Abstract
Titanium and titanium alloy are widely used as orthopedic implants for their favorable mechanical properties and satisfactory biocompatibility. The aim of the present study was to investigate the antibacterial effect and bone cell biocompatibility of a novel implant made with nanotubular anodized titanium coated with gentamicin (NTATi-G) through in vivo study in rabbits. The animals were divided into four groups, each receiving different kinds of implants, that is, NTATi-G, titanium coated with gentamicin (Ti-G), nanotubular anodized titanium uncoated with gentamicin (NTATi) and titanium uncoated with gentamicin (Ti). The results showed that NTATi-G implant prevented implant-related osteomyelitis and enhanced bone biocompatibility in vivo. Moreover, the body temperature of rabbits in NTATi-G and Ti-G groups was lower than those in Ti groups, while the weight of rabbits in NTATi-G and Ti-G groups was heavier than those in NTATi and Ti groups, respectively. White blood cell counts in NTATi-G group were lower than NTATi and Ti groups. Features of myelitis were observed by X-ray films in the NTATi and Ti groups, but not in the NTATi-G and Ti-G groups. The radiographic scores, which assessed pathology and histopathology in bone tissues, were significantly lower in the NTATi-G and Ti-G groups than those in the NTATi and Ti groups, respectively (P<0.05). Meanwhile, explants and bone tissue culture demonstrated significantly less bacterial growth in the NTATi-G and Ti-G groups than in the NTATi and Ti groups, respectively (P<0.01). The bone volume in NTATi-G group was greater than Ti-G group, and little bone formation was seen in NTATi and Ti groups.
Introduction
Osteoarthritis (OA) is one of the most common causes of chronic joint pain and functional disability, which has become a major public health issue globally.Citation1,Citation2 Since the first report of total knee arthroplasty (TKA) in the 1960s,Citation3 TKA and total hip arthroplasty (THA) have been demonstrated to be the most reliable surgical procedures to relieve pain and improve rehabilitation in patients with serious OA. Infection and aseptic loosening are the two major causes of surgical failure, with reported incidences of 25.2% and 16.1%, respectively, in the US, and 38% and 33%, respectively, in South Korea.Citation4,Citation5 On the other hand, poor biocompatibility of the prosthesis with surrounding bone tissues or stress shielding is the main culprit for aseptic loosening, which may subsequently lead to bacterial contamination and infection. Implant removal and revision are the major therapeutic methods for patients suffering from infection or aseptic loosening after primary TKA or THA. The longevity after revision is shortened than that after primary surgeries.Citation6 Meanwhile, orthopedic surgical-site infection prolongs hospital stay by a median of 2 weeks, doubles readmission rate and triples hospital care costs.Citation7 Therefore, it is important to prevent infection and improve biocompatibility of prosthesis during primary TKA and THA.
Various strategies have been developed to minimize the risk of infection in prosthetic joint surgery, such as ultraclean operation rooms and standardized surgical techniques, and prosthetic devices with improved designs are adopted to shorten operation time.Citation8–Citation11 However, infection remains to be prevalent with adverse impacts on the prognosis of patients undergoing joint replacement surgeries. Systemic antibiotic treatment has been the mainstay strategy to prevent and cure infections despite its various drawbacks such as poor penetration into the surgical site due to ischemic and necrotic nature of the posttraumatic and postoperative tissue, and adverse effects such as liver- and nephrotoxicity.Citation12,Citation13 Meanwhile, topical antibiotic administration by alternative local delivery may be a potential approach to maintain high local concentration without systemic toxicity.Citation14 Bacterial adhesion plays a critical role in biofilm formation at the implantation site, which may subsequently lead to local infection. Various antibiotic-eluting devices and materials have been reported in joint arthroplasty, such as nondegradable materials like spacer beads and polymethylmethacrylate bone cements, titanium implants,Citation15–Citation17 biodegradable materials such as poly-(lactide-co-glycolide) copolymers,Citation18–Citation20 polycaprolactone,Citation21,Citation22 polyanhydrides,Citation23,Citation24 polyhydroxybutyrate-co-hydroxyvalerateCitation25,Citation26 and polyhy-droxyalkanoatesCitation27 and natural polymers such as collagenCitation28,Citation29 and chitosan.Citation30,Citation31
Titanium and titanium alloys are widely used as orthopedic implants due to their ideal mechanical properties and satisfactory biocompatibility. The adoption of nanotechnology has promoted the development of novel orthopedic implant materials with ideal properties, such as better cytocompatibility and the ability to be used as specific drug delivery platforms.Citation32 In vitro studies on nanotubular anodized titanium uncoated with gentamicin (NTATi) were performed to examine its bio-compatibility and antibacterial effects using physical drug-loading method, and results indicated that NTATi surface could improve osteogenic activity, and gentamicin coating could lead to significantly improved antibacterial ability. To prolong the duration of continuous antibiotic delivery from NTATi, simulated body fluid (SBF) was used to mix the drug molecules to facilitate their precipitation with calcium phosphate crystals on the surface of NTATi. Results showed that such coprecipitation method could promote continuous drug release for up to 3 weeks.Citation32 To our knowledge, no in vivo study has been performed previously to demonstrate the cytocompatibility properties of NTATi and the effect of NTATi loaded with antibiotics on the prevention of implantation-site infection. The present study aimed to investigate using a coprecipitation drug-loading approach the effects of nanotubular anodized titanium coated with gentamicin (NTATi-G) on infection prevention and bone cell biocompatibility in a rabbit model with Staphylococcus aureus inoculation in the tibial metaphysis.
Materials and methods
Preparation of implant materials
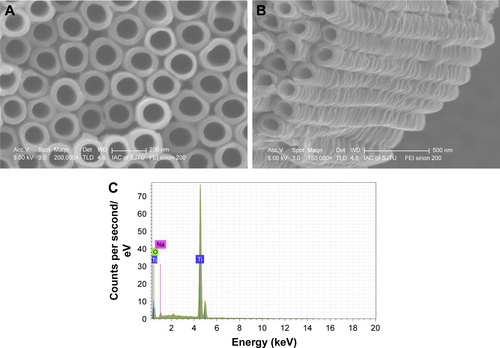
Two implant materials, pure titanium and NTATi, were used in this study. All implant materials were provided by Shanghai Institute of Ceramics, Chinese Academy of Sciences. Pure titanium uncoated with gentamicin (Ti) of 99.5% purity (0.25×0.25 cm) was sonicated with acetone to remove surface oil pollution, and the nanotube arrays were prepared by an electrochemical anodization method. The anodization process was performed employing a two-electrode system; pure Ti served as the anode, and Pt sheet served as the cathode. NH4F (0.09 M) in ethylene glycol solution containing 10 vol% water was used as the electrolyte solution.Citation33 After anodization for 30 min at a constant voltage of 60 V, an NTATi was obtained with a length of 1.05 µm (Figure S1B), an inner diameter of 125 nm and an outside diameter of 170 nm (Figure S1A). An energy dispersive spectrometer attached to the scanning electron microscopy system was used to determine the surface chemistry of the nanotubular anodized titanium. The energy dispersive spectrum of NTATi samples revealed the existence of Ti, O and Na; atom% of Ti:O was about 1:2 (Figure S1C). The characterization of the morphology and chemical composition of the anodized titanium are presented in Figure S1. Gentamicin sulfate (Sangon Biotech A100304-0001) of 10 mg/mL was loaded on implant materials by coprecipitation approach as previously described.Citation32 After anodization, NaOH soaking and heat treatment of the implant materials, the samples were soaked into 1.5× SBF mixed with gentamicin sulfate (10 mg/mL) and incubated for 3 days.Citation32
Preparation of bacteria
S. aureus subsp. aureus Rosenbach (ATCC 25923; American Type Culture Collection, Manassas, VA, USA) was used in this study. The strain is sensitive to gentamicin, erythromycin, ampicillin and cephalexin.
S. aureus was cultured overnight in tryptic soy broth (TSB) (9 mL, caseinpepton–soybean flour–pepton solution; Oxoid Ltd., Basingstoke, UK). The suspension of 100 µL was then transferred into 3-mL sterile tubes and incubated for 3 hours in 37°C incubator to obtain log-phase growth. After incubation, these tubes were centrifuged for 10 min at 3,000 rpm, and the supernatant was disposed. The bacterial sediment was washed three times with phosphate-buffered saline (PBS) and resuspended in PBS. The concentration of the bacteria was adjusted to 3×109 colony-forming units (CFU)/mL with TSB according to the McFarland methodCitation34 for the use in the following procedures.
Animals and surgical procedures
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Second Military Medical University. All operations in this study were performed according to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health of the US.
A total of 36 healthy New Zealand White rabbits with body weight ranging between 2.2 and 2.8 kg were selected for the present study. Each rabbit was kept in separate cage and fed with commercial pelleted diet.
After anesthesia with mainline pentobarbital (30 mg/kg) induction and sterilization, an incision was made at the proximal tibia of the left hind leg. A 2-mm soteotomy was introduced into the intramedullary cavity after a 3- to 5-mm bone slot was made. Bone marrow (1–1.5 mL) was aspirated to obtain sufficient space to insert the implant material. S. aureus (0.1 mL PBS with 3×109 CFU/mL) was then injected into the intramedullary cavity, and bone wax was used to close the bone slot. The fascia and skin were closed with suture after soft tissues were irrigated with entoiodine.
Animals were divided into the following groups, with each group having eight animals:
Group I NTATi-G +3×109 CFU/mL S. aureus 0.1 mL
Group II Titanium coated with gentamicin (Ti-G) +3×109 CFU/mL S. aureus 0.1 mL
Group III NTATi +3×109 CFU/mL S. aureus 0.1 mL
Group IV Ti +3×109 CFU/mL S. aureus 0.1 mL.
Measurements of body temperature, weight and white blood cell (WBC)
Body temperature, body weight and WBC were measured on days 0, 3, 7, 14, 21, 28, 35 and 42 postoperatively. Digital thermometer (Terumo, Zhejiang, People’s Republic of China) and precision scale (TCS, Shanghai, People’s Republic of China) were used to measure the body temperature and weight, respectively. Blood was drawn from the ear veins, and WBC count was measured. Operated rabbits were inspected for clinical signs of infection (appearance of the wound, status of the soft tissues at the entry site of implants, and presence of joint effusion or loss of mobility).
Radiographic evaluation
Anterior–posterior- and lateral-view X-rays of the operative limbs were taken on days 0, 7, 14, 21, 28, 35 and 42 after surgery, using a Mobilett Plus X-ray unit (Siemens AG, Munich, Germany) and digital films (DLR Cassette, Digiscan 2H/2C; Siemens).
Three senior radiologists blinded of the grouping status independently evaluated radiographs taken on days 0, 21 and 42, and assessed the severity of infection according to a modified scoring criteria by An and Friedman:Citation35 1) general impression, 2) periosteal reaction, 3) soft tissue swelling, 4) osteolysis, 5) deformity, 6) sequestrum formation and 7) spontaneous fracture. Criteria 1–5 were judged as 0 (absent), 1 (mild), 2 (moderate) or 3 (severe), whereas criteria 6 and 7 were judged with 0 (absent) or 1 (present). The maximum score was 17.
Sacrifice of rabbits
Rabbits were sacrificed 6 weeks postoperatively through air injection. The proximal end of tibias containing implant materials was harvested and weighed using a precision scale under sterile conditions, which was subsequently used for microbiological and histological evaluations.
Microbiological evaluation
One tibia with implants was randomly chosen from animals in each group to assess the microbiological status. The implant materials were explanted and rolled over in the agar (Columbia® full blood; Becton Dickinson Microbiology Europe, Meylan Cedex, France) before being placed into the 2.5-mL sterile TSB. Both agar plates were incubated with TSB for 24 h in the 37°C incubator. Colonies of bacteria formed on the agar plates were counted, and bacterial growth in TSB was evaluated (cloudy appearance represents positive growth, while clear appearance represents no growth) after 24 h of incubation.
Tibial bone tissues were frozen with liquid nitrogen and pulverized with a bone mill (Tissuelyser-48; Jingxin Tech, Shanghai, People’s Republic of China) under sterile conditions. Powder of bone tissue (500 mg) was added into sterile PBS (6 mL) and agitated for 2 min by vortex mixing. The suspension was centrifuged for 10 s at 10,000 rpm, and supernatant of 100 µL was withdrawn and serially diluted to 10-fold. The supernatants were plated in triplicate on agar and incubated at 37°C for 24 h. The CFU on agar was counted, and the ratio between bacterial level and sample weight (CFU/g bone tissue) was calculated.
Histological and histopathological evaluation
All the remaining tibial tissues were split into two parts for histological and histopathological evaluations, respectively.
The samples were fixed for 24 h in 4% formaldehyde, dehydrated in alcohol solution of different concentration gradient, transparentized in xylene and embedded in methylmethacrylate (Technovit 9100; Heraeus, Hanau, Germany) for histological evaluation. Longitudinal sections perpendicular to the implants were cut at 300-µm thickness by a Leica SM 2500S microtome (Leica, Wetzlar, Germany) with a 40° stainless steel knife before toluidine blue staining. Stained slices were then used for histological evaluation according to the criteria described by Petty et al:Citation44 1) abscess formation, 2) sequestrum formation, 3) enlargement of corticalis, 4) destruction of corticalis and 5) general impression. Parameters 1–4 were judged as absent (0) or present (1), while parameter 5 was judged as absent (0), mild (1) or severe (2). Scoring was done by three independent observers in a blinded manner with the maximum score of 6.
Samples taken for histopathology were fixed for 24 h with 4% formaldehyde and decalcified in EDTA for 28 days.Citation36 The bone tissues were then embedded in paraffin and cut into 5-µm longitudinal sections with a microtome (CUT 6062; SLEE Medical, Mainz, Germany). The sections were then stained with hematoxylin and eosin and evaluated for histopathological changes according to the scoring method described by Smeltzer et al.Citation37 The main factors taken into account were intraosseous chronic inflammation (ICI), intraosseous acute inflammation (IAI), periosteal inflammation (PI) and bone necrosis (BN).Citation37 The criteria for histological parameters and scoring system are shown in detail in the research by Smeltzer et al.Citation37
Statistical analysis
Statistical analysis was conducted by SPSS 16.0 software (SPSS for Windows, version 16.0; SPSS Inc., Chicago, IL, USA). All data were expressed as mean ± standard deviation. Differences among four groups in body temperature, body weight, WBC count and radiographic and histological scores were analyzed by one-way analysis of variance. Differences between two groups were compared by the LSD-t test when the data were normally distributed. Differences in microbiological outcomes among four groups were analyzed using Mann–Whitney U test with balancing with Bonferroni–Holm test if two or more groups were compared. P<0.05 was considered statistically significant.
Results
Clinical evaluation
Two rabbits died during anesthesia on day 0, and another two in Ti group died on days 5 and 13 due to diarrhea and severe infection, respectively. Dead rabbits were replaced. The remaining rabbits tolerated the operation without life-threatening conditions. A total of five rabbits in NTATi group and four rabbits in Ti group had surgical-site infection, while animals in NTATi-G and Ti-G groups did not present signs of local infection.
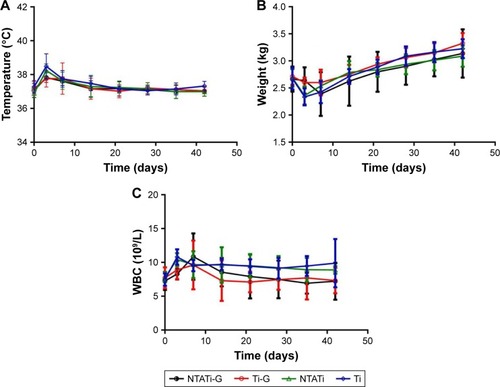
The body temperature in all four groups followed a similar pattern: It gradually increased from day 1 to day 3, followed by gradual decrease from day 3 to day 14 and maintained at a stable level from day 14 to day 42. The body temperature of rabbits in NTATi-G and Ti-G groups was lower than those in Ti groups on day 3 (P<0.05), and there was no difference among four groups from day 3 to day 42 (). The body weights of the rabbits were the lowest on day 3, and rabbits in NTATi-G and Ti-G groups were heavier than those in NTATi and Ti groups on day 3 (P<0.05). From day 3 to day 42, the body weights increased gradually, and there was no difference among four groups (). WBC of rabbits in NTATi and Ti group exhibited a gradually increasing pattern from day 1 to day 3 followed by a gradual decrease from day 3 to day 42, while in NTATi-G and Ti-G groups, WBC increased gradually from day 1 to day 7 and decreased gradually from day 7 to day 42. In general, the WBC count in NTATi-G group was less than that in NTATi and Ti groups on days 3, 35 and 42, while WBC in Ti-G group was less than that in NTATi and Ti groups on days 3, 21 and 42 (P<0.05) ().
Figure 1 Change patterns of the body temperature, weight and WBC in rabbits of all four groups from day 0 to day 42. (A) The body temperature increased gradually from day 1 to day 3, decreased gradually from day 3 to day 14 and remained stable from day 14 to day 42. The body temperature of NTATi-G and Ti-G groups was lower than Ti groups on day 3 (P<0.05), and no significant difference was found among four groups from day 3 to day 42. (B) The weight of rabbits decreased from day 1 to day 3 and reached the lowest on day 3. Rabbits in NTATi-G and Ti-G groups were heavier on day 3 than NTATi and Ti groups (P<0.05). The weight increased gradually afterward, and there was no difference among four groups from day 3 to day 42. (C) The WBC in NTATi and Ti groups increased gradually from day 1 to day 3 and decreased gradually from day 3 to day 42, whereas in NTATi-G and Ti-G group, WBC increased gradually from day 1 to day 7 and decreased gradually from day 7 to day 42. Besides, the WBC count in NTATi-G was significantly less than in NTATi and Ti groups on days 3, 35 and 42 (P<0.05), and WBC count in Ti-G group was less than in NTATi and Ti groups on days 3, 21 and 42 (P<0.05).
Abbreviations: WBC, white blood cell; NTATi-G, nanotubular anodized titanium coated with gentamicin; Ti-G, titanium coated with gentamicin; NTATi, nanotubular anodized titanium uncoated with gentamicin; Ti, titanium uncoated with gentamicin.
Radiograph evaluation
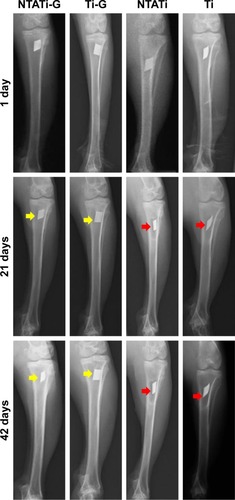
Abnormal radiographic signs such as osteolysis, periosteal reaction, soft tissue swelling and deformity were observed in NTATi and Ti groups on day 21, and these signs became more obvious on day 42 after surgery. In contrast, spontaneous fracture and sequestrum formation were not observed in these groups (). No apparent signs of osteomyelitis were noticed on day 21, while slight periosteal reaction, osteolysis and soft tissue swelling were observed in some rabbits on day 42 in NTATi-G and Ti-G groups after surgery ().
Figure 2 Representative radiographs of four experimental groups on days 1, 21 and 42. Periosteal reaction, osteolysis, soft tissue swelling and deformity were observed in NTATi and Ti groups on day 21 and became more obvious on day 42 after surgery; meanwhile, spontaneous fracture and sequestrum formation were not observed in these groups. We did not observe obvious radiography signs of osteomyelitis on day 21, but slight periosteal reaction, osteolysis or soft tissue swelling was observed in some rabbits on day 42 in NTATi-G and Ti-G groups after surgery.
Abbreviations: NTATi-G, nanotubular anodized titanium coated with gentamicin; Ti-G, titanium coated with gentamicin; NTATi, nanotubular anodized titanium uncoated with gentamicin; Ti, titanium uncoated with gentamicin.
Radiographic features in all groups were quantified and analyzed according to a modified score by An and Friedman.Citation35 The radiographic scores increased gradually with time after surgery in all four groups. The mean scores of NTATi-G, Ti-G, NTATi and Ti were 1.88±0.99, 2.13±0.64, 5±1.78 and 5.62±2 on day 21, respectively, and the mean scores in NTATi-G and Ti-G groups were lower than that in NTATi and Ti groups (P<0.01). The mean scores of NTATi-G, Ti-G, NTATi and Ti were 2.12±0.51, 2.5±0.7, 7.75±0.98 and 8±2.33 on day 42, respectively. Specifically, the mean scores of NTATi-G and Ti-G groups were lower than NTATi and Ti groups (P<0.01). Relevant data are shown in .
Table 1 Mean score of radiographic assessment on days 0, 21 and 42
Microbiological evaluation
Cultures of implants
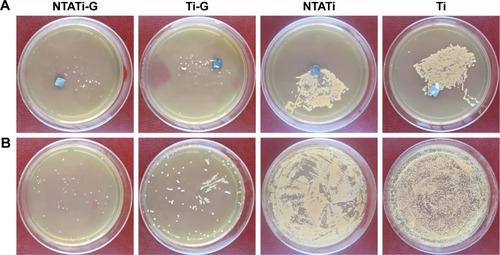
After being detached and rolled over on agar plates, implants were cultured in TSB. The CFU on each agar plate were counted after 24 h of incubation. Bacterial colony formation was noticed on agar plates of all four groups. The average CFU counts in NTATi-G and Ti-G groups were 40.5±12.36 and 73.75±10.69, respectively. The implant cultures in NTATi and Ti groups showed substantial bacterial growth with average CFU counts of more than 1,000, which was significantly higher than that in NTATi-G and Ti-G groups. All these results were corresponding to the implants immersed in TSB; all implants TSB cultures were positive (cloudy appearance) ( and ).
Table 2 Microbiological results and bone weight determined on the day of sacrifice
Figure 3 Results of microbiological evaluation in four groups on day 42. (A) Bacterial colony formation of implants on agar plates. (B) Bacterial colony formation of bone tissues on agar plates.
Abbreviations: NTATi-G, nanotubular anodized titanium coated with gentamicin; Ti-G, titanium coated with gentamicin; NTATi, nanotubular anodized titanium uncoated with gentamicin; Ti, titanium uncoated with gentamicin.
CFU/g bone
The average CFU/g ratios in NTATi-G, Ti-G, NTATi and Ti groups were 7.08±2.5×103, 1.95±0.21×103, 3.15±0.82×106 and 3.48±0.49×106, respectively. Bacterial loads in the NTATi-G and Ti-G groups were significantly lower than those in NTATi and Ti groups ().
Histological and histopathological results
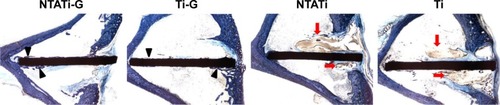
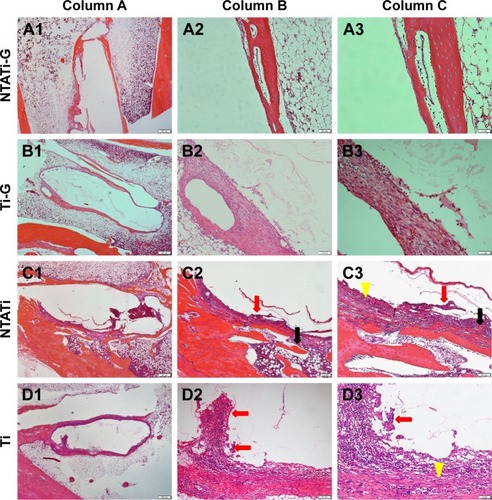
Histological images from different groups are shown in . Signs of infections (development of abscesses, cortical bone destruction, cancellous bone and periosteal new bone formation) were evaluated in NTATi and Ti groups, while NTATi-G and Ti-G groups showed no signs of apparent bone infection. The bone volume was greater in NTATi-G group compared to Ti-G group, whereas little bone formation was seen in NTATi and Ti groups. The quantitative histological scores of NTATi-G, Ti-G, NTATi and Ti groups were 2.25±0.5, 2.5±0.58, 3.5±0.58 and 3.25±0.5, respectively. The histological scores of NTATi-G and Ti-G groups were significantly lower than NTATi and Ti groups, respectively (P<0.05). No significant difference was found between NTATi-G and Ti-G groups, as well as between NTATi and Ti groups.
Figure 4 Photomicrographs of longitudinal sections from proximal tibiae, with toluidine blue staining. The bone volume (black arrowheads) in NTATi-G group was greater than Ti-G group; meanwhile, there was little bone formation in NTATi and Ti groups. Typical signs of infections (red arrows) were observed in NTATi and Ti histological slices, while NTATi-G and Ti-G groups showed no signs of apparent bone infection.
Abbreviations: NTATi-G, nanotubular anodized titanium coated with gentamicin; Ti-G, titanium coated with gentamicin; NTATi, nanotubular anodized titanium uncoated with gentamicin; Ti, titanium uncoated with gentamicin.
The histopathological results of all groups are shown in . Quantitative pathological scores are presented in . The mean total scores were 3.5±1, 4.5±1.3, 11.25±2.06 and 11±0.82 in the NTATi-G, Ti-G, NTATi and Ti groups, respectively. Specifically, the mean IAI scores of NTATi-G and Ti-G groups (1±0.82 and 1.25±0.25, respectively) were significantly lower than those in NTATi and Ti groups (2.75±0.96 and 2.5±0.58, respectively, P<0.05); Similarly, the mean ICI scores of NTATi-G and Ti-G groups (1.25±0.5 and 1.25±0.96, respectively) were significantly lower than those in NTATi and Ti groups (3±0.7 and 2.75±0.5, respectively, P<0.05). The mean PI scores of NTATi-G and Ti-G groups (0.75±0.5 and 1.25±0.96, respectively) were significantly lower than those in NTATi and Ti groups (2.75±0.96 and 2.75±0.96, respectively, P<0.05). The mean BN scores of NTATi-G and Ti-G groups (0.50±0.58 and 0.75±0.5, respectively) were significantly lower than those in NTATi and Ti groups (2.75±0.5 and 3.00±0.82, respectively, P<0.001). No significant difference was found between NTATi-G and Ti-G groups, as well as between NTATi and Ti groups.
Table 3 Mean pathological scores on the day of sacrifice
Figure 5 Photomicrographs of longitudinal sections from the proximal tibiae, with hematoxylin and eosin staining. Column A (A1–D1), overview of signs of osteomyelitis in the proximal metaphysis. Magnification, ×20. Column B (A2–D2), moderate-to-severe inflammation with massive enlargement and destruction of the bone tissue (black arrow) and chronic inflammatory cells (red arrows) in the NTATi and Ti groups and mild inflammation in the NTATi-G and Ti-G groups. Magnification, ×40. Column C (A3–D3), moderate-to-severe inflammation with intramedullary abscesses and acute or chronic inflammatory cells (red arrows) around necrotic bony trabeculae (black arrow) and fibrosis (yellow arrows) in the NTATi and Ti groups, and mild inflammation in the NTATi-G and Ti-G groups. Magnification, ×100.
Abbreviations: NTATi-G, nanotubular anodized titanium coated with gentamicin; Ti-G, titanium coated with gentamicin; NTATi, nanotubular anodized titanium uncoated with gentamicin; Ti, titanium uncoated with gentamicin.
Discussion
Infection and aseptic loosening are two of the major causes of orthopedic surgical failure. Six hours after the implant operation is a critical time window to prevent organism adhesion on implant surface, which is often referred to as a “decisive period”. The decision and intervention at this time point is vital to the long-term success of an implant.Citation38 Infection would become difficult to control if biofilm had already been formed on the surface of implants or sequestrum, since bacteria within the biofilm are protected from host defenses and antibacterial agents. Studies have demonstrated that once biofilm occurred, implants must be removed so that the infection can be resolved.Citation39 Costs of revision would increase four to six times if infection occurred after initial joint replacement.Citation40 Therefore, timely prevention of infection during initial joint replacement is important in decreasing medical cost and reducing adverse outcomes. In addition, biocompatibility is another important issue for orthopedic-related devices, as both mechanical and biological factors are the key to success for orthopedic surgeries. Stress shielding and insufficient bone formation surrounding the implants may occur if there is an elastic mismatch between the implants and bone tissue. Therefore, appropriate choosing and the modification of surface topography of materials are critical to refine the mechanical and/or biological properties of the implants.
The aim of this study is to investigate the cytocompatibility properties and calcium phosphate crystals coating of NTATi orthopedic implant, which may serve as a local antibiotic delivery device for the prevention of implant-related infections. We used an animal model of New Zealand White rabbits.Citation36 Other models inducing chronic myelitis with local osteonecrosis by sodium morrhuate and bacterial inoculation into the bone marrow cavity have been described previously.Citation35 However, in order to better mimic the clinical setting of postoperative infection, no artificial manipulations were conducted to promote the onset of infection in this study. S. aureus was chosen in this study because it is an organism predominantly associated with postoperative infection in the orthopedic department, which is characterized by its high affinity to the skin and bones, resorption of bone matrix and induction of osteonecrosis.Citation41 Gentamicin was used due to its wide antibacterial spectrum and thermostability, and S. aureus strain used in this study was sensitive to gentamicin.Citation34,Citation42
We compared the effectiveness of NTATi-G in the prophylaxis of infection in the rabbit tibial metaphysis with that of Ti-G, NTATi and Ti according to the physical and laboratorial examination, microbiological analysis, radiographic evaluation and histological and histopathological evaluations. Results also indicated fluctuations of body weight, body temperature and WBC in 7 days after operation, and the extent of fluctuation in NTATi and Ti groups was greater than that in NTATi-G and Ti-G groups. These parameters remained stable afterward from days 7 to day 42. Radiographic analysis did not reveal signs of apparent bone infections in the NTATi-G and Ti-G groups, whereas bone infections were obvious in Ti and NTATi groups on days 21 and 42. Significantly lower radiological, histological and histopathological scores were observed in the NTATi-G and Ti-G groups compared with the NTATi and Ti groups. Average CFU counts on agar plates and CFU/g bone ratio in NTATi-G and Ti-G groups were significantly lower than those in NTATi and Ti groups. Histological evaluation results showed better cytocompatibility in NTATi-G group than in Ti-G group. These results further demonstrate that NTATi-G and Ti-G with coprecipitation drug-loading approach can prevent the development of bone infections and that NTATi-G implant has better bone cell responses than Ti-G implant.
Previous studies have reported that orthopedic implant materials processed with advanced nanotechnology can provide optimal bone cell compatibility properties and be used as drug delivery platforms.Citation32 In the present study, we used anodized titanium with nanotubular surface structures. Gentamicin was loaded on pure titanium and NTATi using a coprecipitation drug-loading approach in which gentamicin was mixed with SBF before it was precipitated with calcium phosphate crystals. An in vitro research has demonstrated that such coprecipitated coatings on NTATi could continuously release antibiotics for up to 3 weeks,Citation32 while a previous study has demonstrated that antibiotics coated with physical adsorption can only release for 150 min.Citation43 The study also demonstrated that NTATi-G through physical absorption could significantly improve the antibacterial ability and bone cell activity of orthopedic implants.Citation43 The present study further demonstrated that NTATi-G through a coprecipitation drug-loading method could alleviate infections after bacterial inoculation in the medullary cavity. Notably, the bacteria was not completely eradicated in animals in the NTATi-G and Ti-G groups, which may partly be attributed to the high bacterial dose (109 CFU) inoculated in the medullary cavity of the surgical site; however, in real-life practice, the amount of contaminating bacteria in joint replacement surgeries is much lower. In addition, Ti-G implant could also inhibit bacterial adhesion in rabbit models; however, its biocompatibility was somewhat lower than the NTATi-G implant. Consistent with the previous studies, we have also demonstrated that anodized nanutubular titanium could improve osteoblast compatibility.Citation43
Conclusion
The present study using in vivo rabbit model demonstrated that NTATi-G had significantly better antibacterial effect than NTATi alone or conventional titanium implants. This novel implant also had more optimal biocompatibility compared to conventional titanium implant loaded with gentamicin. This study introduced NTATi-G as a new kind of implant with the potential of preventing local infections for joint replacement surgeries.
Acknowledgments
The authors thank the colleagues of Shanghai Institute of Ceramics, Chinese Academy of Sciences, for technical support. This study was supported by the National Natural Science Fund (81371936, 14JC1493102). No benefit in any form has been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary material
Figure S1 Scanning electron micrographs of the titanium surface after anodization. (A) NTATi surface under high magnification, scale bar 200 nm. (B) Length of the NTATi under high magnification, scale bar 500 nm. (C) Energy dispersive spectrum of NTATi samples revealed the existence of Ti, O and Na; atom% of Ti:O is about 1:2. The condition used for anodization was 60 V for 30 min in 0.09 M NH4F in ethylene glycol solution containing 10 vol% water.
Abbreviations: NTATi, nanotubular anodized titanium uncoated with gentamicin; Ti, titanium uncoated with gentamicin; O, oxide; Na, sodium.
References
- BenzTAngstFLehmannSAeschlimannAAssociation of the sense of coherence with physical and psychosocial health in the rehabilitation of osteoarthritis of the hip and knee: a prospective cohort studyBMC Musculoskelet Disord20131415923641831
- SunYStürmerTGüntherKPBrennerHReliability and validity of clinical outcome measurements of osteoarthritis of the hip and knee – a review of the literatureClin Rheumatol19971621851989093802
- MotaRETarriconeRCianiOBridgesJFDrummondMDeterminants of demand for total hip and knee arthroplasty: a systematic literature reviewBMC Health Serv Res20121222522846144
- BozicKJKurtzSMLauEThe epidemiology of revision total knee arthroplasty in the United StatesClin Orthop Relat Res20104681455119554385
- KohIJChoWSChoiNYKimTKCauses, risk factors, and trends in failures after TKA in Korea over the past 5 years: a multicenter studyClin Orthop Relat Res2013472131632623982406
- LieSAHavelinLIFurnesONEngesaeterLBVollsetSEFailure rates for 4762 revision total hip arthroplasties in the Norwegian Arthroplasty RegisterJ Bone Joint Surg Br200486450450915174543
- WhitehouseJDFriedmanNDKirklandKBRichardsonWJSextonDJThe impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra costInfect Control Hosp Epidemiol200223418318912002232
- BengtsonSBorgquistLLidgrenLCost analysis of prophylaxis with antibiotics to prevent infected knee arthroplastyBMJ198929967017197202508887
- BlackburnWDJrAlarconGSProsthetic joint infections. A role for prophylaxisArthritis Rheum19913411101171984767
- ChingDWGouldIMRennieJAGibsonPHPrevention of late haematogenous infection in major prosthetic jointsJ Antimicrob Chemother19892356766802668244
- YoungEJSugarmanBInfections in prosthetic devicesSurg Clin North Am19886811671803277302
- PriceJSTencerAFArmDMBohachGAControlled release of antibiotics from coated orthopedic implantsJ Biomed Mater Res19963032812868698690
- RuszczakZFriessWCollagen as a carrier for on-site delivery of antibacterial drugsAdv Drug Deliv Rev200355121679169814623407
- ZalavrasCGPatzakisMJHoltomPLocal antibiotic therapy in the treatment of open fractures and osteomyelitisClin Orthop Relat Res20044278693
- WiningerDAFassRJAntibiotic-impregnated cement and beads for orthopedic infectionsAntimicrob Agents Chemother19964012267526799124821
- WalenkampGHKleijnLLde LeeuwMOsteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1–12 yearsActa Orthop Scand19986955185229855236
- ZhangLYanJYinZElectrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infectionsInt J Nanomedicine201493027303625028544
- GarvinKLMiyanoJARobinsonDGigerDNovakJRadioSPolylactide/polyglycolide antibiotic implants in the treatment of osteomyelitis. A canine modelJ Bone Joint Surg Am19947610150015067929497
- OverbeckJPWincklerSTMeffertRTöormäläPSpiegelHUBrugEPenetration of ciprofloxacin into bone: a new bioabsorbable implantJ Invest Surg1995831551627547723
- NieLNicolauDPNightingaleCHBrownerBDQuintilianiRIn vitro elution of ofloxacin from a bioabsorbable polymerActa Orthop Scand19956643653687676828
- RutledgeBHuyetteDDayDAnglenJTreatment of osteomyelitis with local antibiotics delivered via bioabsorbable polymerClin Orthop Relat Res200341128028712782886
- HendricksKJLaneDBurdTAElution characteristics of tobramycin from polycaprolactone in a rabbit modelClin Orthop Relat Res200139241842611716417
- NelsonCLHickmonSGSkinnerRATreatment of experimental osteomyelitis by surgical debridement and the implantation of bioerodable, polyanhydride-gentamicin beadsJ Orthop Res19971522492559167628
- LiLCDengJStephensDPolyanhydride implant for antibiotic delivery – from the bench to the clinicAdv Drug Deliv Rev200254796398612384317
- YagmurluMFKorkusuzFGürselIKorkusuzPOrsUHasirciVSulbactam-cefoperazone polyhydroxybutyrate-co-hydroxyvalerate (PHBV) local antibiotic delivery system: in vivo effectiveness and biocompatibility in the treatment of implant-related experimental osteomyelitisJ Biomed Mater Res199946449450310398010
- RossiSAzghaniAOOmriAAntimicrobial efficacy of a new antibiotic-loaded poly(hydroxybutyric-co-hydroxyvaleric acid) controlled release systemJ Antimicrob Chemother20045461013101815537698
- TüresinFGürselIHasirciVBiodegradable polyhydroxyalkanoate implants for osteomyelitis therapy: in vitro antibiotic releaseJ Biomater Sci Polym Ed200112219520711403236
- LeeJEParkJCLeeKHOhSHKimJGSuhHAn infection-preventing bilayered collagen membrane containing antibiotic-loaded hyaluronan microparticles: physical and biological propertiesArtif Organs200226763664612081522
- ParkSNKimJKSuhHEvaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substituteBiomaterials200425173689369815020144
- AoyagiSOnishiHMachidaYNovel chitosan wound dressing loaded with minocycline for the treatment of severe burn woundsInt J Pharm20073301–213814517049772
- RossiSMarcielloMSandriGWound dressings based on chitosans and hyaluronic acid for the release of chlorhexidine diacetate in skin ulcer therapyPharm Dev Technol200712441542217763146
- YaoCWebsterTJProlonged antibiotic delivery from nanotubular anodized titanium using a co-precipitation drug loading methodJ Biomed Mater Res B Appl Biomater200991258759519582847
- WangPLiHZhangYMorphology of nanotube arrays grown on Ti–35Nb–2Ta–3Zr alloys with different deformationsAppl Surf Sci2014290308312
- LuckeMSchmidmaierGSadoniSGentamicin coating of metallic implants reduces implant-related osteomyelitis in ratsBone200332552153112753868
- AnYHFriedmanRJAnimal models of orthopedic implant infectionJ Invest Surg19981121391469700622
- TanHLAoHYMaRLinWTTangTTIn vivo effect of quaternized chitosan-loaded polymethylmethacrylate bone cement on methicillin-resistant Staphylococcus epidermidis infection of the tibial metaphysis in a rabbit modelAntimicrob Agents Chemother201458106016602325070107
- SmeltzerMSThomasJRHickmonSGCharacterization of a rabbit model of staphylococcal osteomyelitisJ Orthop Res19971534144219246088
- HetrickEMSchoenfischMHReducing implant-related infections: active release strategiesChem Soc Rev200635978078916936926
- CostertonJWBiofilm theory can guide the treatment of device-related orthopaedic infectionsClin Orthop Relat Res2005437711
- SugarmanBYoungEJInfections associated with prosthetic devices: magnitude of the problemInfect Dis Clin North Am198932187198
- CioffiGATerezhalmyGTTaybosGMTotal joint replacement: a consideration for antimicrobial prophylaxisOral Surg Oral Med Oral Pathol19886611241292970053
- DunneNHillJMcAfeePIn vitro study of the efficacy of acrylic bone cement loaded with supplementary amounts of gentamicin: effect on mechanical properties, antibiotic release, and biofilm formationActa Orthop200778677478518236183
- LinWTTanHLDuanZLInhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diametersInt J Nanomedicine201491215123024634583
- PettyWSpanierSShusterJJSilverthorneCThe influence of skeletal implants on incidence of infection. Experiments in a canine modelJ Bone Joint Surg Am1985678123612443902846
