Abstract
The purpose of this study was to develop a novel, highly water-soluble poly(L-γ-glutamyl-glutamine)-paclitaxel nanoconjugate (PGG-PTX) that would improve the therapeutic index of paclitaxel (PTX). PGG-PTX is a modification of poly(L-glutamic acid)- paclitaxel conjugate (PGA-PTX) in which an additional glutamic acid has been added to each glutamic side chain in the polymer. PGG-PTX has higher water-solubility and faster dissolution than PGA-PTX. Unlike PGA-PTX, PGG-PTX self-assembles into nanoparticles, whose size remains in the range of 12–15 nm over the concentration range from 25 to 2,000 μg/mL in saline. Its critical micellar concentration in saline was found to be ~25 μg/mL. The potency of PGG-PTX when tested in vitro against the human lung cancer H460 cell line was comparable to other known polymer-PTX conjugates. However, PGG-PTX possesses lower toxicity compared with PGA-PTX in mice. The maximum tolerated dose of PGG-PTX was found to be 350 mg PTX/kg, which is 2.2-fold higher than the maximum tolerated dose of 160 mg PTX/kg reported for the PGA-PTX. This result indicates that PGG-PTX was substantially less toxic in vivo than PGA-PTX.
Introduction
Due to the high cost and uncertain success of new drug development,Citation1 serious effort is being devoted to developing novel formulations that can improve the therapeutic ratio of established hydrophobic drugs.Citation2,Citation3 Conjugation of such drugs to polymers is one of the means of increasing water-solubility. The concepts underlying the development of polymer–drug conjugates are not new and have been comprehensively reviewed.Citation4,Citation5 The core principle is that when a poorly soluble drug is linked to a water-soluble polymer, it results in a conjugate with a markedly improved aqueous solubility that also has a prolonged plasma half-lifeCitation6 and is passively accumulated in solid tumor tissues via the “enhanced permeability and retention” effect.Citation6,Citation7 Since the earliest studies of polymer– drug conjugates in 1975,Citation8 many groups have investigated the use of polymers for drug delivery, but only a few polymers, such as N-(2-hydroxypropyl)methacrylamide, poly(L-glutamic acid) (PGA), and polyethylene glycol, have been systemically examined or have entered clinical trials.Citation4,Citation5 However, none of these polymer conjugates have been reported to form nanoparticles in aqueous solutions. Encapsulation is another means of solubilizing hydrophobic anticancer drugs. Polymeric micelles comprising block hydrophilic polyethylene glycol and hydrophobically modified poly-aspartate, poly-glutamate, or poly(d,l-lactide) have been used for encapsulating hydrophobic drugs. These are capable of forming nanoparticles when loaded with paclitaxel (PTX),Citation9–Citation12 doxorubicin,Citation13,Citation14 and cisplatin.Citation15–Citation17
The goal of the study reported here was to design a nonblock polymer capable of supporting high drug loading and forming a nanoparticle in aqueous environments. We reasoned that the addition of an amino acid between the polyglutamic acid and a hydrophobic PTX would provide enough flexibility and water-solubility for the conjugate to spontaneously self-assemble into a nanoparticle. After an optimal amino acid was selected, poly(L-γ-glutamyl-glutamine) (PGG) was synthesized and conjugated with a known hydrophobic drug and its solubility experimentally determined. Finally, its in vitro efficacy was examined and compared with that of other known polymer–drug conjugates. We report here the success of this approach that has important implications for the design of polymer–drug conjugates with increased therapeutic effectiveness.
Experimental procedures
Materials
PGA, sodium salt, anhydrous N,N-dimethylformamide, sodium bicarbonate, and 4-dimethylaminopyridine were purchased from Sigma Chemical Co (St Louis, MO). N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide, L-glutamic acid di-tert-butyl ester hydrochloride, and trifluoroacetic acid were purchased from EMD Biosciences (La Jolla, CA). 1-hydroxybenzotriazole was purchased from Spectrum (Gardena, CA). PTX was purchased from NuBlocks (Vista, CA). All the chemicals and reagents were used as received without further purification. 1H-nuclear magnetic resonance (1H-NMR) spectra were recorded at 400 MHz with a Jeol spectrometer at room temperature. 1H chemical shifts are reported in parts per million (ppm).
Size exclusion chromatography–high pressure liquid chromatography (SEC-HPLC) was operated using a ChemStation program with Agilent 1200 analytical series (Quantum Analytics, Inc; Foster city, CA). The column was a Shodex OHpak SB 804HQ (Phenomenex; Torrance, CA) with guard column SB-G 605038. The mobile phase was phosphate buffer solution (50 mM phosphate, 50 mM sodium chloride [NaCl], 200 ppm sodium azide; pH 6.6) and 45% methanol (HPLC- grade) by volume to volume. The injection volume was 10 μL; the column was eluted isocratically at a flow rate of 0.525 mL/min over 40 minutes, and the eluate monitored with a multiwavelength detector at 228 nm.
A gel permeation chromatography with multiangle light scattering (GPC-MALS) detector was operated using a ChemStation program with Agilent 1200 analytical series and ASTRA V program with Dawn Heleos light scattering detector and refractive index detector (Wyatt Technology Corporation; Santa Barbara, CA). The column was Shodex OHpak SB 804HQ (Phenomenex) with a guard column SB-G 605038. The mobile phase was phosphate buffer solution (50 mM phosphate, 50 mM NaCl, 200 ppm sodium azide; pH 6.6) and 20%–50% methanol (HPLC- grade) by volume to volume. The injection volume was 100 μL, and the elution was isocratic at a flow rate of 0.7 mL/min over 60 minutes with detection at 228 nm.
Ultraviolet spectra were recorded on a PerkinElmer Lambda Bio 40 spectrophotometer (PerkinElmer; Fremont, CA). The content of conjugated PTX was estimated using an established methodCitation6 based on a standard curve generated with known concentrations of PTX in methanol (A = 228 nm) with the R2 value of 0.9999.
Particle size measurements were carried out on the Zetasizer ZS (Malvern Instruments, Malvern, UK). Poly(L-γ-glutamyl-glutamine)-paclitaxel conjugate (PGG-PTX) was first dissolved in 0.9% NaCl at 2 mg/mL. The solution was further diluted with 0.9% NaCl to produce a series of diluted solutions, which were measured for their particle sizes using dynamic light scattering method.
Synthesis of poly(L-glutamic acid)- paclitaxel conjugates (PGA-PTX)
Poly(L-glutamate) was purchased from Sigma Chemical Co. Its average molecular weight (Mw) reported based on GPC-MALS is shown in . Synthesis of PGA-PTX was carried out according to the procedure reported in the literatureCitation6 except for the replacement of 1,3-dicyclohexylcarbodiimide coupling agent with N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide coupling agent for the ease of purification and to avoid contact with chloroform. The PTX content of PGA-PTX was about 32%.
Table 1 Characteristics of the PGA and PGG polymers and the PGG-PTX
Synthesis of PGG
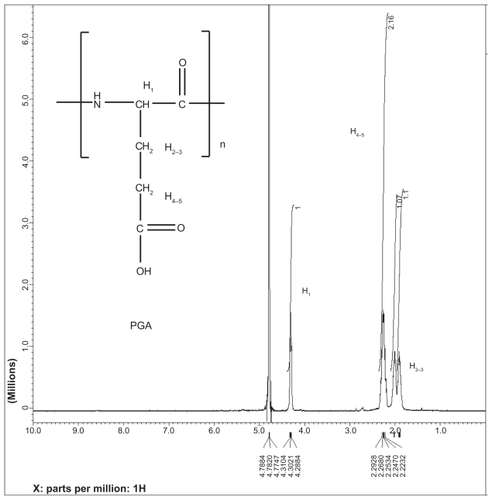
Poly-L-glutamate sodium salt (relative molecular mass 35,000, 10.0 g, 0.066 mol/monounit of polymer) was added to a 1000-mL, round-bottom glass flask equipped with a teflon magnetic stir bar and a septum under argon atmosphere. L-glutamic acid di-tert-butyl ester hydrochloride (38.3 g, 0.148 mol), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (37.0 g, 0.193 mol), 1-hydroxybenzotriazole (10.6 g, 0.074 mol), and anhydrous N,N-dimethylformamide (500 mL) were added to the flask. The mixture solution was stirred at room temperature for 24 hours. The reaction mixture solution was poured slowly into the distilled water (3 L) while stirring. A white precipitate formed. The precipitate was filtered and washed with water (3 × 250 mL). It was then dried under vacuum for 15 hours. All the dried solids were transferred to a 1000-mL, round-bottom glass flask equipped with a teflon magnetic stir bar and a septum under argon atmosphere. Trifluoroacetic acid was added into the flask, and the reaction solution was stirred for 5 hours at room temperature. Trifluoracetic acid was later removed by rotary evaporation. Water (800 mL) was added into the flask, and the mixture solution was stirred for 30 minutes until the solid became completely dissolved. The solution was poured into the dialyzed bags (Mw cut off at 10,000 Da), and PGG was dialyzed against water for 24 hours, including changing water (4 L) 4 times (once every hour for 3 hours and once overnight). The solution was then filtered through a 0.45 μm filter and PGG was lyophilized. The obtained PGG (14.8 g) was characterized using 1H-NMR, GPC-MALS detector, and SEC-HPLC. 1H-NMR (400 MHz, D2O): d 4.40 (br, 1H), 4.22 (br, 1H), 2.45 (br, 4H), 2.15 (br, 3 H), 1.97 (br, 1 H) ppm. GPC-MALS: Zimm Model was used for Mw determination; Mw, 53,180 (0.5%); Mw/Mn, 1.46; 94.7% recovery.
Synthesis of PGG-PTX
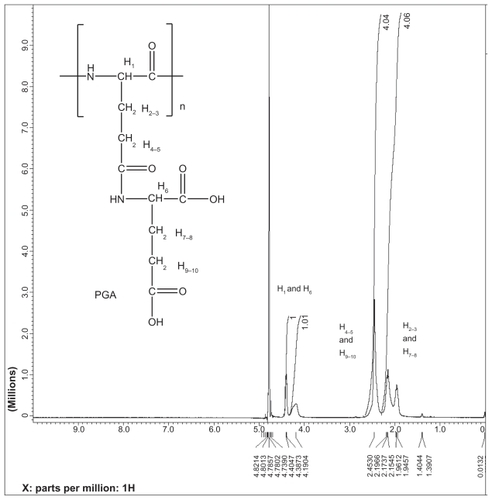
PGG (10.0 g, 0.063 mol/monomer-unit of polymer) was added into a 1000-mL glass flask equipped with a teflon magnetic stir bar and a septum under argon atmosphere. Anhydrous N,N-dimethylformamide (500 mL) was added into the flask, and the solution was stirred at room temperature for an hour to allow complete dispersion. N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (9.4 g, 0.049 mol) and 4-dimethylaminopyridine (2.6 g) were added to the solution, and the mixture solution was stirred at room temperature for 15 minutes to allow complete dispersion. PTX (5.4 g, 0.0063 mol) was added to the mixture solution, and the reaction solution was stirred for 24–28 hours until the PTX was unable to be detected by thin layer chromatography ([TLC], 100% ethyl acetate). The solution was poured slowly into 0.2 M aqueous hydrochloric acid solution (1.5 L) while vigorously stirring. A white precipitate formed. The precipitate was isolated by centrifuging the solution for 10 minutes at 5,000 rpm. The residue was dissolved in 0.5 M sodium bicarbonate solution (1.5 L). PGG-PTX was dialyzed against water for 24 hours, including changing water (4 L) 4 times (once every hour for 3 hours and once overnight). The solution was then filtered through the 0.45 μm filter and lyophilized. PGG-PTX (14.7 g) was obtained and characterized using GPC-MALS detector and SEC-HPLC. The PTX content was determined to be 35.8% by the ultraviolet-visible method.Citation6 GPC-MALS: Zimm Model was used for Mw determination: Mw, 129,400 (0.9%); Mw/Mn: 1.87; 97.7% recovery.
Determination of in vitro cytotoxicity and in vivo toxicity
NCI-H460 cells were purchased from American Type Culture Collection (ATCC HTB 177; Rockville, MD) and were grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and 100 U/mL penicillin at 37°C in 5% CO2. Relative in vitro cytotoxicity was assessed using the tetrazolium reduction assay reported by Monks et alCitation18 Relative in vivo toxicity was assessed by determining the maximum tolerated dose defined on the basis of a 15% reduction in weight. Nude mice (6–8 week old, body weight 21–25 g) were purchased from Charles River Lab (Willington, MA). PGG-PTX was dissolved in saline at 50 mg per mL and administered as an intravenous bolus. Stock solutions were prepared fresh on the day of injection.
Results and discussion
Design and synthesis of PGG-PTX
PGA-PTX (also known as CT-2103) was reported to produce complete regression of established tumors in miceCitation6 and has demonstrated activity in Phase ICitation19,Citation20 and Phase IICitation21 clinical trials. However, the combination of CT-2103 and carboplatin was not superior to PTX and carboplatin in a randomized Phase III trial in patients with lung cancer.Citation22 Furthermore, PGA-PTX has not been reported to self-assemble into nanoparticles. In our study, a new nanoconjugate platform, PGG- PTX, was designed and synthesized without utilizing diblock copolymers. We reasoned that in the case of PGA- PTX, PTX molecules attached directly to the PGA precluding the flexibility needed for the polymer conjugate collapse and for the formation of a nanoparticle. In the case of PGG-PTX, the PGA was first modified by adding another glutamic acid as a side chain to each glutamic acid in the polymer backbone. The PTX was then covalently conjugated to the added glutamic acid side chain. The glutamic acid linker was found to provide additional water-solubility so that the polymer could be loaded to a high level with PTX while having sufficient flexibility through which the hydrophobic PTX moieties could interact and cause the polymer to form a nanoparticle.
PGG-PTX was synthesized in three steps, as shown in , from commercially available PGA sodium salt with a Mw of 24,880 Da as determined by GPC-MALS. When working with polymer–drug conjugates, choosing the right reagents and solvents is a major determinant of success in achieving the final pure product. In this synthesis, only water-soluble reagents and solvents were used for the ease of purification and isolation by precipitation, filtration, and dialysis.
Figure 1 Synthesis of PGG-PTX nanoconjugate.
Abbreviations: HOBt, hydroxybenzotriazole; TFA, atrifluoroacetic acid; DMAP, 4-dimethylaminopyridine; NaHCO3, sodium bicarbonate; PGA, poly(L-glutamic acid); PGG, poly(L-γ-glutamyl-glutamine); PGG-PTX, poly(L-γ-glutamyl-glutamine)-paclitaxel conjugate.
The first goal was to obtain PGG. The synthesis began with poly-L-glutamate coupled to 2.2 equivalence of L-glutamic acid di-tert-butyl ester hydrochloride using coupling agents N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide and 1-hydroxybenzotriazole; the reaction mixture was stirred at room temperature for 24 hours and yielded an intermediate PGG-tBu ester, which was easily purified by precipitation in a large volume of water and filtered through a Buchner funnel. PGG-tBu ester was further treated with trifluoroacetic acid to convert it to PGG (Mw 53,180 Da, yield 87%). The 1H-NMR spectrum showed chemical shifts at δ 4.40 (broad, 1 H), 4.22 (broad, 1 H), 2.45 (broad, 4 H), 2.15 (broad, 3 H), and 1.97 (broad, 1 H) ppm, which were consistent with the expected proton patterns and chemical shifts. The conversion of PGA to PGG was complete, which was confirmed by 1H-NMR integration 1:1 ratio of peaks δ 4.40 (broad, 1 H) and 4.22 (broad, 1 H; Supporting information). The chemistry was found to be robust, and PGG can be produced at the 10–15 g scale.
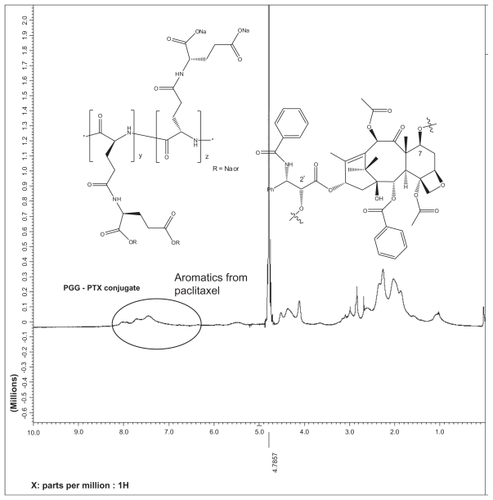
PTX was conjugated to PGG using the coupling agent N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide in the presence of catalytic amounts of 4-dimethylaminopyridine to produce PGG-PTX. The reaction was monitored using TLC with ethyl acetate (Rf of PTX is around 0.8). PTX was completely conjugated to PGG after 20 hours of stirring at room temperature; TLC showed that there was an absence of a spot of Rf around 0.8. The PTX is expected to be randomly distributed along the PGG polymer and attached predominantly at the 2′-O-position of PTX, with a minor amount linked via the 7-O position based on a single-molecule chemistry model of a variety of amino acids coupled to PTX.Citation23 Furthermore, in PGG, two carboxyl groups are capable of conjugating with the 2′-hydroxyl and 7-hydroxyl group of the PTX. As a result, 1H-NMR spectrum could not provide resolvable chemical shifts of the PGG-PTX; however, it could be confirmed that the aromatic protons of the PTX were observed (see Supporting information). The PTX content of PGG-PTX was estimated using another established methodCitation6 and was found to be 35.8% based on a standard curve generated with known concentrations of PTX in methanol (A = 228 nm) with an R2 value of 0.9999. The chemistry for the coupling of PTX to PGG is also very robust and it is possible to produce PGG-PTX at the 10–15 g scale. Conjugation using the dicyclohexylcarbodiimide coupling agent was also investigated; however, under the conditions needed for dicyclohexylcarbodiimide coupling, it was not possible to isolate a pure product due to the side product, dicyclohexylurea, being trapped within PGG-PTX.
Characterization of PGG-PTX
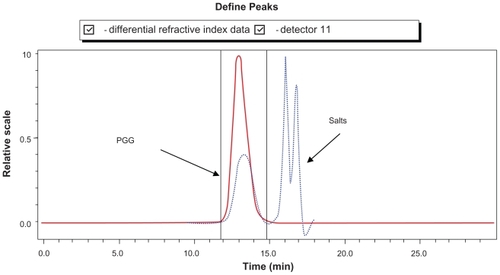
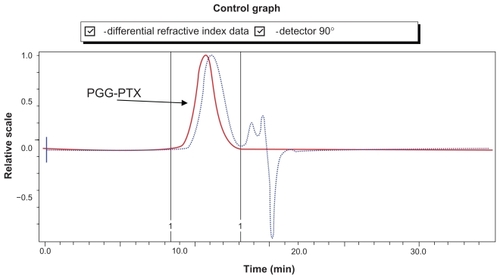
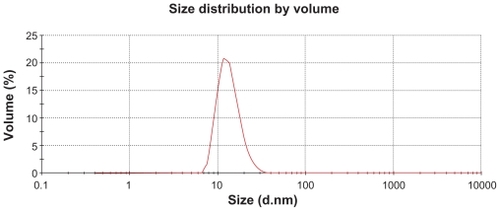
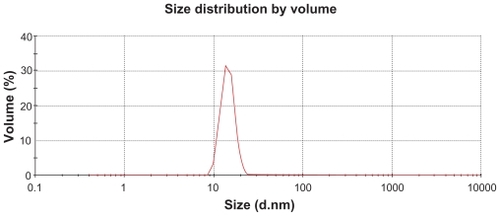
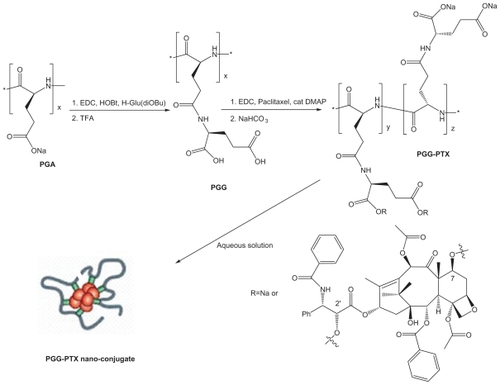
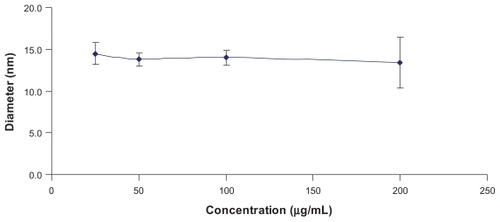
The characterization of PGG-PTX was complicated by the extent of interaction of the conjugate with itself and with the column. The Mw of PGG-PTX was characterized by (GPC-MALS) detection.Citation24 With phosphate-buffered saline as the mobile phase; the recovery was ~25% due to the high binding affinity of the conjugate to the columns. Increasing the methanol concentration from 20% to 40% progressively reduced the binding affinity and the tailing effect. The recovery increased from 25% to 90%; the light-scattering signal was more symmetrical at 40% methanol. Increasing the methanol concentration from 40% to 60% did not significantly affect the Mw, recovery percentage, or further affect the symmetrical light-scattering signal of the PGG-PTX. Based on these findings, phosphate-buffered saline containing 45% methanol was chosen as the mobile phase for GPC-MALS and SEC-HPLC. Since PGA and PGG did not exhibit high affinity for the column, phosphate-buffered saline containing 30% methanol was used as the mobile phase for these two nondrug- loaded polymers. summaries Mws, refractive index increment (dn/dc), and polydispersity (Mw/Mn) for PGA, PGG, and PGG-PTX, respectively. The average Mw of PGG-PTX was found to be 130,000 Da, with polydispersity index (Mw/Mn) of 1.87. PGG-PTX was found to be very pure as determined by SEC-HPLC as shown in . Interestingly, PGG-PTX formed nanoparticles in aqueous solutions. The particle size determined using Malvern Zetasizer Nano-ZS dynamic light scattering method was found to be about 12–15 nm in saline in a range of concentrations from 2 mg/mL down to 0.025 mg/mL. shows that the critical micellar concentration of PGG-PTX in saline was determined to be about 25 μg/mL in 0.9% NaCl at ambient temperature. Due to its unique formation of nanoparticles, PGG-PTX is expected to efficiently target hypervascular tumors that have defective vascular architecture and impaired lymphatic drainage.
Figure 2 SEC-HLPC chromatograms of PGA, PGG, and PGG-PTX. The chromatograms were recorded at 228 nm. A) PGA; B) PGG; C) PGG-PTX.
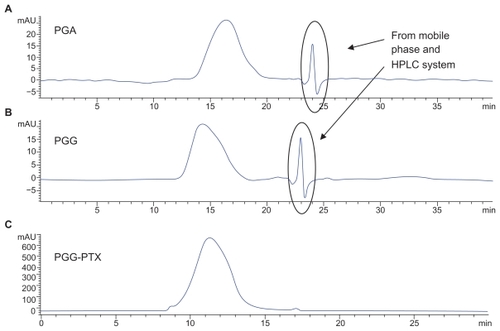
Figure 3 Critical micellar concentration of PGG-PTX nanoconjugate. The DLS could not detect the particle size of PGG-PTX solution below 25 μg/mL. The critical micellular concentration was assumed to be about 25 μg/mL in saline at 25°C. The results are expressed as means ± SD (n = 3).
Abbreviation: DLS, dynamic light scattering.
Comparison of polymer-PTX conjugates and their solubilities
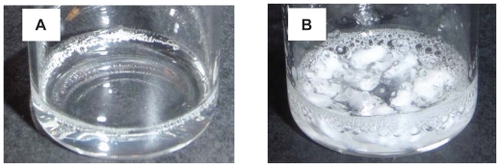
PTXCitation25 is a hydrophobic anticancer drug that is used for the treatment of many common cancers. Because of its hydrophobicity, it is formulated in the solubilizing agent Cremophor, which can produce a variety of adverse events.Citation14 Up to now, PGA was considered to be the most water-soluble and commercially available polymer and has been used to create a PGA-PTX (CT-2103, Xyotax™; Cell Therapeautics Inc; Seattle, WA]) that has now been tested in several mouse modelsCitation6 and human clinical trials.Citation19–Citation22 The characteristics of PGG-PTX were compared with the information on the solubility and therapeutic efficacy of CT-2103. To determine this experimentally, PGG-PTX and PGA-PTX were synthesized. The PTX content of PGG-PTX and PGA-PTX was determined by UV absorbance at 228 nm and was found to be 35% and 33%, respectively. It is important to note that PGG is about twice the size of PGA due to the addition of a glutamate side chain on each glutamyl in the polymer backbone. The addition of another glutamate side chain was confirmed by 1H-NMR analysis of PGG, wherein ratio of integration of the α proton (δ 4.19 ppm) of the PGA backbone to the glutamate side chain (δ 4.40 ppm) is 1:1 (see Supporting information). Thus, when loaded to 35% with PTX, the PGG-PTX contained about twice as much PTX as the PGA-PTX. The solubility of the conjugates was compared in 0.9% NaCl at a concentration of 50 mg/mL. Although PGG-PTX dissolved completely within 20 minutes, the PGA- PTX dissolved only partially, and most of the PGA-PTX remained in suspension as shown in . In order to get PGA-PTX dissolved completely, the concentration had to be lowered to 7 mg/mL or the duration of dissolution increased to >10 hours. Thus, the experimental data confirmed the hypothesis that the addition of a glutamic acid linker would substantially increase the water-solubility of the PTX-loaded polymer. Furthermore, PGG is highly water-soluble; even in the acid form, it can dissolve readily in water, whereas PGA cannot. PGG can potentially be used to deliver other hydrophobic drugs such as camptothecin and doxorubicin. Because of the bidentate ligand of the extra glutamic acid, it may also be capable of chelating cisplatin.
Biological evaluation of polymer-PTX conjugates
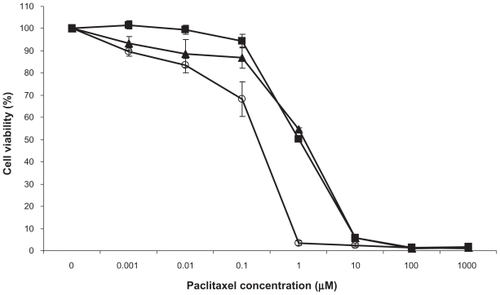
The cytotoxicity of PGG-PTX was compared with that of free PTXCitation25 against human lung cancer H460 cells using a growth inhibition assay. As shown in , free PTX was more potent than either of the polymer conjugates, whereas the 2 conjugates demonstrated equivalent half maximal inhibitory concentration (IC50) values. The IC50 for PGG-PTX was 2.31 ± .01 (standard error of mean [SEM]) μM, whereas that for PGA-PTX was 2.25 ± 0.02 (SEM) μM and that for PTX 0.15 ± 0.02 (SEM) μM. Thus, in vitro, the conjugates were 15-fold less potent than free PTX. The potency of PGG-PTX was comparable to other known polymer-PTX conjugates when tested in vitro against the human lung cancer H460 cell line.
Figure 5 Inhibition of the growth of human lung cancer H460 cells as a function of concentration of PTX (O), PGA-PTX (■) and (▴) PGG-PTX.
Note: Vertical bars, SEM.
To compare their toxicities in vivo, the maximum tolerated dose of each compound was determined in nu/nu mice for a single-dose schedule. PGG-PTX was dissolved in 0.9% NaCl at 50 mg/mL and administered by bolus intravenous injection through the tail vein. The maximum tolerated dose was defined as the dose that produced 15% loss of body weight within 2 weeks. The maximum tolerated single dose of PGG-PTX was found to be 350 mg PTX/kg, which is 2.2- fold higher than the maximum tolerated dose of 160 mg PTX/kg reported for the PGA-PTX CT-2103Citation6 and 4.4-fold higher than the maximum tolerated dose of PTX in Cremophor (80 mg/kg).Citation6 This result indicates that PGG-PTX was substantially less toxic in vivo than either free PTX or PGA-PTX. Studies of pharmacokinetics and biodistribution showed that PGG-PTX polymer significantly prolonged the half-life of total taxanes, extractable taxane, and native PTX in both the plasma and the tumor compartments.Citation26 Furthermore, results of in vivo efficacy studies also showed that PGG-PTX has superior therapeutic activity to that of Abraxane in multiple tumor models.Citation27
We have successfully designed and synthesized a new nanoconjugate platform for the delivery of hydrophobic anti-cancer drug PTX. The particle size of PGG-PTX was about 15 nm, and the size did not vary as the drug was diluted. The experimental data indicated that PGG-PTX was substantially more soluble than PGA-PTX despite the fact that the former contains approximately twice as much PTX per polymer as the latter. Importantly, using the PGG polymer, we were able to develop a PTX conjugate with much higher drug loading compared with CT-2103, the PGA-PTX currently in clinical development.
Supporting information
Spectral characterization data are available.
Acknowledgment
This work was supported by UC Discovery Grant bio06- 10568 and the Nitto Denko Technical Corporation. We wish to thank Dr Gang Zhao for valuable discussion and technical assistance on this project. We also would like to thank Ms Yuan Cao for technical assistance on this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- diMasiJAHansenRWGrabowkiHGThe price of innovation: new estimates of drug development costsJ Health Econ20032215118512606142
- PoutonCWFormulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification systemEur J Pharm Sci20062927828716815001
- AnsariMJKohliKDixitNMicroemulsions as potential drug delivery systems: a reviewPDA J Pharm Sci Technol200862667918402369
- DuncanRThe dawning era of polymer therapeuticsNat Rev Drug Discov2003234736012750738
- HaagRKratzFPolymer therapeutics: concept and applicationsAngew Chem Int Ed Engl2006451198121516444775
- LiCYuD-YNewmanRAComplete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid)- paclitaxel conjugateCancer Res199858240424099622081
- JainRKTransport of molecules in the tumor interstitium: a reviewCancer Res198747303930513555767
- RingsdorfHStructure and properties of pharmacologically active polymersJ Polym Sci Polymer Symp197551135153
- KimSCKimDWShimYHIn vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacyJ Control Release20017219120211389998
- KimT-YKimD-WChungJ-YPhase I and pharmacokinetic study of genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignanciesClin Cancer Res2004103708371615173077
- HamaguchiTMatsumuraYSuzukiMNK105, paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumor activity and reduce the neurotoxicity of paclitaxelBr J Cancer2005921240124615785749
- NegishiTKoizumiFUchinoHNK105, a paclitaxel-incorporating micellar nanoparticle, is a more potent radiosensitising agent compared to fee paclitaxelBr J Cancer20069560160616909136
- NakanishiTFukushimaSOkamotoKDevelopment of the polymer micelle carrier system for doxorubicinJ Control Release20017429530211489509
- MatsumuraYHamaguchiTUraTPhase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicinBr J Cancer2004911775178115477860
- NishiyamaNKataokaKPreparation and characterization of size- controlled polymeric micelle containing cis-dichlorodiammineplatinum(II) in the coreJ Control Release200174839411489486
- NishiyamaNOkazakiSCbralHNovel cisplatin-incorporated polymeric micelles can eradicate solid tumors in miceCancer Res2003638977898314695216
- UchinoHMatsumuraYNegishiTCisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in ratsBr J Cancer20059367878716222314
- MonksAScudieroDSkehanPFeasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell linesJ Natl Cancer Inst1991837577662041050
- VeroneseMLFlahertyKKramerAPhase I study of the novel taxane CT-2103 in patients with advanced solid tumorsCancer Chemother Pharmacol20055549750115711828
- BoddyVAPlummerREToddRA phase I and pharmacokinetic study of paclitaxel poliglumex (XYOTAX), investigating both 3-weekly and 2-weekly schedulesClin Cancer Res2005117834784016278406
- SabbatiniPAghajanianCDizonDPhase II study of CT-2103 in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinomaJ Clin Oncol2004224523453115542803
- LangerCJO’ByrneKJSocinskiMAPhase II trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naïve advanced non-small cell lung cancerJ Thorac Oncol2008362363018520802
- MathewAEMejillanoMRNathJPHimesRHStellaVJSynthesis and evaluation of some water-soluble prodrugs and derivatives of taxol with antitumor activityJ Med Chem1992351451511346275
- Reference of gel permeation chromatography with multi-angle light scattering detection Available from: http://wyatt.com/
- WaniMCTaylorHLWallMECoggonPMcPhailATPlant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus BrevifoliaJ Am Chem Soc197193232523275553076
- WangXZhaoGvanSPharmacokinetics and tissue distribution of PGG-paclitaxel, a novel macromolecular formulation of paclitaxel, in nu/nu mice bearing NCI-460 lung cancer xenograftsCancer Chemother Pharmacol20106551552619593566
- ZhonglingFZhaoGYuLGoughDHowellSBPreclinical efficacy studies of a novel nanoparticle-based formulation of paclitaxel that out-performs AbraxaneCancer Chemother Pharmacol20106592393019685054