalline structures, and almost all the cages and tunnels were filled and/or blocked by the drug molecules. The drug-loading contents of ibuprofen for MIL100 and MIL101 were 58.3 wt% and 2.6 wt%, respectively. The different absorption capacities between MIL100 and MIL101 were attributed to their fine structure inside the particles. The larger pore size of cages in MIL101 made it easier to absorb ibuprofen compared with MIL100. The size of ibuprofen is 6×10.3 Å, whereas some cages in MIL100 were 4.8×5.8 Å (pentagonal), so the latter were not accessible for ibuprofen. The adsorption mechanism of ibuprofen in MIL100 and MIL101 was investigated by Babarao and Jiang through computational studies.Citation114 They suggested that a part of the ibuprofen molecule penetrates MIL101 by forming coordinate bonds with unsaturated chromium molecules in the surrounding regions, and additional ibuprofen molecules weakly interact (ie, π–π interactions and hydrophobic interaction) with existing ibuprofen molecules. The results of molecular simulation in this study were consistent with “two-stage” release profiles observed in Férey et al’s work. Similarly, small-molecule drugs or gas drugs can be either chemisorbed by forming coordination bonds with transition metals or physically adsorbed into coordination polymers by weak intermolecular forces in absorption/desorption-type MOF-based nanomedicines.
For biological application, MOFs can be modified on the surface to increase size, decrease aggregation, and exhibit targeting performance. Horcajada et al developed a series of iron(III)-based MOFs (MIL53, MIL88A, MIL88Bt, MIL89, MIL100, and MIL101_NH2)Citation115–Citation119 with engineered cores and surfaces for retroviral drug delivery.Citation120 As expected, most of these MOFs (ie, azidothymidine triphosphate-loaded MIL101_NH2, cidofovir-loaded MIL101_NH2, urea-loaded MIL100, and urea-loaded MIL53) displayed high drug-loading capacity of over 40 wt%. PEG chains with only one terminal reactive group (amino or carboxyl) were firmly bound to the nanomedicine surface through coordinate covalent bonds of its amino or carboxyl end group with the metal centers. The PEG coating might control the nanomedicine size, protect the nanomedicines from aggregation, and escape sequestration by the reticuloendothelial organs.
Despite the research progress made, the potential application of MOFs in drug delivery is still in its infancy compared with other nanocarriers. Three major existing problems impede MOFs as mature high drug-loading nanomedicine carriers: 1) the relationship between the pore size of cages in different kinds of MOFs and the size of cargo drug molecules needs to be systematically studied and determined; 2) appropriate ligands and low-toxicity metal ions are needed to improve biocompatibility, and more efficient surface functionalization is needed to ensure longer blood circulation and better targeting effects; and 3) more careful and sufficient in vitro and in vivo experiments need to be conducted to expand the application prospects of MOFs in the biomedical field.
Protein NPs as carriers
As potential nanocarriers, protein NPs have intrinsic advantages, including synthesis in natural sources, precise size and uniformity, hollow inter structure, self-assembling ability, biocompatibility, and biodegradation.Citation121 Hydrophobic drug molecules, peptides, and gene drugs are covalently conjugated to the hollow cavity of the protein scaffold or noncovalently loaded into the internal space.Citation122–Citation125 Generally, restricted by the number of attachment sites, most of the protein NP-based nanomedicines prepared by conventional chemical conjugation present the same drawback of lower drug-loading content (<10%).Citation122 One feasible way to enhance the drug-loading capacity of protein nanocarriers is designing and increasing the hydrophobic surface area of the protein NP. Inspired by transport mechanism of multidrug-efflux transporters,Citation126 Ren et alCitation123 implemented a biomimetic approach to enhance hydrophobic drug–protein interactions. Several virus-like protein NPs were redesigned and prepared via self-assembly of the E2 component of the pyruvate dehydrogenase multienzyme complex. Phenylalanine, one of the most hydrophobic amino acids, was systematically introduced to increase the hydrophobicity of E2’s hollow cavity surface. The drug-loading content of protein NPs for Dox reached 13.4%, and the theoretical maximum loading content was thought to be as much as 44.9%.
Although using protein NPs in the fabrication of high drug-loading nanomedicines is potentially feasible, a major concern with protein nanocarrier-based nanomedicines is their immunogenicity. When these nanomedicines are administered for the second time, elevated immune responses can occur and induce rapid clearance or neutralization or create potentially severe inflammatory responses.Citation121 One way to bypass this problem is surface functionalization by introducing PEG and other coatings to reduce immunorecognition.Citation71,Citation72 The other is harnessing this natural effect and using it in immunobased treatments.Citation127
Nanomedicines with drugs as part of carrier
Although absorption/desorption-type high drug-loading nanomedicines with inert porous carriers have attracted heightened interest in fabricating high drug-loading nanomedicines, the inherent limitations of this strategy cannot be ignored. Drug loading and release are mostly achieved by absorption and desorption, which strongly depend on the diffusion rate of drug molecules and drug–carrier interactions in the physiological medium. In the stage of nanomedicine design, the interactions are difficult to predict. Also, the degradation of the inert carrier material may lead to additional toxicity and impose an extra burden on patients to excrete the carriers.
For drugs with stable chemical properties in the circulatory system, the protective function from carriers is not essential. To overcome this limitation and further improve the drug-loading content of nanomedicines, the direct way is gradually reducing or replacing the use of carrier material. With this goal in mind, two popular strategies are proposed to fabricate such high drug-loading nanomedicines. One strategy is to form prodrugs by conjugating drug molecules with polymers, and another is to form ICP I-type nanomedicines, in which drug molecules function as ligands and coordinate with the central metal atom (). The drug and polymer are two indispensable excipients in high drug-loading nanomedicine. Without one of these, the nanostructures cannot be maintained.
Table 2 Overview of two strategies of fabricating high drug-loading nanomedicines with drug as part of carrier
Polymer–drug conjugates
Compared with the physical encapsulation of drug molecules in inert carriers, PDCs, such as liner and branch types, are excellent candidates for high drug-loading nanomedicines, because of their limited usage of inert carrier materials and self-assembly abilities. In addition to the common advantages of other nanomedicines already mentioned, the smart covalent links between the drugs and polymers endow them with multiple advantages: 1) stimuli-triggered release and targeted delivery; 2) protection of drugs from leakage and premature release during intravenous administration; 3) altered drug pharmacokinetics in the whole organism and even at the subcellular level, which may enhance the drug’s therapeutic value; and 4) additionally loading hydrophilic drugs into the core of micelles to achieve multidrug-combination therapy. For stimuli-responsive PDCs, pH-responsive types are the most frequently used. Several pH-responsive covalent links, such as acetal, orthoester, hydrazone, imine, cis-acotinyl, and oxime, are common, due to their rapid hydrolysis in the endosomal compartment (pH about 5). Moreover, a reduction-response covalent link disulfide depends on different redox potentials between the extra- and intracellular environments, especially in tumor cells.Citation128–Citation130
Linear polymer–drug conjugates
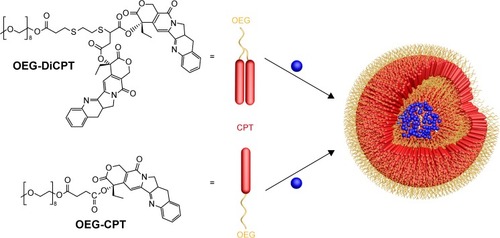
As the name suggests, linear PDCs are block copolymers that have a simple linear monomeric structure. Shen et al formed dual-drug-loading nanomedicines by conjugating one or two strong hydrophobic camptothecin (CPT) molecules to a very short oligomer chain of oligoethylene glycol (OEG) to form biodegradable ester bonds ().Citation131 The amphiphilic prodrugs OEG-CPT and OEG-DiCPT self-assemble into 100–200 nm liposome-like nanocapsules in aqueous solutions. The drug-loading contents for CPT were 40 wt% and 58 wt% in OEG-CPT and OEG-DiCPT, respectively, with undetected burst releases for both. Meanwhile, the liposome-like structures further loaded a water-soluble Dox HCl salt via a dialysis mechanism similar to drug loading into conventional liposomes. The drug-loading efficiency and drug loading content of Dox HCl were 85% and 18.5 wt%, respectively. Once inside the cells, the Dox HCl-loaded amphiphilic CPT prodrug liposomes released both CPT and Dox HCl, thereby providing synergistic cytotoxicity to cancer cells, as confirmed by high in vitro and in vivo antitumor activities. Similarly, Zhang et al synthesized a nanomedicine by conjugating the hydrophobic drug SN38 (7-ethyl-10-hydroxy-CPT), the active metabolite of CPT, to a low-molecular-weight OEG via an ester bond. The amphiphilic OEG-SN38 monomer self-assembled into micelles with a hydrodynamic diameter of 28.74±2.51 nm, and a high drug-loading content of 36 wt% for SN38 was obtained.Citation132 Gou et alCitation133 anchored hydrophobic Dox to a low-molecular-weight PEG chain via a pH-responsive covalent hydrazine bond to form a linear amphiphilic PDC – PEG-Dox. This amphiphilic prodrug self-assembled into micelles with a diameter of about 125 nm in aqueous solution, and the drug-loading content for Dox was as high as 46 wt%. Once the nanomedicines were taken up by cells via endocytosis, the hydrazine bonds were hydrolyzed in the acidic environment of the endosomal compartment, and the Dox molecules were simultaneously released.
Figure 5 Amphiphilic CPT–polymer conjugates (OEG-CPT and OEG-DiCPT) and their self-assembly into nanocapsules to load other hydrophilic drug.
Note: OEG-DiCPT means two CPT molecules were conjugated to one OEG molecule.
Abbreviations: CPT, camptothecin; OEG, oligoethylene glycol.
Furthermore, PDC prodrug micelles can achieve targeted delivery by introducing targeting ligands, such as carbohydrates, folate, transferrin, peptides, and HA, onto the surfaces.Citation134 For example, Guo et al synthesized a linear PDC – FA-PEG-b–poly-ε-caprolactone (PCL)–hydrazone-Dox – to form pH-sensitive folate-functionalized nanomedicines.Citation135 The FA-PEG-b-PCL-hydrazone-Dox conjugates self-assembled into micelles with a hydration diameter of 70.9 nm, according to dynamic light-scattering measurements. In these micelles, Dox molecules anchored to PCL via hydrazone bonds functioning as the hydrophobic part, whereas PEG served as the hydrophilic part. The FA layer on the surface of micelles facilitated the endocytosis of the folate receptor-overexpressing tumor cells.
Branched polymer–drug conjugates
Compared with linear PDCs, branched PDCs, including branched star PDCs, hyperbranched PDCs, dendrimer PDCs, and brush PDCs, exhibit controllable supramolecular morphologies, self-assembly behavior, and thermal, mechanical, rheological, and solution properties.Citation136–Citation139 All properties render branched PDCs viable for high drug-loading nanomedicines.
Branched star polymers are branched polymers containing several linear chains attached to a central core. Star PDCs often have a compact structure, globular shape, and large surface; they also possess enhanced solubility, low melting viscosity, unique rheological properties, and a large number of drug-binding sites.Citation140–Citation143 All these excellent properties depend on the arm’s molecular weight.Citation144 Kowalczuk et alCitation145 synthesized core–shell-type star polymer–cisplatin conjugates with a branched poly(p-[iodomethyl]styrene) acting as a hydrophilic core and PAA arms functioning as a hydrophilic shell. These star-type PDCs exhibited “unimolecular micelle” behavior in aqueous solution, and were globular with a hydration diameter of 12.9–14 nm. The cisplatin molecules were loaded into the unimolecular micelles via the coordinative bonds between the platinum atoms of cisplatin molecules and high density of carboxyl groups on PAA arms. The drug-loading content for cisplatin was 45 wt%. In vitro experiments showed lower cytotoxicity that was consistent with the sustained release of cisplatin molecules and slower cellular uptake of conjugated prodrug micelles compared with free cisplatin. Some examples of star PDCs utilized for nanomedicine fabrication following this strategy have been presented in the literature.Citation146–Citation149 In a recent study, Liang et al designed pH-sensitive star polymer–Dox conjugates, in which a triblock copolymer 2-(N,N-dimethylaminoethyl)- methacrylate-co-p-(methacryloxyethoxy)benzaldehyde-co-2,2-dithiodiethoxyl dimethacrylate served as a hydrophilic core and poly(6-O-methacryloyl-d-galactopyranose) arms acted as a hydrophilic shell.Citation150 Instead of loading drug molecules on the arm as previously mentioned, Dox molecules in this nanomedicine were loaded into the hydrophilic core through acidic–labile imine bonds between the aldehyde groups in p-(methacryloxyethoxy)benzaldehyde and the primary amines in Dox molecules. The drug-loading content of Dox in this nanomedicine was controllable in the range of 7.3–37.6 wt%, depending on the different grafting degrees of Dox. These star polymer–Dox conjugates self-assembled into unimolecular micelles with average diameters of 30–50 nm in aqueous solution. Dox molecules were quickly released at pH 5 and 6 due to the acid lability of aromatic imine linkage between the Dox molecule and star polymer. All these properties were desirable for drug delivery.
Hyperbranched polymers (HBPs) are another kind of branched polymer with a highly three-dimensional dendrite-like architecture, and their degree of branching is 0.4–0.6 (0 for linear polymer and 1 for dendrimer). Similar to branched star polymers, HBPs have serious adjustable physicochemical properties, such as molecular entanglement, solution viscosity, solubility, host–guest interaction capacity, and self-assembly behavior, caused by their modifying branches, end groups, and controllable degree of branching. Given these beneficial properties, HBPDCs are excellent candidates for high drug-loading nanomedicine fabrication.Citation151–Citation153 Prabaharan et alCitation154 synthesized the first HBPDC nanomedicines in 2009. In their work, a folate-targeting unimolecular micelle nanomedicine was synthesized, namely Boltorn H40-poly(l-aspartate-doxorubicin)-b-PEG/FA-conjugated PEG (H40-P[LA-Dox]-b-PEG-OH/FA). This nanomedicine contained a commercial aliphatic dendritic polyester Boltorn H40 core, hydrophobic poly(l-aspartate) inner arm, and a hydrophilic PEG and FA-conjugated PEG outer arm. Dox molecules were conjugated to the inner arm through a pH-sensitive hydrazone linker, resulting in a drug-loading content of 16 wt% for Dox. This block polymer self-assembled into micelles with sizes of 17–36 nm under dynamic light scattering and 10–20 nm under transmission electron microscopy. Following this strategy, Ye et al fabricated HBP–cisplatin conjugates based on hyperbranched polyglycerols (HPGs).Citation13 In this system, HPGs were modified with hydrophobic alkyl (C8/C10) chains into the core, derivatized with methoxy PEG (MePEG) onto the surface, and further functionalized with carboxylate groups on MePEG chains to conjugate and release cisplatin. The drug-loading efficiency of cisplatin was 100%, and its drug-loading content was adjusted depending on the conjugated carboxylate levels. Respectively, the drug-loading content of HPG-C8/C10-MePEG6.5-COOH113 was 10 wt%, whereas that for HPG-C8/10-MePEG6.5-COOH348 was 20 wt%. These HBP-cisplatin conjugates self-assembled into micelles with hydrodynamic diameters of 5–10 nm. These conjugates demonstrated pH-triggered release behavior caused by the coordinate bond between cisplatin and carboxylate groups on MePEG chains. In vitro experiments showed that these nanomedicines presented good biocompatibility and antitumor effects.
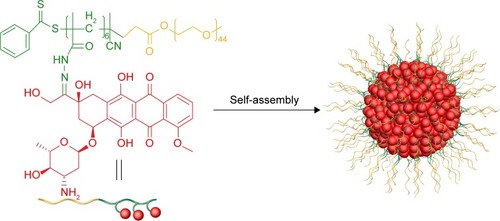
Segmented polymers are a large family. Depending on the chemical differences among main chains, side chains, and grafting density of the side chains, the family of segmented polymers is classified as follows: graft polymer, in which the side chains have a different chemical nature from the main chains and low grafting density; brush-like polymers, in which the side chains have a different chemical nature from the main chains and high grafting density; and comb-like copolymers, in which the side chains have a similar chemical nature to the main chains and lower grafting density than brush-like polymers.Citation155 Graft and brush-like polymers are excellent candidates for high drug-loading nanomedicine fabrication, depending on their side chains’ selectability and modifiability. Xu et al reported a novel graft polymer–Dox conjugate with a Dox-loading content as high as 40 wt%, namely, PEG monomethylether-b-poly(methacrylamide tert-butyl carbazate-Dox) (MPEG-b-Dox) ().Citation156 The MPEG in this conjugate functioned as the hydrophilic part, and methacrylamide tert-butyl carbazate, which was conjugated to Dox via an acid-labile hydrazine bond, acted as the hydrophobic part. The amphiphilic conjugates self-assembled into micelles with average hydrodynamic size of 80 nm. These conjugates also showed minimal drug release at pH 7.4 and high Dox release at pH 5, following the previously mentioned mechanism. In parallel, Zou et al synthesized a brush PDC, PLA-g-Ptx/PEG, through azide–alkyne click reaction of acetylene-functionalized PLA with azide-functionalized Ptx and PEG. The drug-loading content of Ptx reached nearly 23.2 wt%.Citation157
Figure 6 Formation of mPEG-b-Dox micelles.
Abbreviations: mPEG, methoxy polyethylene glycol; Dox, doxorubicin.
Similarly to linear PDC liposomes, branched PDC unimolecular micelles with hydrophobic inner domain can further effectively encapsulate hydrophobic substances into their cores for imaging or codelivery. Tai et alCitation158 reported γ-CPT and Dox dual-drug-loaded nanomedicines prepared via polymerization of γ-CPT–glutamate N-carboxyanhydride on a PEG-based backbone via ring-opening polymerization. The amphiphilic conjugates self-assembled into unimolecular micelles with average hydrodynamic size of 50 nm. Dox molecules were then loaded into the hydrophobic core through physical interactions, such as π–π stacking. The drug-loading contents of CPT and Dox were 25.1 wt% and 30 wt%, respectively. An in vivo study demonstrated that dual-drug-loaded nanomedicines displayed significant synergistic effect on lung cancer-xenograft mice.
PDCs are an emerging one-component strategy for high drug-loading nanomedicine fabrication. This one-component feature with drug as part of carrier is essentially a double-edged sword: 1) for a particular PDC, the influence of the covalent links between drug molecules and biodegradable polymers to drug pharmacokinetics cannot be predicted; 2) the drug-release process can be complicated, which often involves both the nanostructure dissociating into monomeric units and the chemical breakdown of the linker; and 3) the choice of degradable linkers significantly affects the release mechanism of free bioactive drug from assembled nanostructures.
ICP I-type nanomedicines
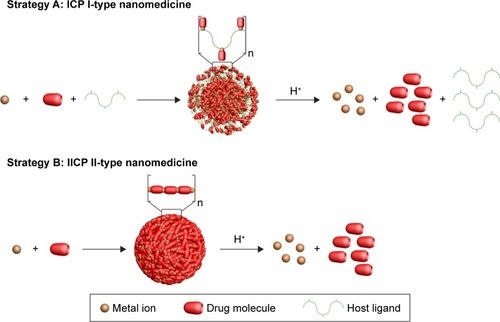
ICPs are one of the classes of coordination polymers. Compared with conventional MOF-based nanomedicines, ICP-type high drug-loading nanomedicines often exhibit certain additional advantages. The ICP-type nanomedicines have a trimmable size and well-defined shapes;Citation159 therefore, a desired particle size can be obtained for intravenous injection compared with absorption/desorption-type MOF-based nanomedicines and MBioFs. The pH-responsive point of nanomedicines can move to a targeted location without any additional dramatic alterations in the polymeric structure, due to the controllable degree of polymerization, chain monodispersion, intermolecular forces, and drug–metal coordination bonding strength.Citation160,Citation161 Several conventional anticancer drugs with autofluorescence often have tunable fluorescence when coordinating with central metal atoms.Citation162,Citation163 ICP-type nanomedicines are believed to be suitable candidates for computational design and combinatorial chemistry for drug content-specific nanomedicine development, because of their high drug-loading content and efficiency.Citation164 This section outlines recent advances in ICP I-type high drug-loading nanomedicines that consist of metal atoms and two kinds of organic ligands, with one of the ligands being the drug molecule.
The first use of ICPs in drug-delivery systems was in 2011 by Xing et al.Citation164 They defined ICP I-type nanomedicines as “host metal–ligand coordination polymer” and ICP II-type nanomedicines as “metal–ligand coordination polymer”. As illustrated in , in ICP I-type nanomedicine, host ligands and drug molecules were coordinated with metal ions. For some “metal–drug” coordination particles with unsuitable bond energy or irregular NP morphology, host ligands, such as PEG, oligochitosan, and Pluronic F127, were introduced to endow the particles with appropriate size and pH sensitivity. For example, mitoxantrone (MX)-Cu NPs did not release MX, even in a highly acidic medium, because of their strong coordination bond. In this case, PEG was introduced as the host molecule to weaken the coordination bond, which was achieved based on the weak coordination binding site provided by the EO moiety of PEG. Although the article did not provide a specific drug-loading content, such values were obviously high in these nanomedicines. With this strategy, Moustaoui et al reported another ICP I-type high drug-loading nanomedicine, PEG-Au(III)-Dox, in a recent work.Citation165 In this nanomedicine, Dox molecules and dicarboxylic PEG molecules were chelated with Au(III) ions from tetrachloroauric acid (HAuCl4). It was demonstrated that nanomedicine depolymerization and effective Dox release were achieved at pH 4, with stability attained at a diameter close to 20 nm in physiological pH. The drug-loading efficiency of Dox in this nanomedicine was up to 85%.
Figure 7 Formation of ICP I-type nanomedicine and ICP II-type nanomedicine and their pH-responsive release.
Abbreviation: ICP, infinite coordination polymer.
The following issues are worth noting. This strategy is only applied to drugs possessing complexation ability, such as carboxylates and phosphonates, including daunorubicin hydrochloride, Dox hydrochloride, MX, alizarin red, 1,10-phenanthroline, cis-platinum, 1,4-bis-([2-{dimethylamino-N-oxide}ethyl]amino)5,8-dihydroxyanthracene-9,10-dione (AQ4N), and gossypol.Citation165–Citation167 The particle size and pH-responsive point of nanomedicines are dependent on the complex combinations of host ligands, drug molecules, and center metal ions. Computational design and combinatorial chemistry can be utilized as primary screening to equip ICP I-type nanomedicines with ideal properties. More studies on cell and animal levels are needed to confirm the outstanding antitumor effects of ICP I-type nanomedicines.
Carrier-free nanomedicines
With the previously mentioned strategies, a significant number of high drug-loading nanomedicines have been developed ( and ). However, an intrinsic problem in these nanomedicines is that their degradation and excipient-excretion rates remain undetermined.Citation168 To circumvent this concern, carrier-free nanomedicines with no excipient were developed, such as traditional DNCs, DDC micelles, MBioFs, and ICP II-type nanomedicines. All these types of nanomedicines often have drug-loading contents higher than 80 wt%. These strategies are summarized in . Obviously, these strategies were applied for nanomedicine loading with drugs that are stable in the circulatory system, which are similar to those shown in .
Table 3 Overview of three strategies of carrier-free nanomedicines
Drug nanocrystals
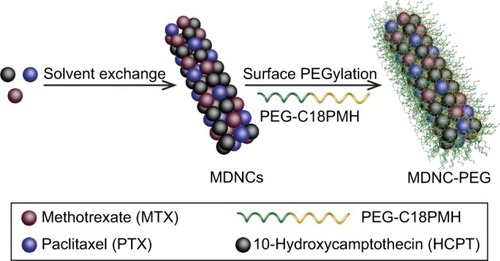
DNCs are a conventional viable strategy to develop hydrophobic drug nanomedicines with the advantages of general applicability and simplicity. Basically, such nanomedicines consist of DNCs dispersed in liquid medium, and are stabilized by surface-active agents, such as the poloxamer family,Citation169–Citation173 Tween,Citation174–Citation176 and polymers.Citation177–Citation180 For oral administration, the high surface:volume ratios of DNCs significantly increase dissolution velocity, thereby leading to an improvement in the gastrointestinal tract absorption based on the Noyes–Whitney equation.Citation181 For intravenous administration, the smallness of this nanomedicine enables it to enter blood circulation and achieve targeted delivery through further surface modification. In general, the conversion of a medical technology converted into a market product takes several decades, but a DNC-type nanomedicine (also called nanosuspensions) only needs several years.Citation182 The first DNC product for oral application (named Rapamune®) was successfully introduced to the market by Wyeth in 2000. Moreover, the first DNC product for intravenous application (named Abraxane®) was placed on the market by American Pharmaceutical Partners. Currently, several products are undergoing clinical trials and will be on the market in the near future. DNC-fabrication techniques are classified as either “top-down” or “bottom-up” methods, based on the size-change process.Citation183 The top-down method starts from large crystals to small NCs in a step-by-step manner during a high-energy process, such as pearl milling and high-pressure homogenization.Citation182,Citation184–Citation186 The bottom-up method involves procedures that start from molecules to NCs, such as solvent exchange,Citation21,Citation187–Citation189 high-gravity-control precipitation,Citation188,Citation190,Citation191 evaporative precipitation,Citation192 rapid expansion of supercritical solution,Citation193 and supercritical antisolvent methods.Citation194 DNCs prepared via bottom-up methods need less energy and are smaller compared with those prepared through top-down methods. Solvent exchange is the simplest, commonest, and most cost-effective method used in laboratory experiments. This method can produce both crystalline and amorphous forms of DNCs. DNCs smaller than 200 nm can be administered intravenously with 100% bioavailability and achieve multifunctionality.Citation195,Citation196 Li et al developed differently shaped 10-hydroxy-CPT (HCPT) NCs, nanospheres (NSs), and nanorods using solvent exchange and further surface modification.Citation197 Polymaleic anhydride-alt-1-octadecene–PEG (C18PMH-PEG) was chosen as a stabilizer, which was introduced on the surfaces of DNCs through noncovalent hydrophobic interaction. The surface PEGylation on HCPT NCs served as a steric repulsion–hydration molecular layer to reduce electrostatic and hydrophobic interactions with biomolecules, endowing them with excellent long-term stability at high salt concentrations and extreme pH.Citation198 The drug-loading contents of PEGylated NSs and nanorods were as high as 91.9 wt% and 93.3 wt%, respectively. In other research, HCPT NCs with targeted delivery ability were prepared using solvent exchange, followed by surface functionalization with C18PMH-PEG-FA.Citation199 This nanomedicine benefited from the high selectivity of FA to folate receptor-positive cancer cells, resulting in enhanced drug efficacy. The drug-loading content in this nanomedicine was approximately 78%. DNCs can also be used for the fabrication of an all-in-one processing system for cancer diagnosis and treatment. Zhou et al developed carrier-free multifunctional multi-DNCs (MDNCs) that consisted of methotrexate (Mtx), HCPT, Ptx, and the amphiphilic polymer stabilizer C18PMH-PEG ().Citation200 The MDNCs were prepared through solvent exchange, and the PEG layer was added on the surface via hydrophobic interaction. In vitro studies demonstrated that the MDNCs showed synergistic effects and improved tolerance. Given the minute amount of red fluorescent dye (TBPT, synthesized by themselves), the MDNCs exhibited high contrast during in vivo imaging. The drug-loading contents of Mtx, HCPT, and Ptx were 24.3 wt%, 49.1 wt%, and 26.6 wt%, respectively.
Figure 8 Preparation and functionalization of MDNCs.
Abbreviations: MDNC, multidrug nanocrystal; PEG, polyethylene glycol; PMH, polymaleic anhydride-alt-1-octadecene.
DNCs are a traditional assembly technology utilized in the fabrication of high drug-loading nanomedicine. Considerable developments have been made in NC-assembly technology over the last decade. However, certain limitations still exist: 1) both top-down and bottom-up methods apply only to the fabrication of hydrophobic high drug-loading nanomedicines, and thus the range of application is limited by the requirement of drug solubility; 2) the problem of removing residual organic solvent cannot be neglected for industrial production; and 3) most studies on crystal nanomedicine have focused on the solubility problem of hydrophobic drugs, and few have involved sustained release, targeting problems, and cell uptake of DNCs. Therefore, research on surface modification and cyclic behavior of nanomedicine crystal in vivo represents an important direction for further DNC development.
Amphiphilic drug–drug conjugates
ADDCs are a class of special PDCs in which the hydrophilic and hydrophobic parts are represented by hydrophilic and hydrophobic drugs, respectively. This class of dual-drug-loaded nanomedicines has the advantages of ultrahigh drug-loading content and synergistic combination chemotherapy because of its inherent nature. This strategy, which was first proposed by Huang et al,Citation201 is considered an excellent candidate for the fabrication of dual-drug synergistic therapy nanomedicines with high and fixed drug loading. Two methods are proposed under this strategy. The first is the “natural method,” which is applied to drug pairs when one drug has a carboxyl group and the other an activated hydroxyl group. The second is the “modified method,” which is applied to any modifiable AD pairs using a linker compound. All these nanomedicines have sizes suitable for intravenous administration. The total drug-loading content is approximately 100 wt%.
For the natural method, a dual-drug-loaded nanomedicine was synthesized from a hydrophilic anticancer drug, irinotecan (Ir), and a hydrophobic anticancer drug, chlorambucil (Cb), via a hydrolyzable ester linkage by Huang et al.Citation201 The ester linkage was formed from the hydroxyl of Ir molecules and carboxyl of Cb molecules through direct esterification using dicyclohexylcarbodiimide/4-dimethylamino-pyridine without any addition (). The amphiphilic Ir-Cb conjugates self-assembled into micelles in aqueous solution with average size of approximately 75.7 nm. After cell uptake of nanomedicine, the ester linkages were hydrolyzed by the existing esterase and low pH in endosomes, causing the drug molecules to be effectively released. In vitro and in vivo antitumor experiments demonstrated that this nanomedicine exhibited high anticancer activity and could circumvent the multidrug resistance of tumor cells in chemotherapy. The same method was also used to fabricate fluorodeoxyuridine–bendamustine (FUDR-BdM) and Ir-BdM conjugates by Huang et al.Citation202,Citation203
Figure 9 Ir-Cb ADDC following natural method (A) and CPT-FUDR ADDCs following modified method (B).
Abbreviations: Ir, irinotecan; Cb, chlorambucil; ADDCs, amphiphilic drug–drug conjugates; CPT, camptothecin; FUDR, fluorodeoxyuridine; DCC, dicyclohexylcarbodiimide; DMAP, dimethylaminopyridine; RT, room temperature; DMSO, dimethyl sulfoxide.
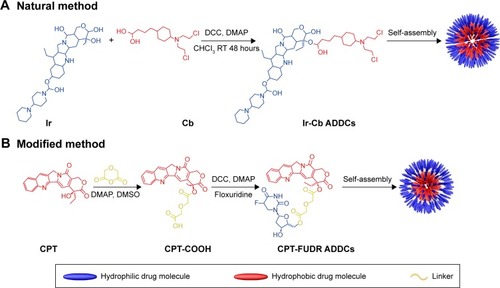
The range of application of this strategy is widely extended with the use of the modified method. For example, in a recent work of Hu et al,Citation204 a dual-drug-loaded nanomedicine, CPT-FUDR conjugates, was synthesized through two-step esterification of hydrophobic CPT and hydrophilic FUDR using a linker compound. As illustrated in , the first step was the introduction of a carboxylic group onto the CPT molecule through esterification of CPT and diglycolic anhydride. Subsequently, the esterification reaction of CPT-COOH and FUDR was carried out, and the amphiphilic CPT-FUDR conjugate linked by a hydrolyzable ester was formed. The resultant amphiphilic conjugate self-assembled into nanomicelles with hydrodynamic size of 36.4 nm. In vitro studies demonstrated that CPT-FUDR conjugates exhibited synergistic anticancer efficacy and similar intracellular behavior to DDC nanomedicines prepared via the natural method.
The following findings are noted. ADDCs are a kind of high drug-loading nanomedicine with narrow applicable range. This requires one of the drugs to be hydrophilic and the other to be hydrophobic, and these two drugs should satisfy a specific structure simultaneously. Few drug pairs possess these conditions while having a synergistic effect at the same time. Although some ADDC pairs have been reported to possess good anticancer efficacy, the influence of the structural modification of drug molecules on anticancer efficacy and pharmacokinetics is impossible to predict, especially the influence of the structural transformation of drug molecules caused by linker compound in the modified method. Further surface modification is difficult, due to the micelle-type structure of ADDC nanomedicines. Therefore, additional research should be concentrated on the surface modification of drug molecules and pharmacokinetics in the future.
Coordination polymer NPs
In this section, we focus on MBioFs and ICP II-type high drug-loading nanomedicines, in which the drug molecule acts as the only ligand coordinating with the central metal atom without any other vehicle components. Similar to ICP I-type nanomedicines, MBioFs and ICP II-type strategies are only applicable to those drugs with complexing ability.
MBioFs
MBioFs are defined as MOFs constructed from biomolecules that serve as organic ligands, such as amino acids, peptides, proteins, nucleobases, saccharides, and drug molecules ().Citation205 MBioFs display more advantages in the fabrication of high drug-loading nanomedicines than MOFs: 1) MBioFs avoid the structural requirements of MOFs, such as pore size and volume; 2) drug release is achieved through nanostructure biodegradation, without any side effects, because no inert material linkers are used; and 3) drug molecules often have many different metal-binding sites, which leads to multiple possible coordination modes and adjustable physical and chemical properties.Citation206 For example, Miller et al developed a small-pore iron (II/III) nicotinate Bio-MIL1, which consists of nicotinic acid (pyridine-3-carboxylic acid, also called niacin or vitamin B3) and nontoxic iron.Citation207 The structure of Bio-MIL1 comprises a three-dimensional connected framework built from trimeric Fe3N3O13 units linked together via nicotinate molecules. These nanomedicines have a drug-loading content as high as 75 wt%, and are rapidly degraded to achieve nicotinic acid release under physiological conditions. To date, MBioF nanomedicines have not been fully explored. Only some examples have been reported, and almost no studies on cell and animal levels can be searched. A possible explanation is that the stability of some MBioFs in aqueous and in vivo environments is not yet known.Citation120,Citation205,Citation208 As such, further in vitro and in vivo studies should be conducted so that MBioFs have wide application prospects in the biomedical field.
ICP II-type nanomedicines
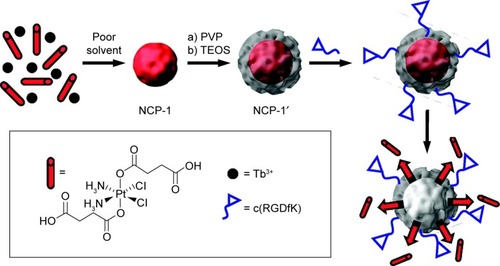
ICP II-type nanomedicines are ICPs prepared directly from drug molecules and metal atoms (). ICP II-type nanomedicines display the same advantages as ICP I-type nanomedicines, and have higher drug-loading content. ICP II-type nanomedicines can be formed from a number of self-assembly processes.Citation209–Citation211 Solvothermal synthesis is the most straightforward method, and consists of metal and ligand molecules heated in a solvent, which can lead to the formation of amorphous or crystalline products. ICP II-type can also be synthesized using reverse microemulsion (water in oil), in which the reactants are suspended in surfactant-stabilized aqueous droplets in the organic phase and the droplets behave as nanoreactors, facilitating ICP assembly. Finally, ICPs can be formed via rapid precipitation that involves precipitating a solution of reactants with a solvent, which usually results in amorphous particle formation. For instance, Rieter et al fabricated an ICP II-type nanomedicine from Tb(III) ions and c,c,t-(PtCl2[NH3]2[O2CCH2CH2CO2H]2) (disuccinatocisplatin, [DSCP]) via rapid precipitation ().Citation212 The Tb(III)-DSCP ICPs displayed a size of 58.3±11.3 nm in diameter using dynamic light scattering. The drug-loading content of DSCP was 36 wt%, which was determined through thermogravimetric analysis. These ICP particles were easily functionalized with a variety of silyl-derived molecules and further grafted to silyl-derived c(RGDfK), which is a small cyclic peptide sequence that is frequently observed in many angiogenic cancers, on the surface to achieve targeted delivery. Huxford et al synthesized serious ICP II-type Mtx nanomedicines via reverse microemulsion.Citation213 Different metal ions, such as Zn2+, Zr4+, and Gd3+, with different coordination numbers were chosen to be coordinated with Mtx. The ICPs were further stabilized and modified using dioleoyl trimethylammonium propane/dioleoyl-l-α-phosphatidylethanolamine–anisamide. These nanomedicines were spherical in appearance with diameters between 40 and 100 nm via transmission electron microscopy. Thermogra-vimetric analysis revealed that the drug-loading content of Mtx reached 79.1 wt%.
Figure 10 Synthesis and functionalization of Tb(III)-DSCP ICPs.
Notes: Reprinted (in part) with permission from Rieter WJ, Pott KM, Taylor KM, Lin WB. Nanoscale coordination polymers for platinum-based anticancer drug delivery. Journal of the American Chemical Society. 2008;130(35):11584–11585. Copyright © 2008 American Chemical Society.Citation212
Abbreviations: DSCP, disuccinatocisplatin; ICPs, infinite coordination polymers; NCP, nano-coordination polymer; PVP, polyvinylpyrrolidone; TEOS, tetraethyl orthosilicate.
Scheme 1 Fabrication strategies of high drug-loading nanomedicines.
Abbreviations: MSNPs, mesoporous silica nanoparticles; MCNPs, mesoporous carbon nanoparticles; MMCNCs, mesoporous magnetic colloidal nanocrystal clusters; MOFs, metal–organic frameworks; LPDCs, linear polymer–drug conjugates; BPDCs, branched PDCs; ICP, infinite coordination polymer; DNCs, drug nanocrystals; ADDCs, amphiphilic drug–drug conjugates; MBioFs, metal–biomolecule frameworks.
MBioF and ICP II-type strategies are not broad-spectrum techniques, due to the requirement of drug-complexing ability, similar to ICP I-type strategy. In addition, controlling the size of these nanomedicines is not easy, because of the absence of host ligands. Fortunately, molecular simulations are possible, because of the one-component feature and ultrahigh drug-loading content and efficiency of these nanomedicines. The application of computational design and combinatorial chemistry will allow the efficient design of MBioF and ICP II-type high drug-loading nanomedicines.
Nanomedicines following niche and complex strategies
Some niche and complex strategies, such as aqueous non-covalent assembly and multiple assemblies, are used for the fabrication of specific multidrug-loaded high drug-loading nanomedicines (). For aqueous noncovalent assembly, the selected drugs should possess opposite static electricity, π–π stacking, and hydrophobic interactions. The biggest difference between solvent exchange for DNC fabrication and noncovalent assembly is that the former is applied for the fabrication of hydrophobic drug-loaded nanomedicines, whereas the latter is utilized for the fabrication of hydrophilic (including water-soluble drugs under certain conditions) drug-loaded nanomedicines. In this strategy, no organic solvent is used in the fabrication process. The molar ratio of multiple drugs is tailorable within a certain range, and the total drug-loading content for nanomedicines is 100 wt%. Zhang et al developed a carrier-free, chemophotodynamic dual-drug-loaded nanomedicine prepared from a chemotherapeutic agent (Dox) and a photosensitizer (chlorine e6 [Ce6]).Citation14 Depending on electrostatic, π–π stacking, and hydrophobic interactions, drug molecules self-assembled into well-defined NSs with an average size of 70 nm in a basic aqueous solution (pH 12). Total drug-loading content was 100 wt%, and drug-loading efficiency of Ce6 and Dox were 95% and 99%, respectively. Further experiments on BALB/c nude mice with xenografted MCF7 tumors demonstrated that this carrier-free, chemophotodynamic dual-drug-loaded nanomedicine was an effective combinational therapeutic modality. Similarly, a novel high drug-loaded nanomedicine is currently being studied by our group.
Table 4 Overview of two strategies used for minority-specific nanomedicines
For multiple assemblies, the selected drug–carrier pairs should possess appropriately matched physical and chemical properties. Notably, this strategy is a comprehensive strategy for the fabrication of multidrug-loaded nanomedicine. Recently, our group reported a mulberry-like dual-drug-loaded nanomedicine following this strategy.Citation214 First, two perfectly matched drug–carrier pairs, apogossypolone–cationic amphiphilic starch (ApoG2-CSaSt) and Dox-HA, self-assembled to form two seeds, namely, ApoG2-CSaSt micelles and Dox-HA NSs (DHA NSs), respectively. These two seeds further self-assembled to form NPs (MLDC NCs) with a mulberry-like shape and dynamic size of 83.1±6.6 nm. The first self-assembly occurred based on the hydrophobic interaction in the ApoG2-CSaSt pair and the electrostatic absorption in the DHA pair. The subsequent self-assembly occurred based on the electrostatic absorption between CSaSt and HA caused by the designed positive charge of CSaSt and the natural negative charge of HA. DHA NSs located on the surface of MLDC NCs functioned as a targeting agent at the same time. The drug-loading contents of ApoG2 and Dox in MLDC NCs were 13.3%±1.2% and 13.1%±3.7%, respectively, and these values were adjustable to a certain extent. For the in vivo tumor-suppression test, the injection dosages of Dox and ApoG2 were both 2 mg/kg, which was only a fifth of the normal dosages in the mouse test, and resulted in enhanced antitumor effects. This result suggested that high drug-loading nanomedicines can effectively realize multidrug synergy and have obvious antitumor advantages compared with low drug-loading nanomedicines.
Deng et al developed an anticancer drug and siRNA-loaded nanomedicine following a similar strategy. First, a Dox-loaded negatively charged phospholipid liposome was developed.Citation215 Consequently, a siRNA-loaded film was generated through alternate deposition of positively charged poly-L-arginine and negatively charged siRNA atop the Dox-loaded liposome. Finally, the NP surface was coated with a layer of HA to achieve targeted delivery. The loading efficiency and content of Dox in the nanomedicine were 97 wt% and 5.5 wt%, respectively. In addition, the poly-l-arginine/siRNA layer-by-layer film loaded approximately 3,500 siRNA molecules per NP per layer. Niche and complex strategies are usually applicable for particular drugs or drug pairs, and their implementation schemes are unsuitable for other nanomedicines. However, the design philosophy, which ingeniously takes advantage of the special physicochemical property of drugs or drug pairs, is worth learning from.
Conclusion and prospects
In the past 30 years, the development of nanocarrier-based platform technologies has led to significant progress in nanomedicine fabrication and applications. However, most of the existing nanomedicines have low drug-loading content (generally lower than 10%), causing extra system toxicity and burden on patients to excrete carrier materials. Therefore, it is difficult for most of the carrier-based nanomedicines to get FDA approval, which limits their applications in the clinic. With this in mind, emergence and fabrication of high drug-loading nanomedicines may not provide the final answer, but present a promising alternative approach to solve this problem, because of the reduced or avoided use of carrier materials.
The three classes of high drug-loading nanomedicines, which can either reduce or avoid the use of carrier materials, are applied to the fabrication of high drug-loading nanomedicines with different drug properties. In addition to common advantages of nanomedicines, some high drug-loading-nanomedicines possess inherent advantages, benefiting from their structure and ultrahigh drug-loading content and efficiency, such as stimulant responsibility, multidrug combined therapy, computational design, and combinatorial chemistry properties. Despite all these benefits, the therapeutic effect of current high drug-loading nanomedicines is not so satisfactory. Furthermore, several theoretical and actual problems remain unsolved. Demonstrating the better in vivo antitumor effect of high drug-loading nanomedicines than low drug-loading nanomedicines is significant. However, most existing high drug-loading nanomedicine studies have focused on design and fabrication and lacked comparison of the antitumor effect at the animal level. As such, these studies failed to demonstrate the complete advantages of high drug-loading nanomedicines. Understanding the differences between the antitumor mechanisms of high drug-loading and low drug-loading nanomedicines is important to improve the design of subsequent high drug-loading nanomedicines. However, existing reports on high drug-loading nanomedicines have mostly focused on nanomedicine design, and almost no research has been conducted on the internalization mechanism, intracellular release, and subcellular level actions. Owing to long conversion times, safety concerns, and associated socioeconomic uncertainties, most concepts investigated are at the laboratory stage, and a few have entered routine clinical application, such as Rapamune and Abraxane, which we discussed in this paper. Despite this, it irresistible that more high drug-loading nanomedicines will be transitioned into clinics in the future.Citation216
Meanwhile, the following issues are worth noting. For some specific drugs, such as gene drugs that are unstable in the circulatory system and brain-targeting drugs that need to cross the blood–brain barrier, carrier materials are essential for the realization of their function. For some carrier materials with function, such as Pluronic block copolymers, biological response modifiers affecting cell-membrane microviscosity and ATPase activity of drug-efflux transporters, the use of carrier materials will enhance the therapeutic effect.Citation217 For most of the high drug-loading nanomedicines, it is hard to realize in vivo controlled release, such as ultrasound-controlled release, magnetism-controlled release, and microwave-controlled release. For nanomedicines with drug-loading content higher than 50%, sustained-release effects are far below expected, and there are fewer pharmacokinetic studies in this category of articles. For nanomedicines with drug-loading content higher than 90%, it is usually hard to achieve surface modification, so further study is needed on the action of those high drug-loading nanomedicines in the complicated physiological environment and highly dynamic and heterogeneous tumor sites. Clearly, the high drug-loading property serves therapeutic effect and function purposes.
Over the past few years, the number of FDA-approved drugs, including protein drugs, oligonucleotide drugs, and small-molecule inhibitors, has increased, such as lixisenatide,Citation218 defibrotide sodium,Citation219 and rucaparib.Citation220 Although these drugs show excellent performance against certain diseases, it cannot be ignored that their inherent drawbacks, such as poor water solubility and unstable properties in the circulatory system, lead to unsatisfactory absorption and frequent injection for patients. A promising approach to overcome these drawbacks is loading them into nanomedicine. Even though much time and high costs are required to develop a new nanomedicine, we still believe that there will be more nanomedicines approved by the FDA in the future. We hope that this review will attract more attention from researchers in this field, and expect that high drug-loading nanomedicines with still-better properties will be prepared by developing novel carrier materials, exploring new fabrication strategies and modification methods and perfecting theoretical research at the cell and animal level. We also believe that high drug-loading nanomedicine-delivery systems will be gradually improved through the joint efforts of chemists, biologists, material specialists, and cancer and pharmaceutical researchers, and will occupy an important position in the field of drug-delivery systems in the future.
Acknowledgments
This work was sponsored in part by the National Natural Science Foundation of China (81271686, 81228011, and 81471771) and grants from the National Key Research and Development Program (2016YFC0100701).
Disclosure
The authors report no conflicts of interest in this work.
References
- KawabataYWadaKNakataniMYamadaSOnoueSFormulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applicationsInt J Pharm2011420111021884771
- LigginsRTBurtHMPolyether-polyester diblock copolymers for the preparation of paclitaxel loaded polymeric micelle formulationsAdv Drug Deliv Rev200254219120211897145
- DuWTHongLYaoTWSynthesis and evaluation of water-soluble docetaxel prodrugs-docetaxel esters of malic acidBioorg Med Chem200715186323633017624790
- LukeDRKasiskeBLMatzkeGRAwniWMKeaneWFEffects of cyclosporine on the isolated perfused rat kidneyTransplantation19874367957993590298
- OnettoNCanettaRWinogradBOverview of Taxol safetyJ Natl Cancer Inst Monogr199315131139
- TorchilinVPNanocarriersPharm Res200724122333233417934800
- FerrariMCancer nanotechnology: opportunities and challengesNat Rev Cancer20055316117115738981
- WagnerVDullaartABockAKZweckAThe emerging nanomedicine landscapeNat Biotechnol200624101211121717033654
- AllenTMCullisPRDrug delivery systems: entering the mainstreamScience200430356651818182215031496
- VermaRKGargSCurrent status of drug delivery technologies and future directionsPharm Technol2001252114
- WuJZhuYJCaoSWChenFHierachically [sic] nanostructured mesoporous spheres of calcium silicate hydrate: surfactant-free sonochemical synthesis and drug-delivery system with ultrahigh drug-loading capacityAdv Mater201022674975320217783
- LiuKPZhangJJChengFFZhengTTWangCMZhuJJGreen and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug deliveryJ Mater Chem201121321203412040
- YeLLetchfordKHellerMSynthesis and characterization of carboxylic acid conjugated, hydrophobically derivatized, hyper-branched polyglycerols as nanoparticulate drug carriers for cisplatinBiomacromolecules201112114515521128674
- ZhangRYXingRRJiaoTFCarrier-free, chemophotodynamic dual nanodrugs via self-assembly for synergistic antitumor therapyACS Appl Mater Interfaces2016821132621326927176934
- TrucilloPCampardelliRReverchonESupercritical CO2 assisted liposomes formation: optimization of the lipidic layer for an efficient hydrophilic drug loadingJ CO2 Util201718181188
- ZhangWWangGFalconerJRStrategies to maximize liposomal drug loading for a poorly water-soluble anticancer drugPharm Res20153241451146125355460
- RibeiroCACastroCEAlbuquerqueLJBatistaCCGiacomelliFCBiodegradable nanoparticles as nanomedicines: are drug-loading content and release mechanism dictated by particle density?Colloid Polym Sci Epub201719
- ZhengSJXieYQLiYDevelopment of high drug-loading nanomicelles targeting steroids to the brainInt J Nanomedicine20149556624379663
- QinSYZhangAQChengSXRongLZhangXZDrug self-delivery systems for cancer therapyBiomaterials201611223424727768976
- CaiKMHeXSongZYDimeric drug polymeric nanoparticles with exceptionally high drug loading and quantitative loading efficiencyJ Am Chem Soc2015137103458346125741752
- ZhangJLiSAnFFSelf-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug releaseNanoscale2015732135031351026199064
- LokichJJZipoliTEMooreCSonnebornHPaulSGreeneRDoxorubicin/vinblastine and doxorubicin/cyclophosphamide combination chemotherapy by continuous infusionCancer1986585102010233731035
- AzadNSPosadasEMKwitkowskiVECombination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activityJ Clin Oncol200826223709371418669456
- BlankCUHooijkaasAIHaanenJBSchumacherTNCombination of targeted therapy and immunotherapy in melanomaCancer Immunol Immunother201160101359137121847631
- JiangHTWangTYWangLHDevelopment of an amorphous mesoporous TiO2 nanosphere as a novel carrier for poorly water-soluble drugs: effect of different crystal forms of TiO2 carriers on drug loading and release behaviorsMicroporous Mesoporous Mater201215314124130
- YuMXueYMaPXMaoCLeiBIntrinsic ultrahigh drug/miRNA loading capacity of biodegradable bioactive glass nanoparticles toward highly efficient pharmaceutical deliveryACS Appl Mater Interfaces20179108460847028240539
- Vallet-RegiMRámilaARealRPPérez-ParienteJA new property of MCM-41: drug delivery systemChem Mater2001132308311
- TarnDAshleyCEXueMCarnesECZinkJIBrinkerCJMesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibilityAcc Chem Res201346379280123387478
- LiXZhangLXDongXPLiangJShiJLPreparation of mesoporous calcium doped silica spheres with narrow size dispersion and their drug loading and degradation behaviorMicroporous Mesoporous Mater20071021–3151158
- ChenHTHuhSLinVSFine tuning the functionalization of mesoporous silicaRegalbutoJCatalyst Preparation: Science and EngineeringNew YorkCRC Press20064573
- SlowingIIVivero-EscotoJLWuCWLinVSMesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriersAdv Drug Deliv Rev200860111278128818514969
- BurleighMCDaiSHagamanEWBarnesCEXueZLStepwise assembly of surface imprint sites on MCM-41 for selective metal ion separationsACS Symp Ser Am Chem Soc2000778146158
- SunLZWangYZhengXNovel chitosan-functionalized spherical nanosilica matrix as an oral sustained drug delivery system for poorly water-soluble drug carvedilolACS Appl Mater Interfaces20135110311323237208
- EltohamyMSeoJWHwangJYJangWCKimHWShinUSIonic and thermo-switchable polymer-masked mesoporous silica drug-nanocarrier: high drug loading capacity at 10°C and fast drug release completion at 40°CColloids Surf B Biointerfaces201614422923727092438
- ZhouGXZhangZLChenBSynthesis of double-shelled hollow silica sphere with single-shelled hollow silica sphere and cetyltrimethyl ammonium bromide as dual templatesIET Micro Nano Lett2017122133135
- EltohamyMShinUSKimHWSilica nanoparticles with enlarged nanopore size for the loading and release of biological proteinsMater Lett2011652335703573
- ZhuYFShiJLLiYSChenHRDongXPHollow mesoporous spheres with cubic pore network as a potential carrier for drug storage and its in vitro release kineticsJ Mater Res20052015461
- KresgeCTLeonowiczMERothWJVartuliJCBeckJSOrdered mesoporous molecular sieves synthesized by a liquid-crystal template mechanismNature19923596397710712
- ZhuYShiJShenWStimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core-shell structureAngew Chem Int Ed Engl200544325083508716015668
- GengHJZhaoYTLiuJHollow mesoporous silica as a high drug loading carrier for regulation insoluble drug releaseInt J Pharm2016510118419427262268
- EbabeERRahmaniSLauretCFunctionalized mesoporous silica nanoparticle with antioxidants as a new carrier that generates lower oxidative stress impact on cellsMol Pharm20161382647266027367273
- FarsangiZJBeitollahiAHattonBOne-pot and controllable synthesis of carboxylic group functionalized hollow mesoporous silica nanospheres for efficient cisplatin deliveryRSC Adv20166726759267598
- De AzaPNGuitianFDe AzaSBioactivity of wollastonite ceramics: in vitro evaluationScr Metall Mater199431810011005
- De AzaPNLuklinskaZBMartinezAAnseauMRGuitianFDe AzaSMorphological and structural study of pseudowollastonite implants in boneJ Microsc2000197Pt 1606710620149
- LinKLZhaiWYNiSYChangJZengYQianWJStudy of the mechanical property and in vitro biocompatibility of CaSiO3 ceramicsCeram Int2005312323326
- WanXHChangCKMaoDLJiangLLiMPreparation and in vitro bioactivities of calcium silicate nanophase materialsMater Sci Eng C Biomim Supramol Syst2005254455461
- XueWCBandyopadhyayABoseSMesoporous calcium silicate for controlled release of bovine serum albumin proteinActa Biomater2009551686169619249262
- WangBXMengWYBiMNiYXCaiQWangJYUniform magnesium silicate hollow spheres as high drug-loading nanocarriers for cancer therapy with low systemic toxicityDalton Trans201342248918892523660815
- ZhaoYNTrewynBGSlowingIILinVSMesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMPJ Am Chem Soc2009131248398840019476380
- LeeSKimMSLeeDThe comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in miceInt J Nanomedicine20128147158
- LuoZHuYXinRHSurface functionalized mesoporous silica nanoparticles with natural proteins for reduced immunotoxicityJ Biomed Mater Res A2014102113781379424288246
- LiuTLLiLLFuCHLiuHYChenDTangFQPathological mechanisms of liver injury caused by continuous intraperitoneal injection of silica nanoparticlesBiomaterials20123372399240722182752
- YamashitaKYoshiokaYHigashisakaKSilica and titanium dioxide nanoparticles cause pregnancy complications in miceNat Nanotechnol20116532132821460826
- YangXFHeCELiJUptake of silica nanoparticles: neurotoxicity and Alzheimer-like pathology in human SK-N-SH and mouse neuro2a neuroblastoma cellsToxicol Lett2014229124024924831964
- TangLChengJJNonporous silica nanoparticles for nanomedicine applicationNano Today20138329031223997809
- KimTWChungPWSlowingIITsunodaMYeungESLinVSStructurally ordered mesoporous carbon nanoparticles as transmembrane delivery vehicle in human cancer cellsNano Lett20088113724372718954128
- ZhangYZZhiZZLiXGaoJSongYLCarboxylated mesoporous carbon microparticles as new approach to improve the oral bioavailability of poorly water-soluble carvedilolInt J Pharm2013454140341123850816
- GuJLSuSSLiYSHeQJShiJLHydrophilic mesoporous carbon nanoparticles as carriers for sustained release of hydrophobic anti-cancer drugsChem Commun (Camb)20114772101210321183990
- ZhangYZWangHGaoCQLiXLiLSHighly ordered mesoporous carbon nanomatrix as a new approach to improve the oral absorption of the water-insoluble drug, simvastatinEur J Pharm Sci201349586487223791638
- NiuXWanLHouZMesoporous carbon as a novel drug carrier of fenofibrate for enhancement of the dissolution and oral bioavailabilityInt J Pharm20134521–238238923688621
- ZhaoPWangLHSunCSUniform mesoporous carbon as a carrier for poorly water soluble drug and its cytotoxicity studyEur J Pharm Biopharm201280353554322193360
- WangXFZhuXJWangSPanYFTianYDirect synthesis of carbon microspheres under acidic conditions and fenofibrate releaseMater Lett2016164156159
- SahaDSpurriAChenJHHensleyDKControlled release of alendronate from nitrogen-doped mesoporous carbonMicroporous Mesoporous Mater2016229813
- ZhangYZWangHLiCJA novel three-dimensional large-pore mesoporous carbon matrix as a potential nanovehicle for the fast release of the poorly water-soluble drug, celecoxibPharm Res20143141059107024287624
- HuBWangKWuLHYuSHAntoniettiMTitiriciMMEngineering carbon materials from the hydrothermal carbonization process of biomassAdv Mater201022781382820217791
- RyooRJooSHJunSSynthesis of highly ordered carbon molecular sieves via template-mediated structural transformationJ Phys Chem B19991033777437746
- DavisMEChenZShinDMNanoparticle therapeutics: an emerging treatment modality for cancerNat Rev Drug Discov20087977178218758474
- MiaoPTangYGHanKWangBDFacile synthesis of carbon nanodots from ethanol and their application in ferric(III) ion assayJ Mater Chem A Mater Energy Sustain20153291506815073
- ZhiXFangHLBaoCCThe immunotoxicity of graphene oxides and the effect of PVP-coatingBiomaterials201334215254526123566800
- PondmanKMSobikMNayakAComplement activation by carbon nanotubes and its influence on the phagocytosis and cytokine response by macrophagesNanomedicine20141061287129924607938
- MoutRMoyanoDFRanaSRotelloVMSurface functionalization of nanoparticles for nanomedicineChem Soc Rev20124172539254422310807
- JungSHJungSHSeongHChoSHJeongKSShinBCPolyethylene glycol-complexed cationic liposome for enhanced cellular uptake and anticancer activityInt J Pharm20093821–225426119666094
- WangHLiXGMaZQHydrophilic mesoporous carbon nanospheres with high drug-loading efficiency for doxorubicin delivery and cancer therapyInt J Nanomedicine2016111793180627175077
- YangKZhangSAZhangGXSunXMLeeSTLiuZAGraphene in mice: ultrahigh in tumor uptake and efficient photothermal therapyNano Lett20101093318332320684528
- ZhangWGuoZYHuangDQLiuZMGuoXZhongHQSynergistic effect of chemo-photothermal therapy using PEGylated graphene oxideBiomaterials201132338555856121839507
- WagnerDSDelkNALukianova-HlebEYHafnerJHFarach- CarsonMCLapotkoDOThe in vivo performance of plasmonic nano-bubbles as cell theranostic agents in zebrafish hosting prostate cancer xenograftsBiomaterials201031297567757420630586
- BurkeADingXSinghRLong-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiationProc Nati Acad Sci U S A2009106311289712902
- ZhouLDongKChenZWRenJSQuXGNear-infrared absorbing mesoporous carbon nanoparticle as an intelligent drug carrier for dual-triggered synergistic cancer therapyCarbon201582479488
- ZhaoYLuYHuYSynthesis of superparamagnetic CaCO3 mesocrystals for multistage delivery in cancer therapySmall20106212436244220878636
- ShinJMAnisurRMKoMKImGHLeeJHLeeISHollow manganese oxide nanoparticles as multifunctional agents for magnetic resonance imaging and drug deliveryAngew Chem Int Ed Engl20091212327330
- LiDTangJGuoJWangSLChaudharyDWangCCHollow-core magnetic colloidal nanocrystal clusters with ligand-exchanged surface modification as delivery vehicles for targeted and stimuli-responsive drug releaseChemistry20121851165171652423108596
- DengYHWangCCShenXZPreparation, characterization, and application of multistimuli-responsive microspheres with fluorescence-labeled magnetic cores and thermoresponsive shellsChemistry200511206006601316047395
- YoonTJKimSJKimGBYuKNChoMHLeeJKMultifunctional nanoparticles possessing a “magnetic motor effect” for drug or gene deliveryAngew Chem Int Ed Engl2005117710921095
- KimJKimHSLeeNMultifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug deliveryAngew Chem Int Ed Engl200847448438844118726979
- DengHLiXLPengQWangXChenJPLiYDMonodisperse magnetic single-crystal ferrite microspheresAngew Chem Int Ed Engl20051171828422845
- GeJPHuYXBiasiniMBeyermannWPYinYDSuperparamagnetic magnetite colloidal nanocrystal clustersAngew Chem Int Ed Engl200746234342434517465432
- LiuJSunZKDengYHHighly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groupsAngew Chem Int Ed Engl20091213259895993
- LuoBXuSLuoAMesoporous biocompatible and acid-degradable magnetic colloidal nanocrystal clusters with sustainable stability and high hydrophobic drug loading capacityACS Nano2011521428143521284377
- LiDTangJWeiCDoxorubicin-conjugated mesoporous magnetic colloidal nanocrystal clusters stabilized by polysaccharide as a smart anticancer drug vehicleSmall20128172690269722674615
- ChengKPengSXuCJSunSHPorous hollow Fe3O4 nanoparticles for targeted delivery and controlled release of cisplatinJ Am Chem Soc200913130106371064419722635
- HuJChenMFangXSWuLWFabrication and application of inorganic hollow spheresChem Soc Rev201140115472549121799974
- HuPYuLJZuoAHGuoCYYuanFLFabrication of monodisperse magnetite hollow spheresJ Phys Chem C Nanomater Interfaces20091133900906
- LiuSHXingRMLuFRanaRKZhuJJOne-pot template-free fabrication of hollow magnetite nanospheres and their application as potential drug carriersJ Phys Chem C Nanomater Interfaces2009113502104221047
- LuoBXuSAMaWFFabrication of magnetite hollow porous nanocrystal shells as a drug carrier for paclitaxelJ Mater Chem2010203471077113
- DrechslerASynytskaAUhlmannPElmahdyMMStammMKremerFInteraction forces between microsized silica particles and weak polyelectrolyte brushes at varying pH and salt concentrationLangmuir20102696400641020038115
- LiHLEddaoudiMO’KeeffeMYaghiOMDesign and synthesis of an exceptionally stable and highly porous metal-organic frameworkNature19994026759276279
- HuxfordRCRoccaJDLinWBMetal-organic frameworks as potential drug carriersCurr Opin Chem Biol201014226226820071210
- WangFLiuXGUpconversion multicolor fine-tuning: visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticlesJ Am Chem Soc2008130175642564318393419
- LeeJFarhaOKRobertsJScheidtKANguyenSTHuppJTMetal-organic framework materials as catalystsChem Soc Rev20093851450145919384447
- FarrussengDAguadoSPinelCMetal-organic frameworks: opportunities for catalysisAngew Chem Int Ed Engl200948417502751319691074
- BellATThe impact of nanoscience on heterogeneous catalysisScience200329956131688169112637733
- CaoYWJinRMirkinCANanoparticles with Raman spectroscopic fingerprints for DNA and RNA detectionScience200229755861536154012202825
- ChenBLYangYZapataFLinGNQianGDLobkovskyEBLuminescent open metal sites within a metal-organic framework for sensing small moleculesAdv Mater2007191316931696
- XiaoBWheatleyPSZhaoXBHigh-capacity hydrogen and nitric oxide adsorption and storage in a metal-organic frameworkJ Am Chem Soc200712951203120917263402
- MckinlayACXiaoBWraggDSWheatleyPSMegsonILMorrisREExceptional behavior over the whole adsorption-storage-delivery cycle for NO in porous metal organic frameworksJ Am Chem Soc200813031104401044418627150
- DietzelPDJohnsenREBlomRFjellvågHStructural changes and coordinatively unsaturated metal atoms on dehydration of honeycomb analogous microporous metal-organic frameworksChemistry20081482389239718203217
- ZhaoYJZhangJLHanBXSongJLLiJSWangQMetal-organic framework nanospheres with well-ordered mesopores synthesized in an ionic liquid/CO2/surfactant systemAngew Chem Int Ed Engl201150363663921226141
- HorcajadaPSerreCVallet-RegíMSebbanMTaulelleFFéreyGMetal-organic frameworks as efficient materials for drug deliveryAngew Chem Int Ed Engl20061183661206124
- FéreyGSerreCMellot-DraznieksCA hybrid solid with giant pores prepared by a combination of targeted chemistry, simulation, and powder diffractionAngew Chem Int Ed Engl20041164664566461
- CaiWChuCCLiuGWangYXMetal-organic framework-based nanomedicine platforms for drug delivery and molecular imagingSmall201511374806482226193176
- WuMWangQLiuXLiuJHighly efficient loading of doxorubicin in Prussian blue nanocages for combined photothermal/chemotherapy against hepatocellular carcinomaRSC Adv20155393097030980
- ZaidiRHadiSMStrand scission in DNA by gossypol and Cu(II): role of Cu(I) and oxygen-free radicalsJ Biochem Toxicol1992742132171293310
- FéreyGMellotdraznieksCSerreCA chromium terephthalate-based solid with unusually large pore volumes and surface areaScience200530957432040204216179475
- BabaraoRJiangJUnraveling the energetics and dynamics of ibuprofen in mesoporous metal-organic frameworksJ Phys Chem C Nanomater Interfaces2009113421828718291
- SurbléSSerreCMellot-DraznieksCMillangeFFéreyGA new isoreticular class of metal-organic-frameworks with the MIL-88 topologyChem Commun (Camb)2006328428616391735
- WhitfieldTRWangXQLiuLMJacobsonAJMetal-organic frameworks based on iron oxide octahedral chains connected by ben-zenedicarboxylate dianionsSolid State Sci20057910961103
- HorcajadaPSurbléSSerreCSynthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large poresChem Commun (Camb)2007272820282217609787
- BauerSSerreCDevicTHigh-throughput assisted rationalization of the formation of metal organic frameworks in the iron(III) aminoterephthalate solvothermal systemInorg Chem200847177568757618681423
- SerreCMillangeFSurbléSFéreyGA route to the synthesis of trivalent transition-metal porous carboxylates with trimeric secondary building unitsAngew Chem Int Ed Engl200443466285628915372643
- HorcajadaPChalatiTSerreCPorous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imagingNat Mater20109217217820010827
- MolinoNMWangSWCaged protein nanoparticles for drug deliveryCurr Opin Biotechnol201428758224832078
- RenDKratzFWangSWEngineered drug-protein nanoparticle complexes for folate receptor targetingBiochem Eng J201489334125018664
- RenDKratzFWangSWProtein nanocapsules containing doxorubicin as a pH-responsive delivery systemSmall2011781051106021456086
- RenDMDalmauMRandallAShindelMMBaldiPWangSWBiomimetic design of protein nanomaterials for hydrophobic molecular transportAdv Funct Mater201222153170318023526705
- ChoiKKimKKwonICKimISAhnHJSystemic delivery of siRNA by chimeric capsid protein: tumor targeting and RNAi activity in vivoMol Pharm2013101182522663765
- OrlowskiSGarrigosMMultiple recognition of various amphiphilic molecules by the multidrug resistance P-glycoprotein: molecular mechanisms and pharmacological consequences coming from functional interactions between various drugsAnticancer Res1999194B3109312310652600
- FangRHZhangLNanoparticle-based modulation of the immune systemAnnu Rev Chem Biomol Eng20167130532627146556
- Duro-CastanoAMovellanJVicentMJSmart branched polymer drug conjugates as nano-sized drug delivery systemsBiomater Sci20153101321133426266272
- WeiHZhuoRXZhangXZDesign and development of polymeric micelles with cleavable links for intracellular drug deliveryProg Polym Sci2013383503535
- RussoADegraffWFriedmanNMitchellJBSelective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugsCancer Res1986466284528482421885
- ShenYQJinELZhangBProdrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug deliveryJ Am Chem Soc2010132124259426520218672
- ZhangHFWangJQMaoWWNovel SN38 conjugate-forming nanoparticles as anticancer prodrug: in vitro and in vivo studiesJ Control Release2013166214715823266448
- GouPFLiuWWMaoWWTangJBShenYQSuiMHSelf-assembling doxorubicin prodrug forming nanoparticles for cancer chemotherapy: synthesis and anticancer study in vitro and in vivoJ Mater Chem B Mater Biol Med201313284292
- Brannon-PeppasLBlanchetteJONanoparticle and targeted systems for cancer therapyAdv Drug Deliv Rev200456111649165915350294
- GuoXShiCLWangJDiSZhouSBpH-triggered intracellular release from actively targeting polymer micellesBiomaterials201334184544455423510854
- ZhouYFYanDYDongWYTianYTemperature-responsive phase transition of polymer vesicles: real-time morphology observation and molecular mechanismJ Phys Chem B200711161262127017243669
- ZhouYFYanDYSupramolecular self-assembly of giant polymer vesicles with controlled sizesAngew Chem Int Ed Engl200443374896489915372639
- ZhouYFYanDYSupramolecular self-assembly of amphiphilic hyperbranched polymers at all scales and dimensions: progress characteristics and perspectivesChem Commun (Camb)2009101172118819240868
- ZhouYFHuangWLiuJYZhuXYYanDYSelf-assembly of hyperbranched polymers and its biomedical applicationsAdv Mater201022414567459020853374
- ZhuWLingJShenZSynthesis and characterization of amphiphilic star-shaped polymers with calix[6]arene coresMacromol Chem Phys20062079844849
- WangFBronichTKKabanovAVRauhRDRooversJSynthesis and characterization of star poly(ε-caprolactone)-b-poly(ethylene glycol) and poly(l-lactide)-b-poly(ethylene glycol) copolymers: evaluation as drug delivery carriersBioconjug Chem20081971423142918564869
- HadjichristidisNRooversJLinear viscoelastic properties of mixtures of 3- and 4-arm polybutadiene starsPolymer198526710871090
- PitsikalisMPispasSMaysJWHadjichristidisNNonlinear block copolymer architecturesAdv Polym Sci199813511137
- SchultzJLWilksESDendritic and star polymers: classification, nomenclature, structure representation, and registration in the DuPont Scion databaseJ Chem Inf Comput Sci19983828599
- KowalczukAStoyanovaEMitovaVStar-shaped nano-conjugates of cisplatin with high drug payloadInt J Pharm2011404122023021078377
- EtrychTKovářLStrohalmJChytilPŘíhováBUlbrichKBiodegradable star HPMA polymer-drug conjugates: biodegradability, distribution and anti-tumor efficacyJ Control Release2011154324124821699933
- EtrychTStrohalmJChytilPBiodegradable star HPMA polymer conjugates of doxorubicin for passive tumor targetingEur J Pharm Sci201142552753921392579
- LidickýOJanouškováOStrohalmJAlamMKlenerPEtrychTAnti-lymphoma efficacy comparison of anti-CD20 monoclonal antibody-targeted and non-targeted star-shaped polymer-prodrug conjugatesMolecules20152011198491986426556320
- YangCShaoQLVenkataramanSStructure-directing star-shaped block copolymers: supramolecular vesicles for the delivery of anticancer drugsJ Control Release20152089310525813888
- LiangQHongCYPanCYDoxorubicin-loaded aromatic imine-contained amphiphilic branched star polymer micelles: synthesis, self-assembly, and drug deliveryInt J Nanomedicine2015103623364026056444
- SideratouZTsiourvasDTheodossiouTFardisMPaleosCMSynthesis and characterization of multifunctional hyperbranched polyesters as prospective contrast agents for targeted MRIBioorg Med Chem Lett201020144177418120621729
- GaoCXuYMYanDYChenWWater-soluble degradable hyperbranched polyesters: novel candidates for drug deliveryBiomacromolecules20034370471212741788
- PrabaharanMGrailerJJPillaSSteeberDAGongSQFolate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn H40, poly(l-lactide) and poly(ethylene glycol) for tumor-targeted drug deliveryBiomaterials200930163009301919250665
- PrabaharanMGrailerJJPillaSSteeberDAAmphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug deliveryBiomaterials200930295757576619643472
- LutzPJPeruchFGraft copolymers and comb-shaped homopolymersMoellerMMatyjaszewskiKPolymer Science: A Comprehensive ReferenceAmsterdamElsevier2012511542
- XuZGZhangKLHouCLA novel nanoassembled doxorubicin prodrug with a high drug loading for anticancer drug deliveryJ Mater Chem B Mater Biol Med20142234333437
- ZouJYuYLiYWell-defined diblock brush polymer-drug conjugates for sustained delivery of paclitaxelBiomater Sci201537867874
- TaiWYMoRLuYJiangTYGuZFolding graft copolymer with pendant drug segments for co-delivery of anticancer drugsBiomaterials201435257194720324875756
- OhMMirkinCAChemically tailorable colloidal particles from infinite coordination polymersNature2005438706865165416319888
- HoskinsBFRobsonRInfinite polymeric frameworks consisting of three dimensionally linked rod-like segmentsJ Am Chem Soc19891111559625964
- TachibanaMIwaizumiMEPR and UV-visible spectroscopic studies of copper(II) and cobalt(II) complexes of hydroxyanthraquinonesJ Inorg Biochem1987302141151
- ZhouGPanJPolarographic and voltammetric behaviour of ofloxacin and its analytical applicationAnal Chim Acta199530714953
- XingLCaoYYCheSASynthesis of core-shell coordination polymer nanoparticles (CPNs) for pH-responsive controlled drug releaseChem Commun (Camb)201248485995599722576702
- XingLZhengHQCheSNA pH-responsive cleavage route based on a metal-organic coordination bondChemistry201117267271727521567490
- MoustaouiHMoviaDDupontNTunable design of gold (III)-doxorubicin complex – PEGylated nanocarrier: the golden doxorubicin for oncological applicationsACS Appl Mater Interfaces2016831199461995727424920
- YangPWangHFFeiGYangBSAntitumor activity of the Cu(II)-mitoxantrone complex and its interaction with deoxyribonucleic acidJ Inorg Biochem19966221371458729800
- RamaswamyHNO’ConnorRTMetal complexes of gossypolJ Agric Food Chem2002176752762
- ChampionJAKatareYKMitragotriSParticle shape: a new design parameter for micro- and nanoscale drug delivery carriersJ Control Release20071211–23917544538
- ZhangDRTanTWGaoLZhaoWFWangPPreparation of azithromycin nanosuspensions by high pressure homogenization and its physicochemical characteristics studiesDrug Dev Ind Pharm200733556957517520449
- Hernández-TrejoNKayserOSteckelHMüllerRHCharacterization of nebulized buparvaquone nanosuspensions-effect of nebulization technologyJ Drug Target2005138–949950716332575
- KassemMARahmanAAGhorabMMAhmedMBKhalilRMNanosuspension as an ophthalmic delivery system for certain gluco-corticoid drugsInt J Pharm20073401–212613317600645
- MoutonJWvan PeerAde BeuleKVan VlietADonnellyJPSoonsPAPharmacokinetics of itraconazole and hydroxyitraconazole in healthy subjects after single and multiple doses of a novel formulationAntimicrob Agents Chemother200650124096410216982783
- CrispMTTuckerCJRogersTLWilliamsROJohnstonKPTurbidimetric measurement and prediction of dissolution rates of poorly soluble drug nanocrystalsJ Control Release2007117335135917239469
- KumarMPRaoYMApteSImproved bioavailability of albendazole following oral administration of nanosuspension in ratsCurr Nanosci200732191194
- KumarMPRaoYMApteSFormulation of nanosuspensions of albendazole for oral administrationCurr Nanosci2008415358
- GrauMJKayserOMüllerRHNanosuspensions of poorly soluble drugs: reproducibility of small scale productionInt J Pharm2000196215515910699708
- LeeJChengYCritical freezing rate in freeze drying nanocrystal dispersionsJ Control Release2006111218519216430987
- HecqJDeleersMFanaraDPreparation and in vitro/in vivo evaluation of nano-sized crystals for dissolution rate enhancement of UCB-35440-3, a highly dosed poorly water-soluble weak baseEur J Pharm Biopharm200664336036816846725
- LeeJDrug nano- and microparticles processed into solid dosage forms: physical propertiesJ Pharm Sci200392102057206814502544
- BadruddozaAZGodfrinPDMyersonASTroutBLDoylePSCore-shell composite hydrogels for controlled nanocrystal formation and release of hydrophobic active pharmaceutical ingredientsAdv Healthc Mater20165151960196827249402
- Merisko-LiversidgeELiversidgeGGCooperERNanosizing: a formulation approach for poorly-water-soluble compoundsEur J Pharm Sci200318211312012594003
- KeckCMMüllerRHDrug nanocrystals of poorly soluble drugs produced by high pressure homogenisationEur J Pharm Biopharm200662131616129588
- ChandumpaiASinghpibulpornNFaroongsarngDSornprasitPPreparation and physico-chemical characterization of chitin and chitosan from the pens of the squid species, Loligo lessoniana and Loligo formosanaCarbohydr Polym2004584467474
- SalazarJMüllerRHMöschwitzerJPCombinative particle size reduction technologies for the production of drug nanocrystalsJ Pharm (Cairo)2014201426575426556191
- Van EerdenbrughBVan den MooterGAugustijnsPTop-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid productsInt J Pharm20083641647518721869
- MöschwitzerJPDrug nanocrystals in the commercial pharmaceutical development processInt J Pharm2013453114215623000841
- SutradharKBKhatunSLunaIPIncreasing possibilities of nano-suspensionJ Nanotechnol201320131112
- SinhaBMüllerRHMöschwitzerJPBottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle sizeInt J Pharm2013453112614123333709
- ZhangJLiangYCLinXSelf-monitoring and self-delivery of photosensitizer-doped nanoparticles for highly effective combination cancer therapy in vitro and in vivoACS Nano20159109741975626390118
- ChanHKKwokPCProduction methods for nanodrug particles using the bottom-up approachAdv Drug Deliv Rev201163640641621457742
- ChenJFZhangJYShenZGZhongJYunJPreparation and characterization of amorphous cefuroxime axetil drug nanoparticles with novel technology: high-gravity antisolvent precipitationInd Eng Chem Res2006452587238727
- SarkariMBrownJChenXSwinneaSWilliamsRO3rdJohnstonKPEnhanced drug dissolution using evaporative precipitation into aqueous solutionInt J Pharm20022431–2173112176292
- MontesAGordilloMDPereyraCde la OssaEJParticles formation using supercritical fluidsNakajimaHMass Transfer: Advanced AspectsRijekaIntech2011461480
- GirotraPSinghSKNagpalKSupercritical fluid technology: a promising approach in pharmaceutical researchPharm Dev Technol2013181223823036159
- GaoLZhangDRChenMHDrug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery systemJ Nanopart Res2008105845862
- ChangTLZhanHLiangDLiangJFNanocrystal technology for drug formulation and deliveryFront Chem Sci Eng201591114
- LiWZhangXJHaoXJJieJSTianBSZhangXHShape design of high drug payload nanoparticles for more effective cancer therapyChem Commun (Camb)20134993109891099124136236
- DobrovolskaiaMAAggarwalPHallJBMcneilSEPreclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistributionMol Pharm20085448749518510338
- LiWYangYLWangCCarrier-free, functionalized drug nano-particles for targeted drug deliveryChem Commun (Camb)201248658120812222766919
- ZhouMZhangXYangYCarrier-free functionalized multi-drug nanorods for synergistic cancer therapyBiomaterials201334358960896723958027
- HuangPWangDLSuYCombination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapyJ Am Chem Soc201413633117481175625078892
- HuangPHuMZhouLSelf-delivery nanoparticles from an amphiphilic covalent drug couple of irinotecan and bendamustine for cancer combination chemotherapyRSC Adv201551058625486264
- ZhangTHuangPShiLLSelf-assembled nanoparticles of amphiphilic twin drug from floxuridine and bendamustine for cancer therapyMol Pharm20151272328233625996874
- HuMXHuangPWangYSynergistic combination chemotherapy of camptothecin and floxuridine through self-assembly of amphiphilic drug-drug conjugateBioconjug Chem201526122497250626497258
- ImazIRubio-MartínezMAnJHSolé-FontIRosiNLMaspochDMetal-biomolecule frameworks (MBioFs)Chem Commun (Camb)201147267287730221503346
- McKinlayACMorrisREHorcajadaPBioMOFs: metal-organic frameworks for biological and medical applicationsAngew Chem Int Ed Engl201049366260626620652915
- MillerSRHeurtauxDBaatiTHorcajadaPGrenècheJMSerreCBiodegradable therapeutic MOFs for the delivery of bioactive moleculesChem Commun (Camb)201046254526452820467672
- AnJHGeibSJRosiNLCation-triggered drug release from a porous zinc-adeninate metal-organic frameworkJ Am Chem Soc2009131248376837719489551
- LinWBRieterWJTaylorKMModular synthesis of functional nanoscale coordination polymersAngew Chem Int Ed Engl200948465065819065692
- DellaRJLiuDLinWBNanoscale metal-organic frameworks for biomedical imaging and drug deliveryAcc Chem Res2011441095796821648429
- Taylor-PashowKMDellaRJHuxfordRCLinWBHybrid nanomaterials for biomedical applicationsChem Commun (Camb)201046325832584920623072
- RieterWJPottKMTaylorKMLinWBNanoscale coordination polymers for platinum-based anticancer drug deliveryJ Am Chem Soc200813035115841158518686947
- HuxfordRCDekrafftKEBoyleWSLiuDLinWBLipid-coated nanoscale coordination polymers for targeted delivery of antifolates to cancer cellsChem Sci201231198204
- LiKLiuHGaoWMulberry-like dual-drug complicated nano-carriers assembled with apogossypolone amphiphilic starch micelles and doxorubicin hyaluronic acid nanoparticles for tumor combination and targeted therapyBiomaterials20153913114425477180
- DengZJMortonSWBen-AkivaEDreadenECShopsowitzKEHammondPTLayer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatmentACS Nano20137119571958424144228
- RössleinMLiptrottNJOwenABoisseauPWickPHerrmannIKSound understanding of environmental, health and safety, clinical, and market aspects is imperative to clinical translation of nanomedicinesNanotoxicology201711214714928055261
- BatrakovaEVKabanovAVPluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiersJ Control Release200813029810618534704
- ElkinsonSKeatingGMLixisenatide: first global approvalDrugs201373438339123558600
- KornblumNAyyanarKBenimetskayaLRichardsonPIacobelliMSteinCADefibrotide, a polydisperse mixture of single-stranded phosphodiester oligonucleotides with lifesaving activity in severe hepatic veno-occlusive disease: clinical outcomes and potential mechanisms of actionOligonucleotides200616110511416584299
- SyedYYRucaparib: first global approvalDrugs201777558559228247266
