Abstract
Cancer is a leading cause of death globally, and it is predicted and projected to continue rising as life expectancy increases. Although patient survival rates for some forms of cancers are high due to clinical advances in treatment protocols, the search for effective cancer vaccines remains the ultimate Rosetta Stone in oncology. Cervarix®, Gardasil®, and hepatitis B vaccines are currently employed in preventing certain forms of viral cancers. However, they are, strictly speaking, not ‘true’ cancer vaccines as they are prophylactic rather than therapeutic, are only effective against the oncogenic viruses, and do not kill the actual cancer cells. On April 2010, a new prostate cancer vaccine Provenge® (sipuleucel-T) was approved by the US FDA, and it is the first approved therapeutic vaccine that utilizes antigen-presenting cell technology involving dendritic cells in cancer immunotherapy. Recent evidence suggests that the use of nanoscale particles like exosomes in immunotherapy could form a viable basis for the development of novel cancer vaccines, via antigen-presenting cell technology, to prime the immune system to recognize and kill cancer cells. Coupled with nanotechnology, engineered exosomes are emerging as new and novel avenues for cancer vaccine development. Here, we review the current knowledge pertaining to exosome technology in immunotherapy and also seek to address the challenges and future directions associated with it, in hopes of bringing this exciting application a step closer toward an effective clinical reality.
Introduction
Cancer is a major global cause of morbidity and mortality, and it is expected to rise in the coming decades.Citation1 Conventional treatments for cancer include the use of chemotherapeutic drugs, radiotherapy, and interventional surgery in the case of solid (and operable) tumors. Recent evidence suggests that the application of dendritic cell-derived exosomes (DEX), tumor cell-derived exosomes (TEX), and ascitic cell- derived exosomes (AEX) could emerge as novel nanoscale immunotherapy treatments for cancer, by priming the body’s immune system to recognize and kill cancer cells ().
Table 1 Overview of the application of exosomes as a nanoscale cancer vaccine
Exosomes are nanoscale (50–100 nm) membrane vesicles, first described by Rose Johnstone in the 1970s, and it is postulated that they are involved in cell–cell communication, although its exact biological function and mechanism has, to this day, yet to be fully elucidated.Citation2 The canonical exosome is of intracellular origin and displays MHC class I and II.Citation3 It has also been shown to contain heat shock proteins and has a lipid composition rich in cholesterol, sphingomyelin, and ceramide.Citation4 Its main protein markers are tetraspanins (CD63, CD9), Alix, and TSG101 and is able to mediate a biological immune response by activating T lymphocytes (via antigen presentation), natural killer (NK) cells (via NKG2D ligand binding), and dendritic cells (via antigen transfer).Citation5 The understanding of exosome biology has increased exponentially in recent years, leading to the creation of online databases such as Exocarta,Citation6 an encyclopedia dedicated to providing original research findings about exosomes and their associated proteins.Citation7
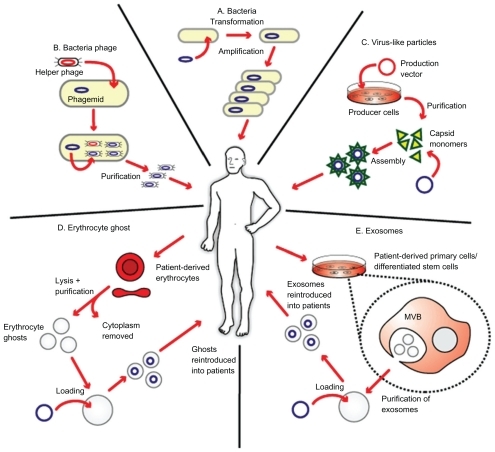
The advent of nanotechnology has generated an immense interest among researchers in its application to medicine. A major goal of the utilization of nanoscale drug delivery systems is to improve its therapeutic index by increasing its potency at specific sites while simultaneously reducing systemic toxicity.Citation8 Nanoscale drug delivery systems approved by the US Food and Drug Administration in cancer treatments are currently available, for example, Doxil® (doxorubicin encapsulated in liposomes)Citation9 and Abraxane® (paclitaxel attached to nanoparticles).Citation10 In addition to being novel drug delivery systems, nanoscale particles can also be specifically engineered to stimulate the immune system, which could form an attractive basis for cancer vaccine development.Citation11 There is increasing evidence that nanoscale particles, for example, recombinant virus-like particles (VLPs), inert nanobeads, and immunostimulating complexes, are being utilized in cancer vaccine development due to their effectiveness at eliciting cellular and humoral immune responses.Citation12 It has been demonstrated that synthetic nanoparticles could be conjugated to biological molecules (eg, exosomes) and could potentially be used as an effective vaccine delivery system.Citation13 Furthermore, there is a growing interest in the utilization of biological molecule delivery systems that serve as conduits to deliver genetic material, drug, or antigen into the body, and attractive candidates are liposomes, VLPs, erythrocyte ghosts, and exosomes ().Citation14
Figure 1 Biological delivery systems. A) Bacteria can be used for gene delivery, and it is used in cancer gene therapy, DNA vaccination, and treatment of some genetic diseases. B) Bacteriophages are viruses that infect bacteria, and they can be genetically engineered and introduced into bacteria for genetic replication. C) Virus-like particles (VLPs) can be engineered from plasmids coding for viral structure proteins. These VLPs can then be linked to antigens and introduced into the body to elicit an immune response. D) Erythrocyte ghosts are red blood cells that have their cytoplasmic contents removed, and they can be used as vehicles for drug delivery. E) Exosomes are nanoscale vesicles released from dendritic cells and tumor cells, and they can be purified and loaded with antigens and introduced into the body to elicit a cell-specific antitumor response. Copyright © 2009, Nature Publishing Group. Reproduced with permission from Seow Y, Wood MJ. Biological gene delivery vehicles: beyond viral vectors. Mol Ther. 2009;17(5):767–777.
Thus in this review, we seek to discuss the evidence pertaining to the use of exosomes as a cell-free (hence, conferring a low immunogenicity and high biosafety profile) nanoscale cancer vaccine and also to explore the potential of combining it with nanotechnology in the hopes of bringing the concept of immunotherapeutic cancer vaccines toward a viable and feasible clinical reality.
The search for a cancer vaccine
Significant clinical advances have been made in the treatment of cancer using the immune system. One of the best examples of this is the use of donor lymphocyte infusions, which is useful in treating chronic myeloid leukemia.Citation15 Bone marrow transplantation has also revolutionized the way hematological malignancies are treated.Citation16 However, the search for a true cancer vaccine remains largely elusive. Using cervical cancer as a prime example, current (prophylactic) treatment involves vaccination with either Cervarix® or Gardasil®, which are developed from and based on VLP technology, and is useful against cancer-causing variants of human papillomavirus (HPV).Citation17 A modified strategy using long synthetic HPV oncopeptides has demonstrated that both CD8+ and CD4+ T-cell responses can be mounted in vivo and are capable of inducing complete remission in some patients with vulvar cancer.Citation18 These results are very encouraging as, for the first time, an antiviral/tumor vaccine has successfully eradicated established tumors. However, these approaches are not ‘true’ cancer vaccines as an antiviral response is what kills the tumor. In the relentless pursuit of a true cancer vaccine, interesting approaches have been adopted by targeting ‘self ’ proteins on cells that are only expressed at particular sites at specific time frames.Citation19 Although these approaches have shown very promising results in murine models, their applicability to humans remain to be seen. In addition, the hepatitis B vaccine protects against hepatitis B virus, which is associated with liver cancer.Citation20 Until recently, these so-called ‘cancer vaccines’ protect against viruses and are prophylactic (must be administered before the viral infection) rather than therapeutic because they do not kill the actual cancer cells.
On April 2010, the Food and Drug Administration approved the use of Provenge® (sipuleucel-T) in the treatment of advanced prostate cancer – the first ‘true’ therapeutic cancer vaccine that stimulates the body to kill cancer cells by eliciting a biological immune response.Citation21 This involves pulsing dendritic cells with prostatic acid phosphatase-containing fusion protein in order to prime the immune system to recognize and kill prostate cancer cells.Citation22 In terms of clinical trials in cancer vaccine development in the European Union and United States,Citation23 this technique of pulsing dendritic cells with cancer antigen bears stark similarity with the clinical trials involving exosomes reviewed here, and therefore gives credence to the proof-of-concept that the application of exosome could be used as a nanoscale cancer vaccine.
Mounting evidence demonstrates that loading/pulsing dendritic cells with tumor antigens (or TEX) results in higher response rates in cancer patients in terms of eliciting an antitumor-specific immunity, compared to other types of vaccine formulations (eg, peptide vaccines and viral vector vaccines).Citation24 This could well form a cornerstone by which cancer vaccines could be effectively developed in the future.
Cancer immunotherapy
For a cell to become cancerous, it must typically gain an oncogenic function (such as among many others, Myc, Bcl-2, and Ras, which promote cell proliferation), lose its tumor suppressor function (such as p53 and Rb, which promote cell arrest), and be immortalized.Citation25 Within the tumor microenvironment, there is extensive interaction between cancer cells and immune cells, and cancer cells may escape immune surveillance by aberrant expression of antigen presentation (eg, lack of MHC I), absence of costimulatory molecules (eg, lack of B7 molecules), and release of immunosuppressive factors (eg, IL-10 and TFGβ).Citation26 However, the ubiquitous question of why and how the immune system tolerates cancer cells in the first place remains poorly understood and has generated a relentless pursuit toward a search for an immunotherapeutic cancer vaccine.
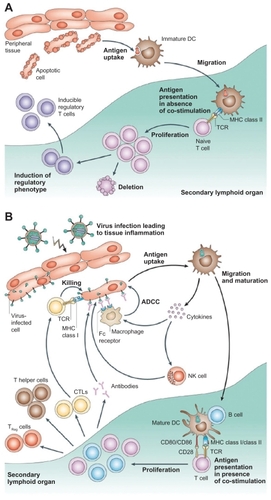
Dendritic cells (or, more accurately, interdigitating dendritic cells) are professional antigen-presenting cells (APCs), which would be activated upon encountering a foreign agent and would interact with T cells to elicit an antigen-specific immune response.Citation27 When immature dendritic cells are activated upon stimulation by foreign antigens, they become mature dendritic cells, which are powerful APCs, priming naïve lymphocytes as well as releasing chemokines to attract T cells ().Citation24 In contrast to using monoclonal antibodies in cancer immunotherapy, which is notorious for its systemic side effects like immunosuppression and hypersensitivity reactions due to by stander effects,Citation28 dendritic cell-based immunotherapy could function as an attractive alternative for cancer treatment as the immune response mounted is more specific.Citation29 Recent evidence shows that artificial APC systems are also emerging as powerful techniques for immunotherapy, and the superior biosafety profile of exosomes has made them an attractive candidate for cancer vaccine development.Citation30
Figure 2 Different functions of immature and mature dendritic cells. A) In the absence of inflammation and costimulation, antigen presentation to immature dendritic cells induces tolerance or anergy (lack of an immune response). This results in deletion or induction of a regulatory phenotype of T cells. B) In the presence of inflammation, immature dendritic cells become activated into mature dendritic cells. Antigen presentation in the presence of costimulatory molecules causes clonal expansion of CD4+ (helper) and CD8+ (cytotoxic) T cells and activation of B cells to produce antibodies and NK cells. Copyright © 2005, Nature Publishing Group. Reproduced with permission from Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296–306.
A nanoscale and cell-free cancer vaccine
An ideal cancer vaccine should be able to prime the immune system to recognize specific tumor antigen and then mount an appropriate immune response toward cancer cells without causing ‘by stander effect’ damage to neighboring cells.Citation31 Evidence for such a vaccine remains few and far between, and clinical trials involving cancer vaccines usually yield less-than-satisfactory results, for example, MyVax® and FavId®, which were hailed as ‘personalized’ vaccines for treating non-Hodgkin’s lymphoma, failed to demonstrate any significant therapeutic response.Citation32 Although Provenge® represents a milestone in cancer vaccine development, the modest increase in survival time (4.1 months) and the exorbitant cost per patient (about US$93,000) for three doses of the vaccine demonstrate a need to find novel yet economical ways of cancer vaccine treatment.Citation33
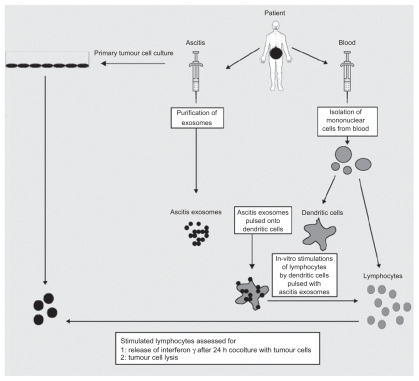
Exosomes interact extensively with cells of the immune system, and it has been shown that they activate dendritic cells, thereby priming the immune system to recognize and kill cancer cells ().Citation5 Exosomes are typically derived and purified using a series of centrifugation steps, not exceeding 100,000 g on a 30% D2O sucrose cushion.Citation34 AEX taken from peritoneal cavity fluid in cancer patients have been shown to cause tumor cell lysis by inducing dendritic cells to prime T lymphocytes via an MHC I-dependent pathway to kill cancer cells and also triggers the release of IFN-γ by peripheral blood lymphocytes in in vitro experimental models ().Citation35
Figure 3 Interactions of exosomes and other nanoscale vesicles with cells of the immune system. A) A variety of cells secrete exosomes, and it can activate different types of immune cells, mainly via antigen presentation. B) Exosomes can also have an inhibitory effect on the immune system, although the mechanisms are not well understood. Copyright © 2009, Nature Publishing Group. Reproduced with permission from Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593.
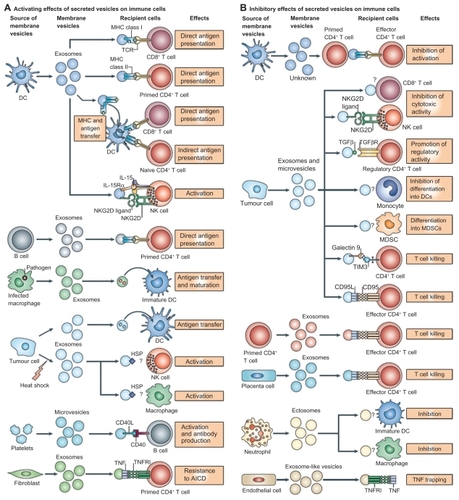
Figure 4 The therapeutic application of AEX. Dendritic cells from peripheral blood monocytes are pulsed with AEX, and cell-specific anti tumor response has been observed. Copyright © 2002, Elsevier. Reproduced with permission from Andre F, Schartz NE, Movassagh M, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305.
Similarly, TEX are exosomes purified from cancerous cells. TEX contains tumor antigens, and they have been shown to stimulate cells of the immune system and reduce tumor growth.Citation36 There is also evidence pointing toward TEX as tumor and RNA transporters, which could serve as a useful biomarker and diagnostic tool ().Citation37 It has been shown that TEX expressing heat shock protein-70 (Hsp70) upregulates T helper cell 1 (Th1)-mediated tumor response.Citation38 Furthermore, it has also been reported that membrane-bound Hsp70 TEX are more efficacious than cytoplasmic Hsp70 TEX.Citation39 Evidence indicates that more potent immune responses are mounted toward vesicle-bound antigen compared to soluble antigen, and the mode of secretion can determine immunogenicity. Citation40 Interestingly, induction of apoptosis in cancer cells via notch signalingCitation41 and PI3K/Akt/GSK-3β survival pathwaysCitation42 is observed when TEX interacts with pancreatic cancer cells. Apart from stimulating the adaptive immune system by cross-priming cytotoxic T cells,Citation43 it has also been demonstrated that TEX can activate the innate immune system by increasing IgG antibody responseCitation44 and NK cells.Citation45
Figure 5 Exosomes are observed on the surface of glioblastoma cells. Tumor-specific peptides or antigens from TEX can be purified and pulsed onto dendritic cells for immunotherapy. Copyright © 2008, Nature Publishing Group. Reproduced with permission from Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476.
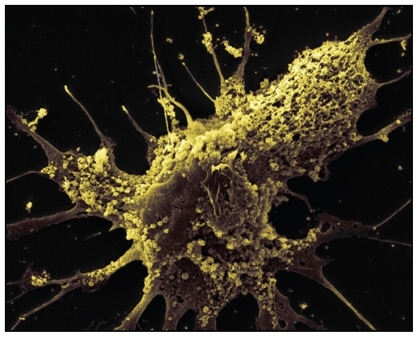
When dendritic cells are pulsed with cancer antigens or tumor peptides,Citation46 DEX has been shown to elicit stronger immune responses toward cancer cells,Citation47 with upregulation of specific antibody release and cytokine production.Citation48 It has also been shown that DEX suppresses tumor growthCitation49 and eradication of established tumorsCitation50 by CD8+ T cellsCitation51 and CD4+ T cells,Citation52 as well as breaking tolerance completely by resistance to secondary tumor challenge.Citation53 Furthermore, it has also been reported that NK cells are involved in the immune response toward cancer cells via NKG2D-dependent NK cell activation and IL-15Rα-dependent cell proliferation.Citation54 In terms of the mode of delivery, it has been shown that intradermal injection of DEX is more efficacious than subcutaneous, leading to suggestions that there might be more dendritic cells in the intradermal area than subcutaneous area.Citation55
To date, three Phase I clinical trials (China, France, and the United States) have been conducted, involving the application of exosomes to elicit immune responses against established tumors (). Dai et alCitation56 reported that using AEX in patients with colorectal cancer induces tumor antigen-specific cytotoxic T-cell responses, with none to minimal side effects, and is well tolerated by patients. Escudier et alCitation57 demonstrated the feasibility of scaling up and purification of clinical grade DEX using good manufacturing practice and restored the number and NKG2D-dependent function of NK cells in patients with melanoma ().Citation54 Furthermore, a study by Morse et alCitation58 indicates a MAGE-specific T cell response and increased NK lytic activity in patients with non-small cell lung carcinoma with the use of DEX.
Figure 6 Clinical grade exosomes in immunotherapy. The process of how DEX can be derived, purified, and utilized in cancer treatment. Creative Commons. Reproduced with permission from Escudier B, Dorval T, Chaput N, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3(1):10.
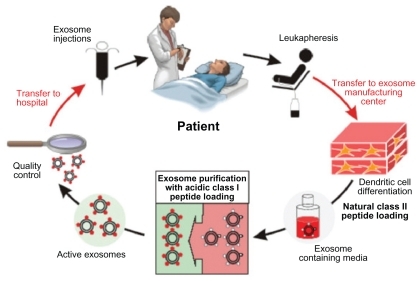
Table 2 The application of exosomes as a cancer vaccine in Phase I clinical trials
Challenges and future directions
Although it has been established from the results in clinical trials that exosomes can be safely administered, their potency in terms of eliciting appropriate immune responses to kill cancer cells leaves much to be desired. The small number of patients involved in the clinical trials calls into question the statistical significance of the results. Most of the experimental evidence reviewed here revolves around solid tumors, and it has not been sufficiently demonstrated that non solid tumors (eg, hematological malignancies) can be treated using exosome technology. Furthermore, taking into account that exosomal immunotherapy relies heavily on the immune system, there remains a need to address the issue of cancer patients who are immunocompromised and/or immunosuppressed due to chemotherapy and radiotherapy regimens and therefore might not be able to sufficiently surmount cancer cells with their immune system alone.
Understandably, the next major goal is to increase the biological magnitude of the immune response, and this could be achieved perhaps by artificially coating and engineering exosomesCitation59 with tumor antigens to make it more recognizable to the immune system. It has also been shown that heat-shocked TEX are more efficacious than TEX alone,Citation60 as it confers a greater immunogenicity and elicits a greater immune response,Citation61 thereby potentially making it a more effective cancer vaccine. It has also been shown that when CpG adjuvants are added alongside exosome therapy, naïve T cells are more effectively primed.Citation62
The application and potential of nanotechnology in medicine heralds a beginning of a new chapter in the treatment of human diseases. Carbon nanotubes (CNTs) are allotropes of carbon, and research reveals that it not only has the potential for imaging and treating cancer cells, it can also be utilized in cancer vaccine delivery systems ().Citation63 There is evidence to suggest that CNTs, when conjugated to tumor antigens, elicits a specific antitumor response in animal models. Citation64 Furthermore, it has been shown that peptide- functionalized CNTs can function as efficient vaccine delivery systems which trigger specific antibody responses.Citation65 In addition, it has been experimentally demonstrated that ex vivo clonal expansion of T cells with antibody-linked CNTs results in T-cell activation and might prove to be a novel immunotherapy technique.Citation66 There are currently no studies that explore the concept of combining CNTs with exosomes, and this could perhaps function as a novel technique for cancer vaccine development. Quantum dots (QDs) are also emerging as novel fluorescent probes for imaging and tracking the behavior of cells and nanoscale particles.Citation67 QDs are semiconductor nanocrystals of about 2–10 nm capable of fluorescing at a greater intensity and are more photostable than conventional chromophores.Citation68 Considering that exosomes are nanoscale spherical objects, it might also be possible to encapsulate or tether QDs with exosomes in order to image and track their progress in killing cancer cells.Citation69 Theoretically, it might also be possible to engineer QDs to fluoresce at different colors to indicate different biological events, and this could be useful in monitoring the interactions of exosomes with T cells, NK cells, and dendritic cells in order to better understand its antitumor mechanism.Citation70 Indeed, it has been experimentally demonstrated that using nanotechnology, exosomes can be engineered to bear an optimal number of MHC I and ligands that would activate T cells, and its in vivo activity can be traced by encapsulating superparamagnetic iron oxide nanoparticles ().Citation71 Exosomes can also carry cytokines, DNA, RNA, adjuvants, labels, costimulatory signals, and gene therapy vectors, which makes it the ultimate Trojan horse. Therefore, engineering the production of exosomes using nanotechnology embodies what we believe is the new platform for cancer vaccines of the future.
Figure 7 Carbon nanotubes in cancer immunotherapy. A) Pristine CNT is the default setting of CNT, which lacks any bioconjugation or biofunctionalization. They have a strong tendency to aggregate and are mostly used in studies to assess nanotoxicity. B) PEG-conjugated CNT can be used in systemic cancer imaging. C) Copolymer or surfactant-coated CNT with PEO or PPO, and it can be used in localized cancer imaging. D) ssDNA-coated CNT, which aids dispersion and separation. E) Chemically functionalized (covalent surface modification) CNT by 1,3 dipolar cycloaddition. F) Chemically functionalized (covalent surface modification) CNT by acid oxidation. Copyright © 2009, Nature Publishing Group. Reproduced with permission from Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol. 2009;4(10):627–633.
Abbreviations: CNT, carbon nanotube; PEG, lipid-polyethylene glycol; PEO, polyethylene oxide; PPO, polypropylene oxide; ssDNA, single-stranded DNA.
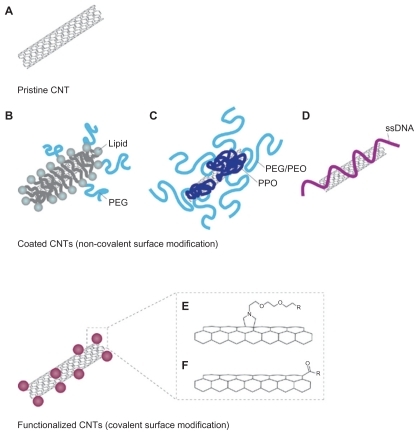
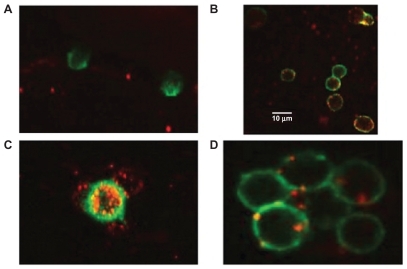
Figure 8 Interaction of engineered exosomes with cells of the immune system. A) Engineered exosomes (red small circles) coated with A2*MHC class I molecules bound to CMV peptides do not bind CD8+ T cell (stained green) from a CMV positive donor who is HLA A2 negative, showing that exosomes do not bind randomly to T cells and that binding is very much MHC dependant. B) However, when the same exosomes carrying A2*MHC/CMV complexes are cocultured with T cell from a CMV+ and A2+ donor, direct interaction between exosomes and CD8+ T cells is clearly seen. C) Engineered exosomes (stained red) also bind to natural APCs (stained green and orange when the red and green color overlaps). This figure shows a natural APC activating three different CD8 T cells via engineered exosomes. D) Exosomes bind to APC naturally whether they are engineered or not as the lipids themselves are the binding sites. Copyright © 2009, Nature Publishing Group. de La Pena H, Madrigal JA, Rusakiewicz S, et al. Artificial exosomes as tools for basic and clinical immunology. J Immunol Methods. 2009;344(2):121–132.
Conclusion
The application of immunotherapy represents a new paradigm in cancer treatment development. The utilization of exosomes in APC systems involving dendritic cells are emerging as powerful techniques to prime the immune system in order to recognize and suppress cancer cell proliferation as well as eradicating established tumors. Despite the modest results of the Phase I clinical trials, the potential for and advancement of the application of exosomes as a nanoscale cancer vaccine are very real. Therefore, larger multicenter clinical trials are needed in order to establish the efficacy of exosomes, and more research has to be conducted to elucidate its mechanism of interaction with the immune system in order to improve its potency. Finally, coupling the use of exosomes with nanotechnology will most likely form the basis where novel nanoscale cancer vaccines will be developed in the future. Taken together, the current literature and the growing body of evidence indicate that exosomes could indeed be utilized as a novel nanoscale and cell-free cancer vaccine, and more research into this area is not only worth pursuing but paramount.
Acknowledgment
We would like to acknowledge the comments from Professor Jill Helms, University of Stanford.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationCancer http://www.who.int/mediacentre/factsheets/fs297/en/index.html. Updated Feb 2009Accessed 2010 Jun 6
- CouzinJCell biology: the ins and outs of exosomesScience200530857301862186315976285
- IeroMValentiRHuberVTumour-released exosomes and their implications in cancer immunityCell Death Differ2008151808817932500
- SimpsonRJJensenSSLimJWProteomic profiling of exosomes: current perspectivesProteomics20088194083409918780348
- TheryCOstrowskiMSeguraEMembrane vesicles as conveyors of immune responsesNat Rev Immunol20099858159319498381
- Exosome protein and RNA databaseExoCarta Available from: http://exocarta.ludwig.edu.au/. Updated 2010 March 29Accessed 2010 Jun 23
- MathivananSSimpsonRJExoCarta: a compendium of exosomal proteins and RNAProteomics20099214997500019810033
- MalamYLoizidouMSeifalianAMLiposomes and nanoparticles: nanosized vehicles for drug delivery in cancerTrends Pharmacol Sci2009301159259919837467
- LeonardRCWilliamsSTulpuleALevineAMOliverosSImproving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet)Breast200918421822419656681
- PetrelliFBorgonovoKBarniSTargeted delivery for breast cancer therapy: the history of nanoparticle-albumin-bound paclitaxelExpert Opin Pharmacother20101181413143220446855
- ZolnikBSGonzalez-FernandezASadriehNDobrovolskaiaMANanoparticles and the immune systemEndocrinology2010151245846520016026
- ScheerlinckJPGreenwoodDLVirus-sized vaccine delivery systemsDrug Discov Today2008131920882887
- RiegerJFreichelsHImbertyAPolyester nanoparticles presenting mannose residues: toward the development of new vaccine delivery systems combining biodegradability and targeting propertiesBiomacromolecules200910365165719203184
- SeowYWoodMJBiological gene delivery vehicles: beyond viral vectorsMol Ther200917576777719277019
- BiernackiMAMarinaOZhangWEfficacious immune therapy in chronic myelogenous leukemia (CML) recognizes antigens that are expressed on CML progenitor cellsCancer Res201070390691520103624
- LeRQBevansMSavaniBNFavorable outcomes in patients surviving 5 or more years after allogeneic hematopoietic stem cell transplantation for hematologic malignanciesBiol Blood Marrow Transplant20101681162117020302959
- GarlandSMSmithJSHuman papillomavirus vaccines: current status and future prospectsDrugs20107091079109820518577
- KenterGGWeltersMJValentijnARVaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasiaN Engl J Med2009361191838184719890126
- JainiRKesarajuPJohnsonJMAltuntasCZJane-WitDTuohyVKAn autoimmune-mediated strategy for prophylactic breast cancer vaccinationNat Med201016779980320512124
- ChenDSToward elimination and eradication of hepatitis BJ Gastroenterol Hepatol2010251192520136972
- US Food and Drug AdministrationFDA approves a cellular immunotherapy for men with advanced prostate cancer Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm210174.htm. Updated 2010 Apr 29Accessed 2010 Jun 6
- HarzstarkALSmallEJImmunotherapy for prostate cancer using antigen-loaded antigen-presenting cells: APC8015 (Provenge)Expert Opin Biol Ther2007781275128017696825
- Rethinking therapeutic cancer vaccinesNat Rev Drug Discov20098968568619721436
- BanchereauJPaluckaAKDendritic cells as therapeutic vaccines against cancerNat Rev Immunol20055429630615803149
- WeinbergRThe Biology of Cancer1st edLondon, UKGarland Science2007
- GrivennikovSIGretenFRKarinMImmunity, inflammation, and cancerCell2010140688389920303878
- DelvesPJMatinSJBurtonDRRoittIMTumour immunologyRoitt’s Essential Immunology11th edOxford, UKBlackwell Publishing2009402
- HanselTTKropshoferHSingerTMitchellJAGeorgeAJThe safety and side effects of monoclonal antibodiesNat Rev Drug Discov20109432533820305665
- FujiiSTakayamaTAsakuraMAkiKFujimotoKShimizuKDendritic cell-based cancer immunotherapiesArch Immunol Ther Exp (Warsz)200957318919819479202
- KimJVLatoucheJBRiviereISadelainMThe ABCs of artificial antigen presentationNat Biotechnol200422440341015060556
- KoosDJosephsSFAlexandrescuDTTumor vaccines in 2010: need for integrationCell Immunol2010263213814720434139
- SasadaTKomatsuNSuekaneSYamadaANoguchiMItohKOvercoming the hurdles of randomised clinical trials of therapeutic cancer vaccinesEur J Cancer20104691514151920413296
- TanneJHFDA approves prostate cancer “vaccine”BMJ2010340c243120442242
- BuNLiQLFengQSunBZImmune protection effect of exosomes against attack of L1210 tumor cellsLeuk Lymphoma200647591391816753878
- AndreFSchartzNEMovassaghMMalignant effusions and immunogenic tumour-derived exosomesLancet2002360932929530512147373
- ChoJAYeoDJSonHYExosomes: a new delivery system for tumor antigens in cancer immunotherapyInt J Cancer2005114461362215609328
- SkogJWurdingerTvan RijnSGlioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkersNat Cell Biol200810121470147619011622
- ChoJALeeYSKimSHKoJKKimCWMHC independent antitumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine modelsCancer Lett2009275225626519036499
- XieYBaiOZhangHMembrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumor immunity than exosomes released from heat-shocked tumor cells expressing cytoplasmic HSP70J Cell Mol Med2009720 [Epub ahead of print]
- ZeelenbergISOstrowskiMKrumeichSTargeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responsesCancer Res20086841228123518281500
- RistorcelliEBeraudEMathieuSLombardoDVerineAEssential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticlesInt J Cancer200912551016102619405120
- RistorcelliEBeraudEVerrandoPHuman tumor nanoparticles induce apoptosis of pancreatic cancer cellsFASEB J20082293358336918511551
- WolfersJLozierARaposoGTumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-primingNat Med20017329730311231627
- TemchuraVVTenbuschMNchindaGEnhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virusVaccine200826293036623672
- YangYXiuFCaiZIncreased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cellsJ Cancer Res Clin Oncol2007133638939917219198
- ZitvogelLRegnaultALozierAEradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomesNat Med1998455946009585234
- AndreFChaputNSchartzNEExosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cellsJ Immunol200417242126213614764678
- BeauvillainCRuizSGuitonRBoutDDimier-PoissonIA vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic miceMicrobes Infect20079141516141622
- GuoFChangCKFanHHAnti-tumour effects of exosomes in combination with cyclophosphamide and polyinosinic-polycytidylic acidJ Int Med Res20083661342135319094445
- HaoSBaiOYuanJQureshiMXiangJDendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomesCell Mol Immunol20063320521116893501
- HaoSBaiOLiFYuanJLaferteSXiangJMature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunityImmunology200712019010217073943
- TaiebJChaputNSchartzNChemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccinesJ Immunol200617652722272916493027
- HegmansJPHemmesAAertsJGHoogstedenHCLambrechtBNImmunotherapy of murine malignant mesothelioma using tumor lysatepulsed dendritic cellsAm J Respir Crit Care Med2005171101168117715764728
- ViaudSTermeMFlamentCDendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15RalphaPLoS One200943e494219319200
- HaoSYeZYangJBaiOXiangJIntradermal vaccination of dendritic cell-derived exosomes is superior to a subcutaneous one in the induction of antitumor immunityCancer Biother Radiopharm200621214615416706635
- DaiSWeiDWuZPhase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancerMol Ther200816478279018362931
- EscudierBDorvalTChaputNVaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trialJ Transl Med2005311015740633
- MorseMAGarstJOsadaTA phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancerJ Transl Med200531915723705
- XiuFCaiZYangYWangXWangJCaoXSurface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomesJ Mol Med200785551152117219095
- ChenWWangJShaoCEfficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cellsEur J Immunol20063661598160716708399
- DaiSWanTWangBMore efficient induction of HLA-A*0201- restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cellsClin Cancer Res200511207554756316243831
- ChaputNSchartzNEAndreFExosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejectionJ Immunol200417242137214614764679
- KostarelosKBiancoAPratoMPromises, facts and challenges for carbon nanotubes in imaging and therapeuticsNat Nanotechnol200941062763319809452
- MengJMengJDuanJCarbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapySmall2008491364137018720440
- PantarottoDPartidosCDHoebekeJImmunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responsesChem Biol2003101096196614583262
- FadelTRSteenblockERSternEEnhanced cellular activation with single walled carbon nanotube bundles presenting antibody stimuliNano Lett2008872070207618547120
- van SchooneveldMMGloterAStephanOImaging and quantifying the morphology of an organic-inorganic nanoparticle at the sub-nanometre levelNat Nanotechnol20105753854420526325
- IgaAMRobertsonJHWinsletMCSeifalianAMClinical potential of quantum dotsJ Biomed Biotechnol20072007107608718317518
- JamiesonTBakhshiRPetrovaDPocockRImaniMSeifalianAMBiological applications of quantum dotsBiomaterials200728314717473217686516
- de La ZerdaAGambhirSSDrug delivery: keeping tabs on nanocarriersNat Nanotechnol200721274574618654423
- de La PenaHMadrigalJARusakiewiczSArtificial exosomes as tools for basic and clinical immunologyJ Immunol Methods2009344212113219345222