Abstract
Imidazoles and their derivatives are compounds with chemotherapeutic applications. In this study, we investigated the chemical functionalization of carboxylated multiwalled carbon nanotubes (MWNT–COOH) by 1,2-phenylendiamine. Multiwalled nanotube (MWNT)–benzimidazole was obtained by an MWNT–amide reaction with POCl3 after 72 hours, which was confirmed by Fourier transform infrared, scanning electron microscopy, thermal gravimetric analysis, and elemental analysis. These functionalizations were chosen due to -NH2 and NHCO active sites in MWNT–amide for future application. Toxicity assays with fibroblast cells and MTT test for measurement of viable cell numbers were also performed. Cellular results did not show any toxicity change in modified samples from that of the reference samples.
Introduction
Incorporation of imidazole and benzimidazole nuclei is an important synthetic strategy in drug discovery.Citation1 Observations suggest that substituted benzimidazoles and related heterocycles, which are the structural isosteres of nucleotides owing to fused heterocyclic nuclei in their structures, allow them to interact easily with biopolymers, and possess potential activity with lower toxicity in the chemotherapeutic approach in man.Citation2,Citation3 The high therapeutic properties of related drugs have encouraged medicinal chemists to synthesize a large number of novel chemotherapeutic agents. The anti-tumoral activity of benzimidazole and its components have been reported in several studies. Furthermore, there are clinical anticancer drugs, known as Hoechst-33258 and Hoechst-33342 dyes, which include a benzimidazole structure.Citation4,Citation5 Carbon nanotubes have been attracting increasing attention from chemists and scientists owing to their electronic, mechanical, optical, and chemical characteristics.Citation6–Citation8 Biomedical applications for MWNTs are being investigated actively because of their useful combination of size and physicochemical properties.Citation9–Citation13 In patients with cancer, MWNTs have potential roles in delivering pharmacologic agents, as diagnostic imaging agents, DNA, silent interfering RNA, oligonucleotides, and proteins to detect or treat cancerous cells.Citation14–Citation16 The application of functionalized carbon nanotubes as new nanovectors for drug delivery was apparent immediately after the first demonstration of the capacity of this material to penetrate into cells. Carbon nanotubes can be used to deliver their cargoes to cells and organs. Two papersCitation17,Citation18 detail the potential use of MWNTs to treat several types of cancer, with minimal or no toxic effects to normal cells.
Compounds such as imidazole are also well known antitumor agents.Citation19–Citation22 Carbon nanotubes also show good antitumoral effects. These materials can diffuse into cells and destroy DNA. Functionalization of carbon nanotubes with imidazole derivatives can leave mutual effects on cancerous cells. In recent years, chemical functionalization of carbon nanotubes has become more interesting because it allows modification of the nanotube surface for subsequent alignment. These surface modifications play an important role for application of nanotubes in composite sensors and many other fields. The chemical modifications of carbon nanotubes have been well summarized in several published review articles.Citation23–Citation29 Amines are among the reagents that have drawn the greatest attention. Haddon et al pioneered the approach of functionalizing the carboxylic groups of carbon nanotubes through amidation with amines bearing long alkyl chains.Citation30–Citation33 On the other hand, acid-chloride-functionalized carbon nanotubes are used for attaching glucosamineCitation34 and didecylamine.Citation35 Amidation of carbon nanotubes has also been carried out with aromatic amines.Citation36–Citation38 In this paper, we investigated the formation of a pentaheterocyclic, MWNT–benzimidazole, on MWNT nanotubes, in addition to developing the amidation of MWNT with bifunctional aromatic amines. Functionalized carbon nanotubes were characterized by Fourier transform infrared (FT-IR), scanning electron microscopy (SEM), thermal gravimetric analysis (TGA), elemental analysis, and toxicity assay.
Materials and methods
Preparation of MWNT–amide
MWNT–COOH 60 mg (20–30 nm; Netvino Co. Ltd) were sonicated in 90 mL of N,N-dimethyl formamide (DMF) for 45 minutes to give a homogeneous suspension. Oxalyl chloride (2.5 mL) was added dropwise to the MWNT suspension at 0°C under nitrogen. The mixture was stirred at 0°C for two hours and followed at room temperature for the same duration. Finally, the temperature was increased to 70°C and the mixture was stirred overnight to remove excess oxalyl chloride. 1,2-phenylendiamine 100 mg dissolved in DMF was added to the MWNT suspension and the mixture stirred at 95°C for 72 hours. After cooling to room temperature, the mixture was filtered and washed thoroughly with DMF, ethyl alcohol, and tetrahydrofuran. Subsequently, the black solid was vacuum-dried at room temperature for five hours.
Preparation of MWNT–benzimidazole
MWNT–amide 30 mg was mixed with 10 mL POCl3 and then stirred at 80°C for 72 hours. After cooling to room temperature, the reaction mixture was separated by centrifugation and washed thoroughly with tetrahydrofuran. Thereafter, the solid obtained was vacuum-dried for four hours.
Instrumental analysis
All reagents and solvents (oxalyl chloride, 1,2-phenylendiamine, phosphoryl trichloride, and DMF) were obtained from Merck Chemical Inc. (Darmstadt, Germany), and MWCNT–COOH (95% purity, 20–30 nm; Netvino Co. Ltd) were purchased and used as received. The FT-IR spectrum was recorded using KBr tablets on a Nexus 870 FT-IR spectrometer (Thermo Nicolet, Madison, WI). SEM was used to study the morphology of the WCNTs. SEM measurement was carried out on the XL30 electron microscope (Philips, Amsterdam, Netherlands). Elemental analyses of carbon, hydrogen, and nitrogen were performed using a Series Π 2400 (Perkin Elmer, Waltham, MA). The samples were investigated by TGA (DuPont Instrument 951; DuPont, Wilmington, DE) in air (10°C/min).
Cytotoxicity analysis
A fibroblast cell suspension (L929) from mouse tails was prepared according to ISO10993standards. The powders to control (TCPS) were well cleaned and sterilized by the autoclave method. Individual samples were placed in Petri dishes using a sterilized pincer; 3 cc of the cell suspension were removed by pipette and poured into the control and experimental samples. Thereafter, all of the samples were separately placed in a Memmert incubator at 37°C for 24 and 48 hours. The samples in the polystyrene Petri dish were removed from the incubator after 24 and 48 hours and studied using a Eclipse TS-100 photonic microscope (100 ×; Nikon, Tokyo, Japan). Cell proliferation was determined by MTT assay for viable cell numbers. The MTT tetrazolium compound is reduced by living cells into a colored formazan product that is soluble in tissue culture medium. The quantity of formazan product is directly proportional to the number of viable cells in the culture. The assays were performed by adding 1 mL of MTT solution (Sigma, St. Louis, MO) and 9 mL fresh medium to each well after aspirating the spent medium and incubating at 37°C for four hours with protection from light. Colorimetric measurement of formazan dye was performed at a wavelength of 570 nm using a Rayto microplate reader.
Results
Elemental analyses of the modified MWNT 1–3 are shown in . Apart from the carbon values, the atomic percentages of hydrogen 1.31% and nitrogen 2.09% of 2 (as compared with 1) indicated that 1 is functionalized with 1,2-phenylendiamine. On the other hand, the percentage decrease in hydrogen from 1.31% to 0.96% confirms the five-ring formation. Based on these data, coupled with the assumption that the atomic percentages of nitrogen and hydrogen originated from the aromatic amine without elimination, we confirmed the functionalization of MWNT–COOH with 1,2-phenylendiamine. shows the synthesis route of the modified MWNT–COOH.
Table 1 Elemental analysis of the modified multiwalled carbon nanotubes (MWNT)
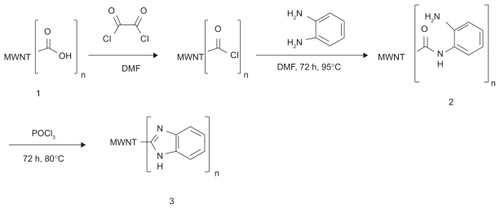
shows the FT-IR spectrum of the modified MWNTs. In spectrum 1, the band at around 1550 cm corresponds to the stretching mode of the C = C double bond that forms the framework of the carbon nanotube sidewall.Citation24,Citation25 The peak at 1730 and 1045 cm apparently corresponds to the stretching modes of the carboxylic acid groups.Citation26 The two bands at 2800–2950 cm which are seen in all spectra are attributed to CH stretching of MWNT–COOH defects. In spectrum 2, the new strong peaks at 3200–3500 can be assigned to the N-H, NH2, and OH stretching modes. The carbonyl peak in the spectrum 2 shift to 1654 cm (as compared with 1730 cm in spectrum 1) is a result of amide C (= O) NH linkage formation. The other peak at around 1610 cm can be assigned to the NH2 scissoring modeCitation27 in 2 that over-laps with the C = C mode. The peaks at around 1520–1590, 1400–1480, 1200–1350, and 1100 cm correspond to C = C stretching nanotubes, aromatic ring modes, and C-N and C-O stretching modes, respectively. In spectra 3, the peaks of the amide group disappear and three peaks at around 1580, 620, and 1150 cm appear which can be assigned to imidazole ring stretching.Citation28 The two peaks at 1710 and 1700 cm in spectra 2 and 3, respectively, can be related to residual carboxylic acids on the nanotube. Thus, FT-IR spectra confirmed that MWNT–COOH had been successfully modified by an aromatic amine.
Figure 2 Fourier transform spectra (after baseline correction) of functionalized carbon nanotubes. 1. carboxylated multiwalled carbon nanotubes, 2. multiwalled amide nanotubes, and 3. multiwalled benzimidazole nanotubes.
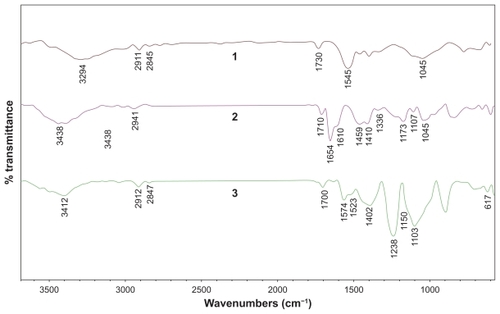
Raman spectroscopy is a powerful tool used to provide structural information about MWNT–COOH before and after functionalization. As shown in , the D and G bands of the MWNT at around 1348 and 1578 cm, attributed to defects, disorder-induced peaks, and tangential- mode peaks,Citation29–Citation31 can be clearly observed for both MWNT–COOH and MWNT–benzimidazole. Additionally, the intensity ratio (ID/IG) of the D and G bands for MWNT–benzimidazole is 1.15, which is greater than that for MWNT–COOH (0.64). The increase in intensity of the defect mode at 1348 cm was related to spCitation3 hybridization of carbon, and is used as an evidence of the disruption of the aromatic system of π electrons by the attached molecules.Citation29
Figure 3 Raman spectra of carboxylated multiwalled carbon nanotubes and benzimidazole multiwalled carbon nanotubes.
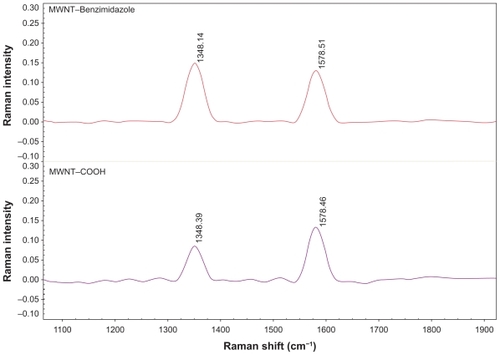
More direct evidence for the functionalization of MWNTs comes from the SEM images. indicates that the MWNT–COOH has a smooth surface. The changes in morphology are remarkable. A uniform tubular layer due to a covalently bonded aromatic amine on the surface of the MWNT (the rough part) is observable. It seems that the diameters of MWNT–benzimidazole are slightly increased in comparison with MWNT–COOH. This may be due to interaction between the grafted molecules of benzimidazole. The third structure is quite different from those of the starting MWNT–COOH, in which the tube surface is relatively smooth and clean, as shown in .
Figure 4 Scanning electron microscopy images of A carboxylated multiwalled carbon nanotubes and B benzimidazole multiwalled carbon nanotubes.
provides quantitative information on nanotube functionalization from the TGA results. In TGA graphs of MWNT–amide and MWNT–benzimidazole, two distinct decompositions are observable. The first one (below 360°C) can be assigned to the aromatic amine and benzimidazole of MWNT–amide and MWNT–benzimidazole, respectively, while the second one (above 360°C) is related to the nanotube as compared with the MWNT–COOH thermogram. If the mass loss of the MWNT–COOH at 360°C (0.88%) is used as the reference, the mass loss of functionalized MWNT by 1,2-phenylendiamine and benzimidazole of MWNT–amide and MWNT–benzimidazole at 360°C is about 19% and 11.4%, respectively. These results indicate that there is one amide group for MWNT–amide per 47.9 and one benzimidazole group for MWNT–benzimidazole per 75.8 carbon atoms of MWNT, respectively. shows cellular images for this sample. Half of the cells in all three cases live. MTT analysis was also corroborated, as shown in .
Figure 5 Thermal gravimetric analysis curves of modified multiwalled carbon nanotubes in air (10°C/min).
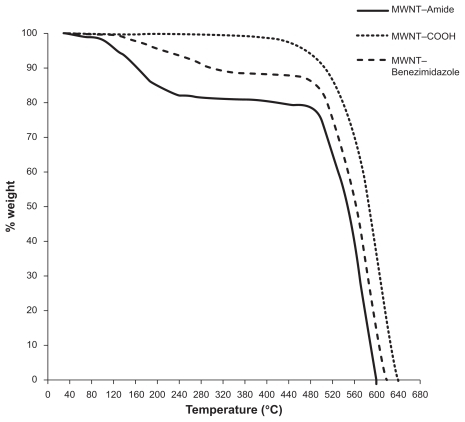
Figure 6 Cell growth on the samples. Control A) carboxylated multiwalled carbon nanotubes B) multiwalled benzimidazole carbon nanotubes C) and multiwalled amide carbon nanotubes D).
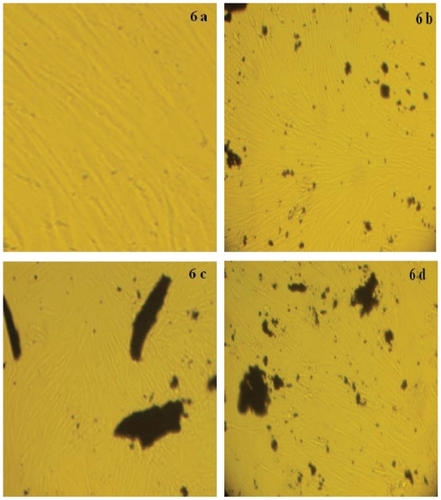
Table 2 MTT analysis of the samples
Conclusion
In summary, we have introduced benzimidazole groups onto the surface of nanotubes via reaction of MWNT–amide with POCl3. Functionalization was demonstrated by their SEM images, as well as FT-IR, elemental analysis, and TGA. The results show successful functional groups and no change in toxicity in functional samples compared with the primary sample, with half of the cell growth on modified samples. In subsequent studies, antitumoral investigations of modified samples will be evaluated.
Acknowledgment
Funding for this research was provided by the Research Vice Presidency of Science and Research Branch, Islamic Azad University and Iranian Nanotechnology Initiative (Government of Iran).
Disclosure
The authors report no conflicts of interest in this work.
References
- TownsendLBImidazole nucleosides and nucleotidesChem Rev1967675335634863398
- HaugwitzRDAngelRGJacobsGAAntiparasitic agents. 5. Synthesis and anthelmintic activities of novel 2-heteroaromatic-substituted iso-thiocyanatobenzoxazoles and benzothiazolesJ Med Chem1982259699747120286
- HisanoTIchikawaMTsumotoKTasakiMSynthesis of benzoxazoles, benzothiazoles and benzimidazoles and evaluation of their antifungal, insecticidal and herbicidal activitiesChem Pharm Bull19823029963004
- KamalARamulPSrinivasQRameshGKumarPPSynthesis of C8-linked pyrrolo[2,1-c][1,4]benzodiazepine-benzimidazole conjugates with remarkable DNA-binding affinityBioorg Med Chem Lett2004144791479415324909
- AlperSTemiz-ArpaciOAki-SenerEYalcinISome new bi-and ter-benzimidazole derivatives as topoisomerase I inhibitorsFarmaco20035849750712818688
- BonardJ-MKindHStöckliTNilssonLOField emission from carbon nanotubes: The first five yearsSolid State Electron200145893914
- BaughmanRHZakhidovAAde HeerWACarbon nanotubes – the route toward applicationsScience200229778779212161643
- AvourisPHCarbon nanotube electronicsChem Phys2002281429
- IijimaSHelical microtubules of graphitic carbonNature19913545658
- BachtoldAFuhrerMSPlyasunovSScanned probe microscopy of electronic transport in carbon nanotubesPhys Rev Lett20008426 Pt 16082608510991129
- McEuenPLFuhrerMSParkHSingle-walled carbon nanotube electronicsIEEE Trans Nanotech2002178185
- DurkopTGettySACobasEFuhrerMSExtraordinary mobility in semiconducting carbon nanotubesNano Lett200443539
- BachiloSMStranoMSKittrellCStructure-assigned optical spectra of single-walled carbon nanotubesScience20022982361236612459549
- ZhangZYangXZhangYDelivery of telomerase reverse transcriptase small interfering RNA in complex with positively charged single-walled nanotubes suppresses tumor growthClin Cancer Res2006124933493916914582
- KamNWO’ConnellMWisdomJADaiHCarbon nanotubes as multifunctional biological transporters and nearinfrared agents for selective cancer cell destructionProc Natl Acad Sci U S A2005102116001160516087878
- KamNWLiuZDaiHFunctionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencingJ Am Chem Soc2005127124921249316144388
- GannonGICarbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency fieldCancer20071102654265517960610
- LiuZSunXNakayama-RatchfordNDaiHThe supramolecular chemistry of organic-inorganic hybrid materialsACS Nano20071505619203129
- SavaGBergamoAZorzetSGavaBCasarsaCInfluence of chemical stability on the activity of the antimetastasis ruthenium compound NAMI-AEur J Cancer20023842743511818210
- KepplerBKWeheDEndersHRuppWSyntheisis, antitumor activity, and X-ray structure of bis (imidazolium) imidazole-pentachloro ruthenate (III), (ImH)2 (RuImCl5)Inorg Chem198726844846
- KepplerBKRuppWJuhlUMEndresHNieuRBlazerWSSynthesis, molecular structure and tumor-inhibiting property of imidazolium-transbis(imidazole) tetra chlororuthenate (III) and its methyl substituted derivativesInorg Chem19872643664370
- SavaGGangliyardiRBergamoAAlessioEMestroniGTreatment of metastases of solid mouse tumors by NAMI-A; comparison with cisplatin, cyclophosphamide and decarbazineAnticancer Res19991996997210368640
- NiyogiSHamonMAHuHZhaoBBhowmikPSenRChemistry of single-walled carbon nanotubesAcc Chem Res2002351105111312484799
- HuC-YXuY-JDuoS-WZhangR-FLiMSOne-step electrodeposited carbon nanotube/zirconia/myoglobin filmJ Chin Chem Soc200956234238
- HolzingerMVostrowskyOHirschAHennrichFKappesMWeissRSidewall functionalization of carbon nanotubes. This work was supported by the European Union under the 5th Framework Research Training Network 1999, HPRNT 1999–00011 FUNCARSAngew Chem Int Ed Engl2001404002400512404474
- SunYPFuKLinYHuangWFunctionalized carbon nano-tubes: Properties and applicationsAcc Chem Res2002351096110412484798
- BanerjeeSHemraj-BennyTWongSSCovalent surface chemistry of single-walled carbon nanotubesAdv Mater2005171729
- BanerjeeSKahnMGCWongSSRational chemical strategies for carbon nanotube functionalizationChem Eur J2003918981908
- HirschAFunctionalization of single-walled carbon nanotubesAngew Chem Int Ed Engl20024185385912491358
- HamonMAChenJHuHDissolution of single-walled carbon nanotubesAdv Mater1999118348340
- HamonMAHuHBhowmikPEnd-group and defect analysis of soluble single-walled carbon nanotubesChem Phys Lett2001347348
- ChenJHamonMAHuHSolution properties of single-walled carbon nanotubesScience199828295989756485
- ZhaoBHuHNiyogiSChromatographic purification and properties of soluble single-walled carbon nanotubesJ Am Chem Soc200112311673116711716724
- PompeoFResascoDEWater solubilization of single-walled carbon nanotubes by functionalization with glucosamineNano Lett20022369373
- LiuLZhangSHuTSolubilized multi-walled carbon nanotubes with broadband optical limiting effectChem Phys Lett2002359191195
- ChenW-YChenC-YHsuK-YWangC-CLingYCReaction monitoring of polyaniline film formation on carbon nanotubes with TOF-SIMSAppl Surf Sci20042312845849
- D’EsteMNardiMde MennaEA co-functionalization approach to soluble and functional single-walled carbon nanotubesEur J Org Chem20061125172522
- KirikovaMNIvanovASSavilovSVLuninVVModification of multi-walled carbon nanotubes by carboxy groups and determination of the degree of functionalizationRuss Chem Bull Int Ed2008572298303