Abstract
Herein, DNA duplex was constructed through the hybridization of adenosine triphosphate (ATP)-responsive aptamer and its cDNA in which GC-rich motif could be used to load doxorubicin (DOX), and then, cationic polymer PEI25K was used as a carrier to simultaneously condense DOX-Duplex and Bcl-2 siRNA to prepare the ternary nanocomplex polyethylenimine (PEI)/DOX-Duplex/siRNA. The ATP concentration gradient between the cytosol and extracellular environment could achieve the stable loading of DOX in duplex and the rapid drug release in an ATP-responsive manner. Using human prostate tumor cell line PC-3 as a model, an obvious induction of cell proliferation could be detected with a cell viability of 53.3%, which was stronger than single cargo delivery, indicating the synergistic effect between these two components. The enhanced anti-proliferative effect of ternary nanocomplex could be attributed to the improved induction of cell apoptosis in a mitochondria-mediated pathway and cell-cycle arrest at the G2 phase. Overall, the ATP-responsive nanocarrier for co-delivering DOX and Bcl-2 siRNA has been demonstrated to be a smart delivery system with favorable anti-proliferative effect, especially for solving the multidrug resistance of tumors.
Introduction
In the cancer treatment, chemotherapy still plays an important role despite great advances in the field of surgery and radiotherapy.Citation1 However, an important limitation associated with chemotherapy is the emergence of multidrug resistance,Citation2,Citation3 which can be divided into two types, pump and non-pump resistance.Citation4 The pump resistance is caused by drug efflux pumps on the cell membrane, which decrease the intracellular concentration of drugs and ultimately lead to the failure of chemotherapy,Citation5–Citation7 while the non-pump resistance is based on the anti-apoptotic defense, which enables the cancer cells to survive against the chemotherapeutics.Citation8–Citation10 The Bcl-2 protein has been considered to be a key executor for non-pump resistance, which can inhibit the release of cytochrome C after the initiation of apoptosis and block the downstream propagation of death signal, thereby promoting the cell survival.Citation11 Previous research has shown that decreasing the expression level of Bcl-2 by the siRNA technique could efficiently trigger the apoptosis of cancer cells.Citation12,Citation13 More importantly, the combination of chemotherapy and gene therapy such as Bcl-2 siRNA has been demonstrated to enhance the efficacy and improve the therapeutic outcome,Citation14–Citation17 owing to the synergistic anticancer activity or enhanced sensitivity to chemotherapeutics.
Up to now, the co-delivery of chemotherapeutic agents and siRNA mediated by nanocarriers has been demonstrated to be effective in both in vitro and in vivo studies.Citation18 To further enhance the biological specificity and therapeutic efficacy, great efforts have been devoted to construct stimuli-responsive delivery systems, which can improve the accumulation of nanocarriers at tumor sites and rapidly release the payloads within tumor cells.Citation19 External signals such as magnetic field, light and temperature or internal ones such as pH, redox potential and enzymatic activities have been widely used to construct stimuli-responsive nanocarriers for improving the drug delivery efficiency.Citation20,Citation21 However, complex design processes and accurate experimental conditions could not be avoided in the construction of these stimuli-responsive nanocarriers.Citation21 Thus, it is still of great challenge to explore more facial and efficient stimuli-responsive nanocarriers, especially for achieving the co-delivery of drug and gene. Recently, adenosine triphosphate (ATP)-responsive nanocarriers for co-delivering drugs and genes have been successfully developed,Citation20–Citation27 based on the higher ATP concentration in the intracellular fluids (1–10 mM) than in the extracellular environment (<0.4 mM).Citation28,Citation29 For instance, Mo et alCitation20 constructed an ATP-triggered nanocarrier for doxorubicin (DOX) release through an ATP-responsive duplex (the ATP aptamer and its complementary single-stranded DNA), whose GC-rich motif was used to intercalate DOX into the aptamer duplex. In an ATP-rich condition, ATP molecules will trigger the rapid release of DOX and high anti-proliferative effect owing to the dissociation of aptamer duplex.
In the present research, an ATP-triggered nanosystem for achieving the co-delivery of DOX and Bcl-2 siRNA was constructed based on an ATP-responsive aptamer duplex, in which the rapid release of DOX and Bcl-2 siRNA could be achieved under ATP-rich conditions. Then, the nanosystem-mediated inhibition of cell proliferation was systematically evaluated, including cell apoptosis and cell-cycle arrest, using human prostate tumor cell line PC-3 as a model.
Materials and methods
Materials
DOX in the form of hydrochloride salt (>99%) was purchased from Huafeng Biotech. Co. (Beijing, People’s Republic of China) and used as received. Bcl-2 siRNA (sense: 5′-UGU GGA UGA CUG AGU ACC UGA dTdT-3′; antisense: 5′-UCA GGU ACU CAG UCA UCC ACA dTdT-3′) and 5-carboxyfluorescein (FAM)-labeled Bcl-2 siRNA (FAM-siRNA) was synthesized by GenePharma (Suzhou, People’s Republic of China). ATP-responsive aptamer (5′-ACC TGG GGG AGT ATT GCG GAG GAA GGT-3′) and its cDNA (5′-ACC TTC CTC CGC AAT ACT CCC CCA GGT-3′) were synthesized by Sangon Biotech (Shanghai, People’s Republic of China). Polyethylenimine (PEI) with a molecular weight of 25 kDa (PEI25K) was obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Amersco (Solon, OH, USA). Diethyl pyrocarbonate (DEPC)-treated water, bicinchoninic acid (BCA) protein assay kit and 4′,6-diamidino-2-phenylindole (DAPI) were obtained from DingGuo Biotech. Co. (Beijing, People’s Republic of China). LIVE/DEAD® Viability/Cytotoxicity kit was purchased from Thermo Fisher Scientific. Annexin V-fluoresceine isothiocyanate (FITC)/PI apoptosis detection kit, cell-cycle detection kit and caspase-3, -8 and -9 activity assay kits were purchased from Bestbio (Shanghai, People’s Republic of China). Polyvinylidene fluoride (PVDF) membrane was purchased from EMD Millipore (Billerica, MA, USA). Antibodies against β-actin, Bcl-2, procaspase-3 and horseradish peroxidase (HRP)-labeled goat anti-mouse IgG were purchased from Abcam (Cambridge, UK).
Construction and characterization of PEI/DOX-Duplex/siRNA nanocomplex
The ATP aptamer and its cDNA were first dissolved in DEPC-treated water to achieve a concentration of 20 µM. Then, these two solutions (160 µL) were mixed together and incubated at room temperature for 15 min to obtain duplex, into which 320 µL of DOX solution (20 µM in DEPC-treated water) was added. The mixture was incubated at room temperature for another 15 min to construct DOX-Duplex. Bcl-2 siRNA solution (0.264 µg/µL in DEPC-treated water) was mixed with DOX-Duplex in different ratios, and PEI25K was added into the mixture at predetermined mass ratios. The sample was incubated at room temperature for 30 min to obtain PEI/DOX-Duplex/siRNA nanocomplex.
The DOX loading was monitored by comparing the fluorescence spectra of DOX solution (320 µL, 20 µM) and DOX-Duplex solution, both of which were diluted to 3 mL using DEPC-treated water before detection. The ATP-triggered DOX release was detected by observing the fluorescence intensity changes of DOX-Duplex treated with different concentrations of ATP (0, 0.4 and 4.0 mM) in the dark for 15 min. The fluorescence spectra were performed on the Shimadzu RF-5301 fluorescence spectrometer (Kyoto, Japan) with excitation and emission wavelengths of 480 and 500–700 nm, respectively. The condensation of PEI25K for DOX-Duplex and Bcl-2 siRNA and the nanocomplex stability in the serum were detected by gel retardation assay. The nanocomplex was incubated at 37°C for 3 h in the presence or absence of 50% FBS and analyzed by 1% agarose gel electrophoresis in Tris-acetate-EDTA (TAE) buffer (120 V, 20 min). The particle size and zeta potential of nanocomplex were determined by Malvern Nano ZS90 Zetasizer (Malvern Instruments, Malvern, UK).
Cellular uptake of PEI/DOX-Duplex/siRNA
The PC-3 cells were obtained from the Shanghai Institute of Cell Bank (Shanghai, People’s Republic of China) and cultured in DMEM supplemented with 100 IU/mL penicillin, 100 mg/mL streptomycin and 10% FBS at 37°C in a humidified atmosphere of 5% CO2. For cellular uptake assay, the cells were cultured in six-well plates at 37°C for 24 h at an initial density of 3.0×105 cells/well, treated with PEI/DOX-Duplex/siRNA for 6 h and observed through IX71 fluorescence microscopy (Olympus Corporation, Tokyo, Japan). In addition, the intracellular DOX release was monitored at different incubation times.
Inhibition of cell proliferation by PEI/DOX-Duplex/siRNA
The inhibition of cell proliferation mediated by PEI/DOX-Duplex/siRNA was evaluated by the MTT assay. Briefly, PC-3 cells were seeded in 96-well plates at a density of 8.0×103 cells/well and cultured to 80% confluence. Then, the medium was removed from each well, and 200 µL of FBS-free DMEM containing different nanocomplexes (10 µg/mL PEI25K, 5 µg/mL DOX-Duplex and 15 µg/mL siRNA) was added into the wells. After 24 h treatment, the cells in each well were incubated with 20 µL of MTT solution (5 mg/mL in PBS) for an additional 4 h. The formed formazan crystals were dissolved by 150 µL dimethyl sulfoxide (DMSO) for 10 min after removing the MTT solution. The cell viability was calculated as the ratio of absorbance values at 492 nm of treatment and control groups, which were measured by the GF-M3000 microplate reader (Caihong Corporation, Shandong, People’s Republic of China). Additionally, Live/Dead staining was conducted in six-well plates to directly observe the induction of cell proliferation induced by nanocomplex. The cells were cultured in an initial density of 2.0×105 cells/well, and the treatment was carried out using nanocomplex with the same DOX or siRNA concentration as the MTT assay. Then, the cells were stained with live/dead reagents for 20 min according to the manufacturer’s instructions, washed with PBS twice and observed through IX71 fluorescence microscopy (Olympus Corporation).
Induction of cell apoptosis by PEI/DOX-Duplex/siRNA
Briefly, PC-3 cells were seeded in six-well plates at a density of 2.0×105 cells/well and cultured at 37°C for 24 h before transfection. As described earlier, the cells were treated with different nanocomplexes in 2 mL of FBS-free DMEM for 24 h, with DOX-Duplex and siRNA concentrations of 5 and 15 µg/mL, respectively. According to the protocol provided in Annexin V-FITC/PI apoptosis detection kit, the harvested cells were washed with PBS twice, re-suspended in binding buffer and incubated with Annexin V-FITC and propidium iodide (PI) solutions at room temperature for 10 min in the dark. Finally, the cell apoptosis was analyzed by FACSCalibur (BD Biosciences, San Jose, CA, USA) with 15,000 ungated cells used.
Induction of cell-cycle arrest by PEI/DOX-Duplex/siRNA
The cell culture and PEI/DOX-Duplex/siRNA treatment were conducted as described in the cell apoptosis analysis. For cell-cycle assay, the harvested cells were first fixed in 70% ethanol at 4°C overnight and then washed with PBS twice and incubated with 20 µL of RNase A solution at 37°C for 30 min and 300 µL of PI solution at 4°C for 1 h in the dark. Finally, the cell cycle was measured by analyzing 15,000 ungated cells through FACSCalibur.
Western blot assay
The cell culture and PEI/DOX-Duplex/siRNA treatment were conducted as described in the cell apoptosis analysis. Prior to Western blot analysis, PC-3 cells were collected, washed with PBS twice and treated with radioimmunoprecipitation assay (RIPA) lysis buffer on ice for 2 h. The supernatants were obtained through the centrifugation of lysates at 12,000 rpm for 10 min. After determining the protein concentration by BCA protein kit, SDS-PAGE following transfer to PVDF membrane via electroblotting was performed using an equal amount of proteins. The membrane was blocked in PBS containing 10% non-fat milk and 0.1% Tween-20 for 2 h and incubated with the primary antibodies (antibodies against β-actin, Bcl-2 and procaspase-3) at 4°C overnight. After washing with PBS containing 0.1% Tween-20 (PBST) three times, the membrane was treated with HRP-labeled secondary antibody at room temperature for 1 h and washed with PBST twice. Finally, the expression levels were normalized against β-actin and detected through enhanced chemical luminescence (TransGen Biotech, Beijing, People’s Republic of China).
Caspase-3, -8 and -9 activities assay
The cell culture and PEI/DOX-Duplex/siRNA treatment were conducted as described in the cell apoptosis analysis, and caspase-3, -8 and -9 activities were assayed using the corresponding kits. After washing with PBS twice, the cells were further treated with 100 mL lysis buffer, after which the supernatants were centrifuged at 10,000 g for 10 min. Finally, 10 mL supernatant was used to detect the caspase-3, -8 and -9 activities according to the manufacturer’s instructions, in which the absorbance at 405 nm was recorded on the GF-M3000 microplate reader.
Results and discussion
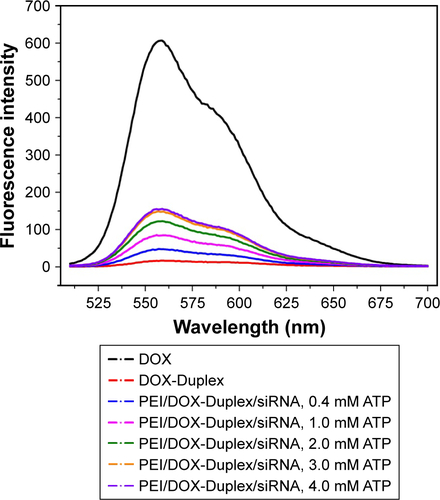
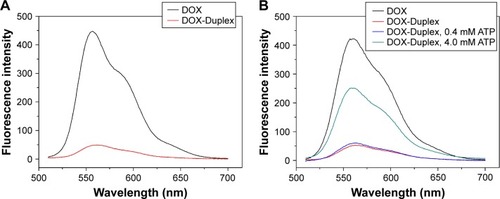
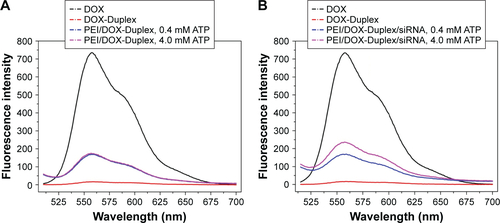
The duplex was first prepared through the hybridization of ATP aptamer and its cDNA, and its GC-rich motif could be used to load the antitumor agent DOX. To detect whether DOX was intercalated into the DNA duplex, the fluorescence spectra analysis of DOX and DOX-Duplex was conducted. As shown in , free DOX exhibited strong fluorescence absorption at 570 nm, while the fluorescence intensity of DOX-Duplex was dramatically reduced, which meant the successful loading of DOX in the duplex. The decreased fluorescence intensity was caused by the resonance energy transfer between DOX molecules when interacted into the DNA duplex.Citation30,Citation31 In the present study, ATP-binding aptamer and its cDNA were used to construct the DNA duplex, and ATP molecules could specially recognize and activate the aptamer, which would directly promote the release of loaded DOX molecules. Thus, the ATP-triggered DOX release was then studied through the incubation of DOX-Duplex in the presence of ATP at different concentrations (). A low concentration of ATP (0.4 mM) could not induce the DOX release as the fluorescence intensity was almost identical to that without ATP treatment. Nevertheless, a high ATP concentration (4.0 mM) could efficiently achieve the release of DOX, indicating the drug release in an ATP-responsive manner. The ATP concentration exhibited a significant difference between the intracellular fluids (1–10 mM) and extracellular environment (<0.4 mM).Citation28,Citation29 Thus, the ATP concentration gradient could be used for realizing the stable loading of DOX in the DNA duplex and the rapid drug release in the cytosol, and these characteristics made it probably an ideal smart delivery system.
Figure 1 Fluorescence spectra of DOX and DOX-Duplex (A) and ATP-triggered DOX release from DOX-Duplex through the incubation at different concentrations of ATP (0, 0.4 and 4.0 mM) for 15 min (B).
Abbreviations: DOX, doxorubicin; ATP, adenosine triphosphate.
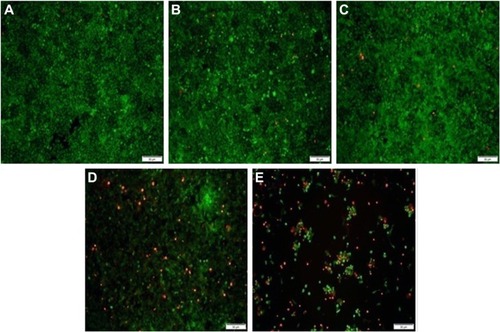
The ATP-responsive co-delivery system was then constructed through the incubation of PEI25K, DOX-Duplex and Bcl-2 siRNA at room temperature for 30 min, namely PEI/DOX-Duplex/siRNA. The cationic carrier PEI25K has been demonstrated to possess superior gene transfection efficiency both in vitro and in vivo, as its high positive charge and proton-buffering capacity can condense nucleic acids to form stable nanocomplex, protect them from the degradation in the serum and facilitate the efficient cellular uptake and rapid release from endosomes.Citation32,Citation33 Agarose gel electrophoresis was conducted to detect the condensation ability of PEI25K with DOX-Duplex and siRNA. As shown in , PEI25K could realize the complete gel retardation of siRNA and DOX-Duplex at a mass ratio of 1.0, and it could simultaneously condense these two components at mass ratios of 1:1:1 and 2:1:1 to form stable nanocomplexes. Furthermore, the carrier PEI25K could protect the cargos from the degradation in the presence of 50% FBS, no matter for the single cargo loading and the co-delivery system, in which 4 mg/mL heparin was used to destroy the nanocomplex to release the oligonucleotides (). Owing to the loading of DOX, PEI25K could not condense the aptamer duplex as efficiently as Bcl-2 siRNA, which would lead to a much higher particle size of PEI/DOX-Duplex (). Nevertheless, the addition of Bcl-2 siRNA was benefi-cial for the binding and condensation of DOX-Duplex by PEI25K, achieving a particle size of 360.8 nm for the ternary nanocomplex PEI/DOX-Duplex/siRNA (mass ratio of 1:1:1). Meanwhile, the ternary nanocomplex exhibited a positive state with zeta-potential of +9.85 mV (). These results demonstrated that the ternary nanocomplex was favorable for achieving an efficient cellular uptake and transfection efficiency. The fluorescence spectra of DOX release from PEI/DOX-Duplex and PEI/DOX-Duplex/siRNA after the treatment with 0.4 or 4.0 mM ATP showed that ATP could not trigger the cargo release from these delivery systems (Figure S1). These results demonstrated that PEI25K could efficiently condense DOX-Duplex and siRNA into stable nanocomplex and protect the cargo release. After the cellular uptake of nanoparticles, PEI25K could achieve the endosomal escape and rapid release of DOX-Duplex and siRNA through the proton sponge effect, and the released DOX-Duplex could rapidly release DOX molecules through the response to high ATP concentration in the cytosol. Meanwhile, PEI/DOX-Duplex/siRNA nanocomplex exhibited a weak ATP responsive ability in the ATP concentration of 0.4–4.0 mM (Figure S2). The cellular uptake of ternary nanocomplex PEI/DOX-Duplex/siRNA was then studied through its incubation with PC-3 cells for 6 h, in which DOX (red) and siRNA (green) could be obviously observed in the cells in a co-location manner (). Meanwhile, the fluorescence intensity of DOX exhibited an increasing tendency with the elongation of incubation time (), implying the gradual release of DOX in the intracellular environment. Additionally, the released DOX was specifically accumulated into the nuclei, which was consistent with previous research.Citation20 Combining the in vitro release analysis of DOX, the results elucidated that DOX could be successfully released from the duplex, which was probably caused by the transformational changes in duplex induced by the interaction between ATP aptamer and the high concentration of ATP in the cytosol.
Figure 2 Agarose gel electrophoresis for the condensation ability of PEI25K toward Bcl-2 siRNA and DOX-Duplex (A) and the stability of nanocomplex in the presence of 50% FBS (B).
Notes: (A) Lane 1: DOX-Duplex, lane 2: siRNA, lane 3: PEI/DOX-Duplex at a mass ratio of 1.0, lane 4: PEI/siRNA at a mass ratio of 1.0 and lanes 5 and 6: PEI/DOX-Duplex/siRNA at mass ratios of 1:1:1 and 2:1:1. (B) siRNA (lanes 1 and 2), DOX-Duplex (lanes 3 and 4), PEI25K/siRNA (mass ratio of 1.0, lanes 5 and 6), PEI25K/DOX-Duplex (mass ratio of 1.0, lanes 7 and 8) and PEI/DOX-Duplex/siRNA (mass ratio of 1:1:1, lanes 9 and 10) in the absence and presence of 50% FBS. The samples were pretreated with 4 mg/mL heparin before the agarose gel electrophoresis except for lanes 5, 7 and 9.
Abbreviations: DOX, doxorubicin; FBS, fetal bovine serum; PEI, polyethylenimine.
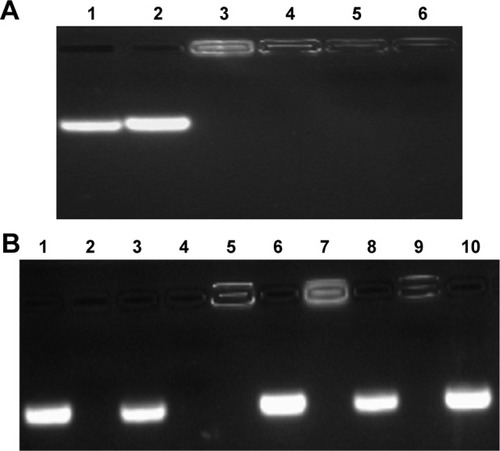
Figure 3 Particle size (A) and zeta potential (B) of different nanocomplexes.
Abbreviations: PEI, polyethylenimine; DOX, doxorubicin.
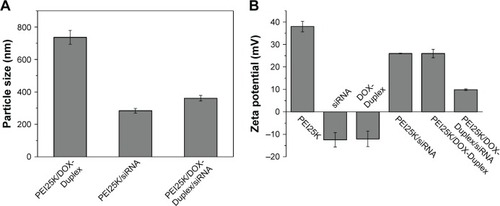
Figure 4 The cellular uptake of ternary nanocomplex PEI/DOX-Duplex/siRNA for 6 h through fluorescence microscopic analysis.
Notes: (A) Red: DOX, (B) green: FAM-siRNA, (C) blue: DAPI for nuclei staining and (D) merge. Scale bar 50 µm.
Abbreviations: PEI, polyethylenimine; DOX, doxorubicin; FAM, 5-carboxyflu-orescein; DAPI, 4′,6-diamidino-2-phenylindole.
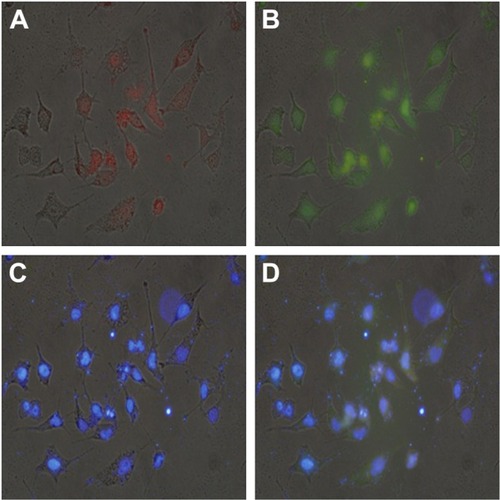
Figure 5 The intracellular DOX release determined by fluorescence microscopy for 0 h (A), 6 h (B), 12 h (C) and 24 h (D). Scale bar 100 µm.
Abbreviation: DOX, doxorubicin.
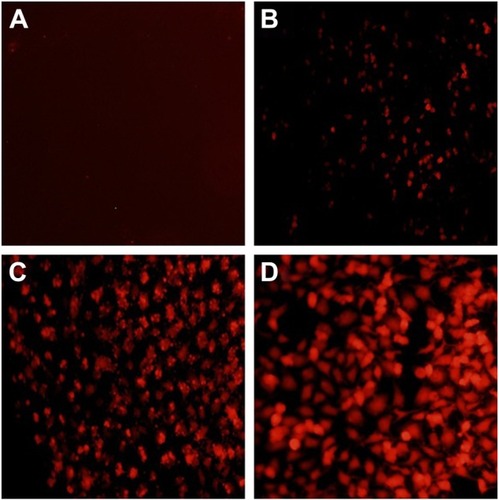
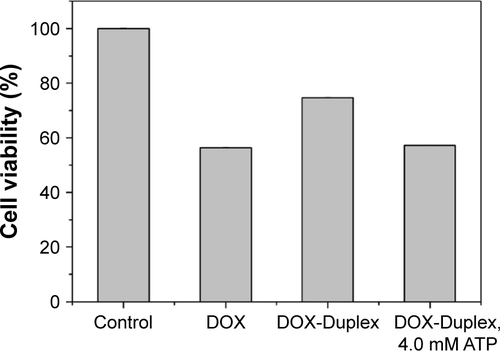
To assay the anti-proliferation effect, the cell viabilities after the treatment with different nanocomplexes were measured through MTT. As shown in , compared to the control, DOX-Duplex and PEI25K/siRNA exhibited almost no obvious effects on the cell proliferation with cell viability values of 98.9% and 77.5%, in which the latter was mainly caused by the cytotoxicity of PEI25K (cell viability of 83.3%). However, PEI25K-mediated delivery of DOX-Duplex could significantly inhibit the cell proliferation with cell viability of 62.5%, which was probably attributed to the efficient cellular uptake and subsequent DOX release quickly in the cytosol. Notably, the co-delivery system showed an enhanced induction of cell proliferation (cell viability of 53.3%), mainly owing to the synergistic anti-proliferative effect between these two components. Meanwhile, DOX-Duplex after the treatment with 4.0 mM ATP exhibited a similar inhibition effect to free DOX (Figure S3), indicating that the dissociation of aptamer duplex and subsequent DOX release could be achieved in an ATP-responsive manner. Through Western blot analysis, the expression level of Bcl-2 could be observed to be decreased after the treatment with PEI25K/siRNA and PEI/DOX-Duplex/siRNA (). The knockdown of Bcl-2 would contribute to the intrinsic inhibition of cell proliferation and even the reduction of anti-apoptotic defense,Citation34 which was promising for improving the antitumor efficacy of chemotherapeutics and decreasing the side effects through the reduced dosage, especially for solving the multidrug resistance of tumors. Live/Dead assay was conducted to evaluate the anti-proliferative effect of ternary nanocomplex, in which live and dead cells were stained green by the intracellular enzymatic hydrolysis of calcein AM and red by the intercalation of ethidium homodimer to DNA, respectively.Citation35 As shown in , more death cells could be detected in PEI/DOX-Duplex/siRNA than in other groups. It was worthy to be noted that stronger induction of cell death could be achieved in the delivery of ternary nanocomplex than PEI25K/DOX-Duplex, implying the facilitated induction ability of cell death of Bcl-2 siRNA.
Figure 6 Cell viabilities of PC-3 cells treated with different nanocomplexes for 24 h.
Note: Data were expressed as mean value ± SD of three experiments.
Abbreviations: PEI, polyethylenimine; DOX, doxorubicin.
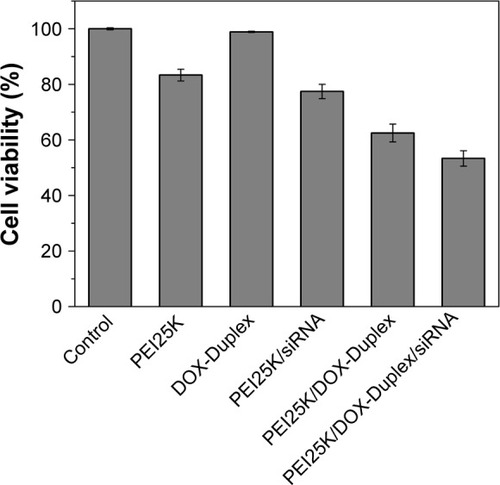
Figure 7 Expression level of Bcl-2 and procaspase-3 in PC-3 cells after the treatment with different nanocomplexes for 24 h using Western blot: 1, control; 2, Bcl-2 siRNA; 3, DOX-Duplex; 4, PEI25K/DOX-Duplex; 5, PEI25K/siRNA and 6, PEI/DOX-Duplex/siRNA.
Abbreviations: DOX, doxorubicin; PEI, polyethylenimine.
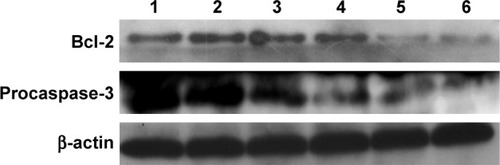
Figure 8 Live/Dead assay of PC-3 cells treated with different nanocomplexes for 24 h: (A) control; (B) DOX-Duplex; (C) PEI25K/DOX-Duplex; (D) PEI25K/siRNA and (E) PEI/DOX-Duplex/siRNA.
Notes: The live and dead cells exhibited green and red fluorescence, respectively. Scale bar 200 µm.
Abbreviations: DOX, doxorubicin; PEI, polyethylenimine.
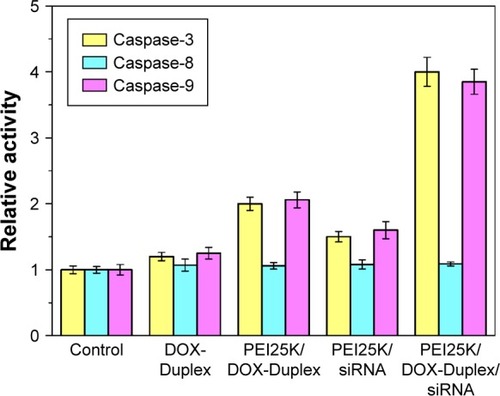
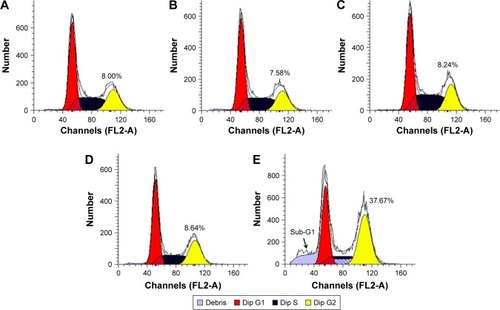
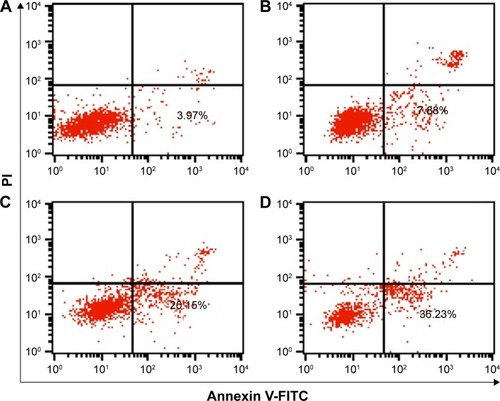
To get a deeper insight into the anti-proliferative mechanism, flow cytometry was conducted to monitor the cell apoptosis and cell-cycle arrest induced by the treatment of ternary nanocomplex. As shown in , compared to the control group (3.97%), obvious early apoptosis could be achieved in PEI25K/siRNA and PEI25K/DOX-Duplex groups, and meanwhile, PEI25K/DOX-Duplex exhibited a more powerful ability of inducing cell apoptosis (20.15%) than PEI25K/siRNA (7.88%). Surprisingly, the co-delivery of these two cargos showed a much stronger ability of inducing cell apoptosis than single cargos with an early apoptotic ratio of 36.23%. These results were consistent with the MTT assay, indicating the synergistic effect between DOX and Bcl-2 siRNA. Procaspase-3, the caspase-3′s precursor, has been widely accepted to play a key role in the caspase family-related apoptotic cascade.Citation36,Citation37 The expression level of procaspase-3 was dramatically decreased after the treatment with PEI/DOX-Duplex/siRNA (), indicating the activation of caspase-3, which would subsequently catalyze the hydrolysis of many protein substrates for achieving the cell apoptosis. Furthermore, the treatment with PEI/DOX-Duplex/siRNA could obviously increase the activities of caspase-3 and caspase-9 and did not influence the caspase-8 activity (). The caspase-9 can target procaspase-3 in the mitochondrial apoptosis pathway,Citation38 which meant the ternary nanocomplex PEI/DOX-Duplex/siRNA could activate the mitochondria-mediated pathway. However, the nanocarrier had no profound effects on death receptor-mediated pathway, in which caspase-8 possesses a death receptor domain and acts as an initiator caspase.Citation38 Similarly, more activation of caspase-3 and caspase-9 could be obtained in PEI/DOX-Duplex/siRNA than in other groups. Finally, cell-cycle arrest assay indicated that PEI25K/siRNA did not induce obvious cell-cycle arrest, while the ternary nanocomplex could improve the G2 ratio (37.67%) and achieve the cell-cycle arrest at the G2 phase (). Additionally, obvious sub-G1 phase could be observed in the PC-3 cells treated with DOX and Bcl-2 siRNA, which was a typical sign of cell apoptosis and consistent with the induction of cell apoptosis. Overall, our results elucidated that the enhanced anti-proliferative effect of ternary nanocomplex could be attributed to the induction of cell apoptosis and cell-cycle arrest in a synergistic manner of chemotherapy and gene therapy.
Figure 9 Flow cytometric analysis of the cell apoptosis of PC-3 cells treated with different nanocomplexes for 24 h: (A) control, (B) PEI25K/siRNA, (C) PEI25K/DOX-Duplex and (D) PEI/DOX-Duplex/siRNA.
Abbreviations: PEI, polyethylenimine; DOX, doxorubicin; PI, propidium iodide; FITC, fluoresceine isothiocyanate.
Conclusion
Based on an ATP-responsive aptamer, a nanocarrier for co-delivering DOX and Bcl-2 siRNA has been successfully constructed. The ATP-triggered rapid release of cargos in the cytosol and the synergistic effect between these two cargos made it an ideal delivery system for achieving an efficient anti-proliferative effect through the induction of cell apop-tosis and cell-cycle arrest. The strategy of combining chemotherapy and gene therapy in an ATP-responsive manner could potentially be used in the construction of smart delivery systems with favorable antitumor efficacy, especially for overcoming the multidrug resistance of tumors.
Acknowledgments
The authors gratefully acknowledge the financial supports from the Natural Science Foundation of China (Nos 81373344, 81473142, 81673502 and 51403074), Science & Technology Department of Jilin Province (Nos 20140101140JC, 20160520144JH and 20160520146JH), Education Department of Jilin Province (No 2015469), Youth Fund of Health and Family Planning Commission of Jilin Province (No 2013Q026) and Norman Bethune Program of Jilin University (Nos 2015324 and 2015423).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Figure S1 Fluorescence spectra of DOX release from PEI/DOX-Duplex/siRNA through the incubation at different concentrations of ATP (0.4–4.0 mM) for 15 min.
Abbreviations: DOX, doxorubicin; PEI, polyethylenimine; ATP, adenosine triphosphate.
References
- GandhiNSTekadeRKChouguleMBNanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advancesJ Control Release201419423825625204288
- GuYLiJLiYNanomicelles loaded with doxorubicin and curcumin for alleviating multidrug resistance in lung cancerInt J Nanomedicine2016115757577027843316
- KachalakiSEbrahimiMMohamed KhosroshahiLMohammadinejadSBaradaranBCancer chemoresistance; biochemical and molecular aspects: a brief overviewEur J Pharm Sci201689203027094906
- PakunluRIWangYTsaoWPozharovVCookTJMinkoTEnhancement of the efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense: novel multicomponent delivery systemCancer Res200464176214622415342407
- LeslieEMDeeleyRGColeSPMultidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defenseToxicol Appl Pharmacol2005204321623715845415
- Kapse-MistrySGovenderTSrivastavaRYergeriMNanodrug delivery in reversing multidrug resistance in cancer cellsFront Pharmacol2014515925071577
- WuDWangCYangJImproving the intracellular drug concentration in lung cancer treatment through the codelivery of doxorubicin and miR-519c mediated by porous PLGA microparticleMol Pharm201613113925393327684197
- TsourisVJooMKKimSHKwonICWonYYNano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancersBiotechnol Adv20143251037105024924617
- LingGZhangTZhangPSunJHeZSynergistic and complete reversal of the multidrug resistance of mitoxantrone hydrochloride by three-in-one multifunctional lipid-sodium glycocholate nanocarriers based on simultaneous BCRP and Bcl-2 inhibitionInt J Nanomedicine2016114077409127601896
- SaadMGarbuzenkoOBMinkoTCo-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancerNanomedicine20083676177619025451
- WalenskyLDBCL-2 in the crosshairs: tipping the balance of life and deathCell Death Differ20061381339135016763614
- WuDYangJXingZPhenylboronic acid-functionalized polyamidoamine-mediated Bcl-2 siRNA delivery for inhibiting the cell proliferationColloids Surf B Biointerfaces201614631832527371891
- BehCWSeowWYWangYEfficient delivery of Bcl-2-targeted siRNA using cationic polymer nanoparticles: downregulating mRNA expression level and sensitizing cancer cells to anticancer drugBiomacromolecules2009101414819072631
- HuCMZhangLNanoparticle-based combination therapy toward overcoming drug resistance in cancerBiochem Pharmacol20128381104111122285912
- LiJWangYZhuYOupickýDRecent advances in delivery of drug-nucleic acid combinations for cancer treatmentJ Control Release2013172258960023624358
- XueWDahlmanJETammelaTSmall RNA combination therapy for lung cancerProc Natl Acad Sci U S A20141113435533561
- WuDHanHXingZIdeal and reality: barricade in the delivery of small interfering RNA for cancer therapyCurr Pharm Biotechnol201617323724726511977
- CreixellMPeppasNACo-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistanceNano Today2012736737926257819
- RapoportNPhysical stimuli-responsive polymeric micelles for anticancer drug deliveryProg Polym Sci200732962990
- MoRJiangTDiSantoRTaiWGuZATP-triggered anticancer drug deliveryNat Commun20145336424618921
- WangGHuangGZhaoYATP triggered drug release and DNA co-delivery systems based on ATP responsive aptamers and polyethylenimine complexesJ Mater Chem B2016438323841
- HeXZhaoYHeDWangKXuFTangJATP-responsive controlled release system using aptamer-functionalized mesoporous silica nanoparticlesLangmuir20122835129091291522889263
- MoRJiangTSunWGuZATP-responsive DNA-graphene hybrid nanoaggregates for anticancer drug deliveryBiomaterials201550677425736497
- ZhuXZhangBYeZShiHShenYLiGAn ATP-responsive smart gate fabricated with a graphene oxide-aptamer-nanochannel architectureChem Commun2015514640643
- LiaoWCSohnYSRiutinMThe application of stimuli-responsive VEGF- and ATP-aptamer-based microcapsules for the controlled release of an anticancer drug, and the selective targeted cytotoxicity toward cancer cellsAdv Funct Mater20162642624273
- ZhaoPZhengMLuoZOxygen nanocarrier for combined cancer therapy: oxygen-boosted ATP-responsive chemotherapy with amplified ROS lethalityAdv Healthc Mater20165172161216727253453
- ZhangYLuYWangFATP/pH dual responsive nanoparticle with d-[des-Arg10]kallidin mediated efficient in vivo targeting drug deliverySmall20171331602494
- GribbleFMLoussouarnGTuckerSJZhaoCNicholsCGAshcroftFMA novel method for measurement of submembrane ATP concentrationJ Biol Chem200027539300463004910866996
- GormanMWFeiglEOBuffingtonCWHuman plasma ATP concentrationClin Chem200753231832517185366
- KimDJeongYYJonSA drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancerACS Nano2010473689369620550178
- XiaoZJiCShiJDNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapyAngew Chem Int Ed2012511185311857
- GodbeyWTWuKKMikosAGTracking the intracellular path of poly(ethylenimine)/DNA complexes for gene deliveryProc Natl Acad Sci U S A19999695177518110220439
- TianHLinLChenJChenXParkTGMaruyamaARGD targeting hyaluronic acid coating system for PEI-PBLG polycation gene carriersJ Control Release20111551475321281679
- TaghaviSHashemNiaAMosaffaFAskarianSAbnousKRamezaniMPreparation and evaluation of polyethylenimine-functionalized carbon nanotubes tagged with 5TR1 aptamer for targeted delivery of Bcl-xL shRNA into breast cancer cellsColloids Surf B Biointerfaces2016140283926731195
- TanakaAFukuokaYMorimotoYCancer cell death induced by the intracellular self-assembly of an enzyme-responsive supramolecular gelatorJ Am Chem Soc2015137277077525521540
- YangTJHaimovitz-FriedmanAVerheijMAnticancer therapy and apoptosis imagingExp Oncol201234326927623070012
- ZhangJWuDXingZN-Isopropylacrylamide-modified polyethylenimine-mediated p53 gene delivery to prevent the proliferation of cancer cellsColloids Surf B Biointerfaces2015129546225829127
- KirazYAdanAKartalMYMajor apoptotic mechanisms and genes involved in apoptosisTumour Biol2016378471848627059734