Abstract
Breast cancer is one of the most common cancers affecting women worldwide. The controlled release of drugs to the precise site of the disease using a nanocarrier vehicle increases the therapeutic efficiency of the drugs. Nanotechnology-based approaches used to endorse clinical improvement from a disease also help to understand the interaction of malignant cells with their microenvironment. Receptor-based targeting is another approach for drug delivery which is undergoing clinical trials. Nanoparticles (NPs) delivery has been proven to promise high loading capacity, less toxicity, and stability of the drugs or biomolecules compared to traditional chemotherapeutic drugs. The goal of this review is to present the current problems of breast cancer therapy and discuss the NP-based targeting to overcome the hurdles of conventional drug therapy approach.
Introduction
Breast cancer is one of the most common cancers affecting women worldwide. In 2016, a total of 246,660 new cases of breast cancer and 14% of deaths due to breast cancer were reported in the US.Citation1 The majority of the deaths from breast cancer are due to its drug resistance and potential of metastasis to distant organs, such as the lymph nodes, bone, lung, and liver.Citation2,Citation3 It is well known that an ATP-binding cassette (ABC) family protein plays an important role in drug resistance in multiple malignancies, and its higher expression is proportional to higher resistance towards chemotherapy. Multidrug resistance (MDR) due to high expression of proteins such as P-glycoprotein (P-gp/ABCB1), ABCG2, and BCRP is a major hurdle in breast cancer prognosis and treatment. Although recent advances in immunotherapy have been made with the development of small molecules, proteins, and peptides, controlled-release drug delivery and targeting are still not achieved.
As indicated, the majority of the deaths from breast cancer are due to its potential of metastasis to distant organs.Citation2,Citation3 There are several pathways involved in the modulation of breast cancer and its progression to metastasis.Citation4–Citation6 There has been progress made in understanding the biological behavior of estrogen receptors (ERs), progesterone receptors (PRs), and human epidermal growth factor receptor 2 (HER-2) for multiple subtypes of breast cancer. Nanoparticles (NPs) carrying anticancer agents can be delivered actively or passively to targeted tumor to serve in diagnosis and treatment of breast cancer. NPs have multifunctional properties. In recent years, controlled release of therapeutic compounds from NPs has been achieved to determine the efficacy of the drug and to overcome MDR.
We previously reported the use of nanocapsules, nanospheres, and polymeric NPs for drug delivery, in which the drugs were physically and uniformly dispersed.Citation7 NPs size and distribution are measured by photon correlation spectroscopy and verified by scanning or transmission electron microscopy (TEM) to determine the diameter of the particles.Citation4 This technology of drug delivery using nanometer-sized particles could be the major therapeutic approach for future cancer patients. A recent progress in drug delivery technology is the design of surface-modified NPs that can improve the poor specificity and toxicological problems of antitumor therapy and act as a major therapeutic approach for patients. Primarily, the drug carriers used during the treatment act slowly for a longer period of time using specific stimuli. The conventional chemotherapeutic drugs affect both normal and cancer cells, whereas NP-coated drugs accumulate in tumors through enhanced permeability.Citation8
The application of NPs in medicine has enabled the development of nano-formulated drug delivery approach. There are several types of drug carriers commonly available such as polymeric dendrimers, micelles, microspheres, liposomes, quantum dots (QDs), nanoemulsions, gold nanoparticles (GNPs), and hydrogels, which require various methods of drug attachment including encapsulation, covalent binding, and adsorption.Citation9,Citation10 For example, lipid-based NPs can activate the secretion of glucagon-like peptide 1 for the treatment of human diabetes mellitus type-2.Citation11 Several other applications of nanomaterials such as carbon nanotubes, silver, and silica nanocarriers have been promoted in the biomedical field, and these nanomaterials are used during the treatment of various neurological and cancer diseases.Citation12–Citation14 Recently, next-generation nano-formulated platinum-based drug delivery has been used in clinics for cancer.Citation15
The receptor (HER-2, epidermal growth factor receptor [EGFR], vascular endothelial growth factor receptor [VEGFR], insulin-like growth factor I receptor [IGF-IR])-based targeting is the approach which is correlated with the breast tumorigenesis and is undergoing clinical trials (Phases II and III).Citation16 Among them, the most common type of breast cancer, which affects one in five women, is HER-2-positive breast cancer caused due to the overexpression of HER-2 on the surface of the breast tumor and characterized by poor prognosis and aggressive growth. An overview of the ongoing breast cancer clinical trials based on targeted therapy and chemotherapy drugs is presented in . Moreover, antibody–NP conjugates also facilitate the loading of higher drug concentrations for targeted delivery. For example, liposomes formulated with the monoclonal antibodies act against tumor cell antigens.Citation17 Nanocarrier-based and clinically approved therapeutic drug conjugates used to target metastatic breast cancer are shown in and . Targeted drug nanocarriers could replace the current method of treatment of chemoresistant tumor cells and cure cancer. In this review, we discuss the various drug delivery platforms (systemic, localized, and receptor-based) used for the model of breast cancer therapy ().
Table 1 Overview of ongoing breast cancer clinical trials based on targeted and chemotherapeutic drugs
Table 2 Nanocarrier-based therapeutic drug conjugates to target metastatic breast cancer
Table 3 Additional clinically approved chemotherapeutic drug combination for metastatic breast cancer prevention
Systemic drug delivery approaches
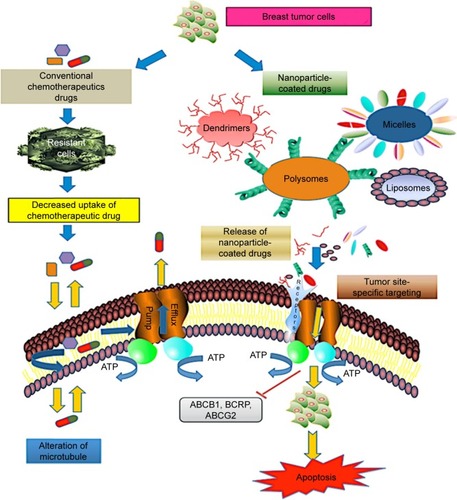
Nanocarrier-based drug delivery systems for chemotherapeutic drugs act efficiently on multiple malignant sites. The most common drug delivery approaches are based on organic and inorganic particles. The organic particles used for drug delivery application are micelles, liposomes, polymers, dendrimers, and nanogels. They have versatile surface building blocks for efficient endocytosis and loading. NPs have a multifunctional surface-modifying property that directs the cell to the tumor vasculature. The technology of encapsulating chemotherapeutic drugs using a nano-scale device is the best approach with regard to decreased side effects and improved bioavailability of drugs for breast cancer. While emerging technologies of systemic drug delivery using NPs promise early treatment of breast cancer, at present, limited options are available to the patients with metastatic breast cancer. Based on nanocarrier platforms, the most relevant strategy for precise site targeting in drug-resistant breast tumor cells is shown in .
Figure 2 Schematic representation of nanoparticle-based drug delivery mechanism in drug-resistant breast cancer cells. The most common mechanism of drug efflux in cancer cells is mediated by ABC transporters P-glycoprotein, BCRP, and ABCG2. Multidrug resistance protein consisting of nuclear-binding domain and transmembrane domain binds to the receptors on the surface of target tumor cells and functions in efflux of chemotherapeutic drugs such as taxol and anthracycline. However, delivery approaches using targeted drug nanocarriers (dendrimers, liposomes, micelles, polysomes) overcome the chemoresistance in tumor cells by activation of proapoptotic mediators, resulting in cell death.
Abbreviation: ABC, ATP-binding cassette.
Organic drug delivery approaches
Micelles
Polymeric micelles (PMs) are colloidal particles prepared from conjugates of water-soluble polymers with phospholipids or long-chain fatty acids and other surfactants. Micelles are used for the delivery of water-insoluble chemotherapeutic drugs. They were first proposed by Paul Ehrlich for targeted drug delivery to diseased cells. Micelles accumulate at poorly vascularized tumors and enhance permeability and retention, and increase the half-life of anticancer agents.Citation44 They have been shown to overcome P-gp efflux, act through receptor-mediated endocytosis, and increase intracellular drug concentration with enhanced cytotoxicity in MCF-7/doxorubicin-resistant cells.Citation45,Citation46 Moreover, fabricated immune micelles (antibodies bound to the surface of micelles) were also used in breast adenocarcinomas. Treatment of HER-2-positive breast cancer was performed with anti-HER-2 monoclonal antibody (mAb), fabricated with antibody-conjugated lysosomal P (LA-co-TMCC)-g-PEG-furan micelles.Citation47 Use of anti-HER-2 antibody complex micellar formulation in HER-2-positive and HER-2-negative cells has shown high efficacy of taxol compared to simple lipid-based protein.Citation48 In another study of the use of paclitaxel PM formulation in metastatic breast cancer patients, the Genexol-PM response rate was observed to be 58.5% compared to plain drugs that are in clinical trial Phases I and II. However, in SK-BR-3 cells, antibody-decorated NPs have shown 53.4% and 38.6% higher cellular uptake than the plain micelles in Phases I and II, respectively.Citation49
Liposomes
Liposomes are spherical vesicle microparticles that contain single or multiple bilayered membrane structures and were first described in 1965.Citation8,Citation50 Their size varies from 50–200 nm and they have a tendency to accumulate in tumor cells with an enhanced permeability and retention. Liposomes are classified based on size and composition and influenced by several factors such as bilayered fluidity, surface charge, surface hydration, and methods of preparation.Citation51 Liposomes have been reported to encapsulate lipophilic and hydrophobic drugs which are stable, nontoxic, biocompatible, biodegradable, and non-immunogenic.Citation52 Moreover, liposomes are reported to play a role in direct inhibition of P-gp by anionic membrane lipids. A previous study on Rhodamine retention using P-gp and BCRP substrate in breast cancer cell line MCF-7 showed that liposome encapsulation was increased in MCF-7/P-gp cells compared to MCF-7/wild-type cells.Citation53 Liposomes are reported as an effective delivery system for siRNA- or oligonucleotide-based therapy, and liposome-based drug formulations are currently used in clinical protocols.Citation54 The encapsulation of drugs in liposomes reduces the toxicity through biodistribution. The therapeutic application of liposomes as a drug carrier for the delivery of paclitaxel has also been evaluated in human ovarian cancer.Citation55,Citation56 Due to their small size and prolonged circulation, liposomes (including PEGylated liposomes) can provide protection from mononuclear phagocytes. PEGylated liposomes formulation with NPs coated on the surface using polyethylene glycol (PEG) improved the efficacy of drug delivery to target cells.Citation57 Yang et al reported that PEGylated formulation of paclitaxel increased the half-life compared to conventional liposomes formulation.Citation58 The combinations of doxorubicin and cyclophosphamide utilizing non-PEGylated liposomes were used for the treatment of metastatic breast cancer.Citation59 Moreover, liposome-conjugated antibody that overexpresses the HER-2 has been developed and reported as delivering 22-fold more calcein to mammary epithelial cells.Citation60 The synergistic effects of combined drug delivery of quercetin and vincristine through liposomes were reported for treatment of ER-negative breast cancer.Citation61
Polymers
Polymeric NPs (size 3–200 nm) that are formulated by binding a copolymer to a polymer matrix are widely used as drug delivery carriers. Polymers are classified as natural, synthetic, biodegradable, and nonbiodegradable forms. The most commonly used natural polymers are cellulose, chitosan, alginate, and gelatin, which are mildly immunogenic in nature. In addition, modified polymers with precise chemical composition increase the efficacy of site-specific targeting. Moreover, synthetic polymers such as poly-ε-caprolactone (PCL), poly-(lactic-co-glycolide), and polylactide (PLA) have a high rate of solubility and permeability. Such polymers are biocompatible and biodegradable with slow degradation rate, with good drug stability and release. Numerous polymer–drug conjugates, such as poly(d,l-lactide-co-glycolide) (PLGA), PEG, dextran, and N-(2-hydroxypropyl) methacrylamide (HPMA), have been tested in drug delivery research.Citation24 Chemotherapeutic drugs like paclitaxel, doxorubicin, camptothecins, and platinates have been clinically tested in drug conjugates for multiple cancers. It has been shown that polymeric NPs have a higher loading capacity for poorly water-soluble drugs, more stability, and more physicochemical properties (solubility, stability) compared to liposomes. The hybrid PM, developed by coating a PEG–phospholipid copolymer envelope on a nuclear PLGA NP, has improved therapeutic index with reduced toxicity.Citation62 A cisplatin-modified Pt(IV)-based PLGA-PEG NP was also reported with a significantly improved efficacy in breast cancer patients.Citation63 Lee and Nan proposed a novel combination of the drug delivery system for HER-2-overexpressing metastatic breast cancer via HER-2-targeted HPMA copolymer conjugates in combination with a tyrosine kinase inhibitor (PKI-166). Their study on targeting HER-2 receptors via extracellular (via TRZ binding) and intracellular (via PKI-166 binding) kinase domains suggested the synergistic effect from a drug conjugate delivery system for anticancer activity.Citation24 Therefore, a novel drug delivery system using a polymer with different mechanisms of action can bring forth a promising targeted therapy to overcome the limitations of the individual drug.
Dendrimers
Dendrimers are highly branched macromolecules possessing low polydispersity index. In 1978, Vogtle first described the nanotechnology platforms for drug delivery using dendrimers.Citation64 Like other nanocarriers, the biocompatibility and pharmacokinetics of dendrimers are easy to predict and can be controlled. There are various drug platforms that have been synthesized as delivery vehicles such as polyether-hydroxylamine (PEHAM), polyamidoamine (PAMAM), polyesteramine, polypropyleneimine, and polyglycerol.Citation65 The biopermeability of cationic PAMAM-NH2 (G0–G4) dendrimers across the biological membranes was evaluated for oral drug delivery which revealed that they crossed the membrane by paracellular and endocytosis pathways.Citation66,Citation67 The water solubility and size of dendrimers increased by PEGylation which helped to improve the retention and biodistribution characteristics. Several groups have shown that cell toxicity strongly correlates with the dendrimers end. The surface functional groups of dendrimers are amines that are decorated with protons, benzyloxycarbonyl- or tert-butoxycarbonyl-protecting group’s ethylenediamine ligands, or dansyl fluorescence labels. The doxorubicin-containing polyion complex micelle accumulates in the nucleus of drug-resistant MCF-7 cells and is also considered to have a potent antiproliferative effect on targeted tumor.Citation68 The cytotoxicity of MCF-7 breast cancer cells was examined in vitro using low-generation (G0, G1, and G2) PAMAM-like polymers.Citation69 However, dendrimers–drug conjugate has an antineoplastic agent and is covalently attached to the peripheral groups of the dendrimers, and has distinct advantages over drug-encapsulated systems. For local delivery in breast cancer, doxorubicin-G4-PAMAM complexes were encapsulated into the liposomes. These were formulated with HEPC and showed enhanced activity towards the MDA-MB435 breast cells compared to the individual dendrimers.Citation65 Thus, the methods for delivering the dendrimers-based NPs for transport of drugs into the specific area of malignant cells could be the best approach for delivery of NPs and to treat cancer cells.
Inorganic drug delivery approaches
Gold nanoparticles
GNPs are used in chemotherapy for several cancers. Due to their small size (approximately 130 nm) and specificity, they circulate throughout the tumor cells. GNP coating acts as a biomarker for the cancer diagnosis and is used as a probe for transmission electron microscopy and antimicrobial agents. There are several methods available for GNP production; the most common is the one involving citrate reduction of gold in water and the Brust–Schiffrin method.Citation70 The conjugation of GNPs to transferrin molecules was tested in breast cancer cells, and the results showed higher cellular uptake of transferrin molecules bound to GNPs in comparison to unbound molecules.Citation71 PEG-conjugated liposomes were used for anticancer drug delivery.Citation72 Balakrishnan et al targeted the breast cancer EGFR/VEGFR-2 signaling pathway using AuNPs-Qu-5, and reported its role in inhibition of migration, invasion, angiogenesis, and metastasis of breast cancer cells. This group has studied significant inhibition of multiple proteins such as p-PI3K, Akt, Snail, Slug, vimentin, N-cadherin, and p-GSK3β with treatment with AuNPs-Qu-5.Citation73 Eissa et al investigated 120 patient samples for ER, PR, and HER-2 status and reported that histidine-rich glycoprotein RNA-AuNPs had 90% sensitivity and specificity and can act as a diagnostic marker for breast cancer prognosis.Citation74 Another report showed that triple-negative breast cancer MDA-MB-231 cells were inhibited by phytochemical compounds such as gallic acid capped with GNPs.Citation75
SPIO-NPs
Superparamagnetic iron oxides (SPIOs) are used in tissue repair, immunoassay, and for cellular imaging in a magnetic field. They are also used as magnetic resonance contrast agents, controlling the direction of magnetic force to allow monitoring of the physiological and molecular changes in the body. SPIO nanoparticles (SPIO-NPs) have the ability to control the physical and chemical properties of particles such as their shape, size, and surface chemistry. SPIO-NPs have several applications in detection of inflammatory diseases and targeting of surface markers on tumors. SPIO consists of two components, an iron oxide core and a hydrophilic coating of the magnetic particle biomolecule, which allow it to deliver nano-derived biomolecules in a targeted area.Citation76 Biopolymers such as PEG, polyacrylic acid, dextran, alginate, polyethylene imine, and poly (vinyl alcohol) (PVA) are used as coating reagents for the surface stabilization of SPIOs.Citation77 They bind to tumor sites for delivery of antibodies, enzymes, proteins, drugs, or nucleotides. The uptake of SPIO-loaded PLA-tocopheryl PEG succinate (SPIO-PNPs) by MCF-7 breast cancer cells was confirmed through TEM in several experiments.Citation78 SPIO-targeted biomarkers have been developed for tumor cell imaging and detection. SPIO-Herceptin detects overexpression of HER-2/neu (c-erbB-2) tyrosine kinase receptor in the metastatic breast cancer.Citation79 SPIOs most efficiently used in magnetic resonance imaging and macrophage processing. However, knowledge concerning breast cancer and metastatic lymph nodes injection of SPIOs is lacking and needs to be explored. In future, SPIO-NPs could be applied as an effective treatment agent in breast cancer therapy.
Quantum dots (QD)
QD nanocrystals have a tunable wavelength, high brightness, anti-photo bleach, and optical properties, and are used as probes for many biological and biomedical applications. The conjugation of surface-modified QDs with antibodies, peptides, or other biomolecules enables their application in clinical oncology targeting. Bae et al synthesized a bimodal imaging nanoprobe by conjugating monoclonal antibodies and perfluorocarbon (PFC)/QD nanoemulsions for the detection of surface antigens on breast cancer cells (SK-BR-3, MCF-7, MDA-MB468) and also proposed that PFC/QD nanoemulsion had a great capacity for imaging therapy of tumor cells.Citation80 Several studies have shown the potential of QDs usage for various applications, including imaging, cell tracking, immunolabeling, in situ hybridization, and other in vitro- and in vivo-related technologies.
Localized drug delivery approaches
The current treatment for recurrent breast cancer is based on chemotherapeutic drugs, radiation, or surgery depending on location and the stages of the tumor. Localized drug delivery has more impact as a therapeutic option for early-stage cancers compared to the systemic drug. There are natural (dextran, chitosan, hyaluronic acid, gelatin, collagen polypeptides) and synthetic polymers that are used intratumorally in the cancerous tissue for drug delivery to cure breast cancer.Citation81 Furthermore, hydrogel formation of the NPs or polymers system, nanofiber with versatile morphology and tensile strength, and intraductal injection using microcatheter enhance the performance of ongoing smart drug delivery therapy.
Nanofibers
A nanofiber is a cross-linked polymer characterized by tensile strength and chemical nature. Biodegradable polymers such as PEG, PLGA, chitosan, PVA, PLA, polyethylene oxide, and PCL are used for preparing nanofibers for drug delivery applications. Another type of nanofiber, electrospun, was found to be bioactive and biocompatible similar to a human extracellular matrix, which supports diverse cells to grow into fabricated tissues.Citation82 A nanofiber-based platform has been prepared to evaluate migration of metastatic breast cancer cells. Curcumin-loaded PCL nanofibers were tested in breast cancer cell line MCF-7, and exhibited 15% more cytotoxicity compared to the commercial drug.Citation16 The use of a nanofiber model could allow testing the efficacy of an anticancer drug in diagnostic tools for multiple malignancies.
Hydrogels
Hydrogels are water-insoluble molecules, chemically or physically linked into a polymer chain. They allow controlled release of a drug within the body. Drugs enclosed within a hydrogel correspond to swell, diffusion, and control of the chemicals. Hydrogels for tumor cell therapy are developed in the form of microspheres or NPs. They are fabricated using protein and glycosaminoglycan components of breast tissue, which stimulate the growth of human breast cells. The most common endothermal hydrogel was based on chitosan, which is formed in tumor tissue after intratumor injection.Citation83 Chitosan hydrogels based on temperature-responsive hydroxyl butyl, poly (vinyl alcohol), thermo sensitive poly (ethylene glycol)-grafted, chitosan chloride/glycerophosphate and chitosan/bifunctional aldehyde have been investigated, but not tested in the preclinical trial for breast cancer application. However, only a few of the hydrogels have in situ gelling properties. Another platform for local and sustained delivery with high efficiencies in in vitro and in vivo breast cancer mice model was reported via siRNA encapsulation in oligopeptide-terminated poly(β-amino ester) NPs. Moreover, sustained delivery was enhanced when NPs were embedded in a hydrogel scaffold.Citation84
Intraductal injection
The molecular and morphological changes that occur in breast ductal epithelium cells are associated with a high risk of breast cancer. Ductal carcinoma in situ is a noninvasive early cancer, occurring in the lining of the breast milk duct, and represents 80% of breast cancers diagnosed. The use of microcatheter for collecting ductal cells can improve the ductal epithelium cells detection in abnormal breast cells. Ductal lavage procedure using microcatheter has been reported for cytological analysis with high efficiency for collecting breast epithelial cells. A study of 507 women who had a high risk of breast cancer was conducted to evaluate nipple aspirate fluid and ductal lavage and proved that ductal lavage was more sensitive and safer than nipple aspiration.Citation85 Moreover, chemotherapy through localized drug delivery was achieved by intraductal injection of chemotherapeutic drugs 5-fluorouracil and estradiol into mammary papilloma for the improvement of the immune response.Citation86 Detection of ductal cellular abnormalities can provide additional information to reduce the risk of breast cancer and also help in ongoing drug therapy.
Receptor-based drug delivery approaches
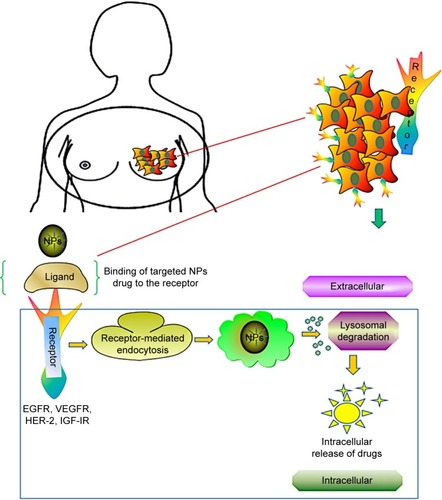
Breast cancer growths are regulated by multiple receptors, and inhibition at the receptor provides a new avenue for cancer therapy. Studies on receptor targeting are being used in clinical trials in patients with metastatic breast cancer. Although multiple cytotoxic drugs, such as gemcitabine, nab-paclitaxel, doxorubicin, etoposide, and vinorelbine, have been developed, the overall survival rates are still less.Citation87 Several studies have focused on receptors HER-2, EGFR, IGF-IR, and VEGFR, which revealed specific targets for breast cancer cells. According to those studies, HER-2 belongs to EGFR family and is poorly differentiated in triple-negative breast cancer; IGF-IR is regulated by tyrosine kinases, whereas VEGFR works as a stimulus for angiogenesis. The receptor-based targeting approach is illustrated in .
Figure 3 Receptor-mediated drug delivery to metastatic breast cancer cells. Nanocarrier-based drug targeting using receptor-mediated pathways governs the major therapeutic approach for the active sites in tumor cells. Ligand–nanoparticle conjugate binds to the receptors (EGFR, VEGFR, HER-2, IGF-IR) on the membrane, mediates internalization of nanoparticles through endocytosis, and releases the drugs by lysosomal degradation to the active sites of tumor cells.
Abbreviations: EGFR, epidermal growth factor receptor; VEGFR, vascular endothelial growth factor receptor; HER-2, human epidermal growth factor receptor 2; IGF-IR, insulin-like growth factor I receptor; NPs, nanoparticles.
HER-2
HER-2 has been reported to be overexpressed in breast cells. It belongs to the EGFR family and strongly correlates with tumorigenesis. Anti-HER-2 therapy using nanocarrier drugs and antibody-directed therapy for the antigen-binding site could be an effective treatment for breast cancer. Trastuzumab (mAb) has shown an overall response rate of 15%–30% against the extracellular domain of HER-2-positive breast cancer cells when given individually, but in combination with taxanes or vinorelbine, the response rates were 50%–80%.Citation88 In a randomized clinical trial Phases II and III, the overall percentage survival and the response rate were demonstrated to be high when combination therapies of trastuzumab with chemotherapeutic drugs were given to breast cancer patients.Citation16 Blockade of receptor using inhibitors may improve the treatment of trastuzumab-resistant tumors. A tyrosine kinase inhibitor, such as lapatinib, blocks the expression of EGFR (ErbB1) and HER-2 (ErbB2), which are co-expressed in 30% of breast cancers. The Phase II trial has shown 33% response rate after treatment of HER-2-positive metastatic breast cancer with lapatinib.Citation89 Another inhibitor gefitinib (an EGFR tyrosine kinase inhibitor) and pertuzumab (mAb) were reported to be able to block the overexpression and hetero-dimerization of HER-2 receptor family.Citation90 Moreover, adjuvant therapy of chemotherapeutic drugs can enhance the overall survival rate of breast cancer patients.
EGFR
Overexpression of EGFR has been reported in poorly differentiated triple-negative and inflammatory breast cancer cells. EGFR gene was identified in the early 1980s, and the clinical interest in the gene began in the late 1990s with the development of inhibitors.Citation91 There are several members of EGFR family reported, including EGFR (also known as ErbB1 and HER-1), HER-2 (also known as HER-2/neu and ErbB2), ErbB3 (HER3), and ErbB4 (HER4). Out of these, HER-2 was overexpressed in breast cancer.Citation92,Citation93 It has been proven that the EGFR expression was correlated with an increased copy number of the gene and protein overexpression in breast cancer. The increased EGFR gene copy number and protein overexpression were observed in ER-negative, PR-negative, HER-2 negative (triple-negative) breast cancer patients. Although drugs including cetuximab, lapatinib, gefitinib, and others have been developed to target the EGFR, the overall clinical outcome is poor. EGFR signaling and the relationship between triple-negative and inflammatory breast cancer-targeted therapies are the current topic of interest in the field of breast tumor therapy. Several clinical trials investigating vascular endothelial growth factor (VEGF), EGFR, Src, and mTOR molecular markers, for the treatments of triple-negative breast cancer, are ongoing; other inhibitors of the PI3K/AKT/mTOR pathway for deregulation in triple-negative breast cancer are in early-phase clinical trials.
IGF-IR
Breast cancer growth is regulated by receptor tyrosine kinases (RTKs), and the inhibition of the receptors, thus, could be the targets for anticancer therapy. The growth and differentiation of normal breast cells are mediated by IGF-IR signaling. Additionally, it stimulates mitogenesis and apoptosis of tumor cells. RTKs contain two domains – intracellular tyrosine kinase domain and extracellular ligand-binding domain. The ligands binding to the IGF-IR activate tyrosine kinases and induce conformational changes.Citation94 After activation, antiapoptotic effects of the IGF-IR are mediated via the Akt/PI3K pathways and IGF-IR is overexpressed in many cell types.Citation95 RTKs such as IGF-IR and c-erbB-2/HER-2/neu (HER-2/neu) have been reported for the breast cancer cell growth. Inhibition of these RTKs helps in reduction of cell growth and drug development.Citation96 Several reports demonstrate the evidence for overexpression and hyperactivation of the IGF-IR in the early stages of breast cancer.Citation97–Citation99 The negative expression of IGF-IR, using monoclonal antibodies, antisense IGF-IR, catechols, and transfection methods, can inhibit the tumor growth in breast cancer.Citation100,Citation101 Nordihydroguaiaretic acid is a phenolic compound and is reported as a direct inhibitor of both IGF-IR and the HER-2/neu receptor in breast tumor cells and induces apoptosis. Thus, negative expression of IGF-IR with a potential inhibitor can play an important role in breast cancer therapy.
VEGF
VEGF serves as a primary stimulus of angiogenesis when upregulated by various hormones, cytokines, and transforming growth factors. It is associated with the development, progression, and metastasis of breast cancer, through receptors such as VEGFR-2 (also known as flk/kdr), VEGFR-1 (also known as flt), and VEGF-C (homolog of VEGF gene family). VEGF-2 (flk) and VEGF-1 (flt) are expressed on vascular endothelial or non-endothelial cell-specific receptor;Citation102 VEGFR-1 (flt) is found on monocytes, whereas VEGF-C receptor (flt-4) is expressed on endothelial cells of lymphatic vessels.Citation103 VEGFRs play an important role in the antiapoptotic mechanisms in breast cancer cells.Citation104 The receptor-based monoclonal antibodies have been reported to inhibit VEGF activity, which downregulates the growth of tumors and their blood vessels.Citation105 The siRNA as well as anti-VEGF antibody therapy is already in clinical trials for regulation of VEGF activity.Citation106 Therefore, targeting of endothelial growth factor either alone or in combination with a target agent could be the future therapeutic strategy for metastatic breast cancer.
Conclusion
Drug carrier systems allow for the controlled release of drugs at the desired sites, thus altering the pharmacokinetics and biodistribution of the drugs. In this sense, nanoparticles are intrinsically advantageous over conventional particles. This study provides an overview of all aspects of drug delivery mechanisms using nanocarriers for metastatic breast cancer treatment. Precise drug release into highly specific targets involves miniaturizing the delivery systems to be much smaller than their targets. It is highly expected that these minute drug delivery system can be realized through the advances in nanotechnology. The integration of nanotechnology products, such as nanoparticles, with therapeutic agents, has recently created a new therapeutic trend that would not otherwise be possible.
Acknowledgments
The authors thank Ms Angela Wimes for her critical review of the manuscript. This study was supported in part by the National Cancer Institute of the National Institute of Health under Award Number SC1CA193758 and by National Institute of Health under Award Number 5G12MD007602.
Disclosure
The authors report no potential conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- CartyNJFoggittAHamiltonCRRoyleGTTaylorIPatterns of clinical metastasis in breast cancer: an analysis of 100 patientsEur J Surg Oncol19952166076088631404
- GrobmyerSRZhouGGutweinLGIwakumaNSharmaPHochwaldSNNanoparticle delivery for metastatic breast cancerNanomedicine20128Suppl 1S21S3022640908
- DaviesEHiscoxSNew therapeutic approaches in breast cancerMaturitas201168212112821144683
- ErolesPBoschAPérez-FidalgoJALluchAMolecular biology in breast cancer: intrinsic subtypes and signaling pathwaysCancer Treat Rev201238669870722178455
- KreikeBvan KouwenhoveMHorlingsHGene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomasBreast Cancer Res200795R6517910759
- SinghRLillardJWJrNanoparticle-based targeted drug deliveryExp Mol Pathol200986321522319186176
- BanghamADLiposomes: the Babraham connectionChem Phys Lipids1993641–32752858242839
- ZhangSChuZYinCZhangCLinGLiQControllable drug release and simultaneously carrier decomposition of SiO2-drug composite nanoparticlesJ Am Chem Soc2013135155709571623496255
- BhattacharjeeHBalabathulaPWoodGCTargeted nanoparticulate drug-delivery systems for treatment of solid tumors: a reviewTher Deliv20101571373422833959
- BeloquiAAlhouayekMCarradoriDA mechanistic study on nanoparticle-mediated glucagon-like peptide-1 (GLP-1) secretion from enteroendocrine L cellsMol Pharm201613124222423027934480
- KafaHWangJTRubioNTranslocation of LRP1 targeted carbon nanotubes of different diameters across the blood-brain barrier in vitro and in vivoJ Control Release201622521722926809004
- VenugopalKRatherHARajagopalKSynthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticumJ Photochem Photobiol B201716728228928110253
- KhosravianPShafiee ArdestaniMKhoobiMMesoporous silica nanoparticles functionalized with folic acid/methionine for active targeted delivery of docetaxelOnco Targets Ther201697315733027980423
- JohnstoneTCSuntharalingamKLippardSJThe next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugsChem Rev201611653436348626865551
- MartyMCognettiFMaraninchiDRandomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study groupJ Clin Oncol200523194265427415911866
- KontermannREImmunoliposomes for cancer therapyCurr Opin Mol Ther200681394516506524
- MilaneLDuanZFAmijiMPharmacokinetics and biodistribution of lonidamine/paclitaxel loaded, EGFR-targeted nanoparticles in an orthotopic animal model of multi-drug resistant breast cancerNanomedicine20117443544421220050
- WangYGaoSYeWHYoonHSYangYYCo-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymerNat Mater200651079179616998471
- NishimuraYMiedaHIshiiJOginoCFujiwaraTKondoATargeting cancer cell-specific RNA interference by siRNA delivery using a complex carrier of affibody-displaying bio-nanocapsules and liposomesJ Nanobiotechnology2013111923800313
- BaeYDieziTAZhaoAKwonGSMixed polymeric micelles for combination cancer chemotherapy through the concurrent delivery of multiple chemotherapeutic agentsJ Control Release2007122332433017669540
- WongMYChiuGNLiposome formulation of co-encapsulated vincristine and quercetin enhanced antitumor activity in a trastuzumab-insensitive breast tumor xenograft modelNanomedicine20117683484021371568
- ChenYBathulaSRLiJHuangLMultifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancerJ Biol Chem201028529226392265020460382
- LeeJHNanACombination drug delivery approaches in metastatic breast cancerJ Drug Deliv2012201291537522619725
- RobertNLeyland-JonesBAsmarLRandomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancerJ Clin Oncol200624182786279216782917
- BartschRWenzelCAltorjaiGCapecitabine and trastuzumab in heavily pretreated metastatic breast cancerJ Clin Oncol200725253853385817679724
- BaselgaJTrigoJMBourhisJPhase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neckJ Clin Oncol200523245568557716009950
- CameronDCaseyMOlivaCNewstatBImwalleBGeyerCELapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trialOncologist201015992493420736298
- Di LeoAGomezHLAzizZPhase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancerJ Clin Oncol200826345544555218955454
- MoyBGossPELapatinib-associated toxicity and practical management recommendationsOncologist200712775676517673607
- AdemuyiwaFOMillerKDIncorporation of antiangiogenic therapies in the treatment of metastatic breast cancerClin Breast Cancer20088Suppl 4S151S15619158035
- GatzemeierUPluzanskaASzczesnaAPhase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation TrialJ Clin Oncol200725121545155217442998
- JoensuuHHolliKHeikkinenMCombination chemotherapy versus single-agent therapy as first- and second-line treatment in metastatic breast cancer: a prospective randomized trialJ Clin Oncol19981612372037309850014
- BriaEGiannarelliDFeliciATaxanes with anthracyclines as first-line chemotherapy for metastatic breast carcinomaCancer2005103467267915637696
- AlbainKSNagSMCalderillo-RuizGGemcitabine plus Paclitaxel versus Paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatmentJ Clin Oncol200826243950395718711184
- BurzykowskiTBuyseMPiccart-GebhartMJEvaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancerJ Clin Oncol200826121987199218421050
- ChanSRomieuGHuoberJPhase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancerJ Clin Oncol200927111753176019273714
- O’ShaughnessyJMilesDVukeljaSSuperior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial resultsJ Clin Oncol200220122812282312065558
- WangJFanYXuBIxabepilone plus capecitabine for Chinese patients with metastatic breast cancer progressing after anthracycline and taxane treatmentCancer Chemother Pharmacol201066359760320490795
- BonadonnaGValagussaPMoliterniAZambettiMBrambillaCAdjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-upN Engl J Med1995332149019067877646
- HainsworthJDMitoxantrone, 5-fluorouracil and high-dose leucovorin (NFL) in the treatment of metastatic breast cancer: randomized comparison to cyclophosphamide, methotrexate and 5-fluorouracil (CMF) and attempts to improve efficacy by adding paclitaxelEur J Cancer Care (Engl)199764 Suppl499460336
- AcklandSPAntonABreitbachGPDose-intensive epirubicin-based chemotherapy is superior to an intensive intravenous cyclophosphamide, methotrexate, and fluorouracil regimen in metastatic breast cancer: a randomized multinational studyJ Clin Oncol200119494395311181656
- BlackwellKLBursteinHJStornioloAMRandomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancerJ Clin Oncol20102871124113020124187
- ZhangYHuangYLiSPolymeric micelles: nanocarriers for cancer-targeted drug deliveryAAPS PharmSciTech201415486287124700296
- MohajerGLeeESBaeYHEnhanced intercellular retention activity of novel pH-sensitive polymeric micelles in wild and multidrug resistant MCF-7 cellsPharm Res20072491618162717385015
- YuanYCaiTXiaXZhangRChibaPCaiYNanoparticle delivery of anticancer drugs overcomes multidrug resistance in breast cancerDrug Deliv20162393350335727098896
- SadatSMSaeidniaSNazaraliAJHaddadiANano-pharmaceutical formulations for targeted drug delivery against HER2 in breast cancerCurr Cancer Drug Targets2015151718625564255
- LeeALWangYChengHYPervaizSYangYYThe co-delivery of paclitaxel and Herceptin using cationic micellar nanoparticlesBiomaterials200930591992719042015
- LeeKSChungHCImSAMulticenter phase II trial of Genex-ol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancerBreast Cancer Res Treat2008108224125017476588
- WangAZLangerRFarokhzadOCNanoparticle delivery of cancer drugsAnnu Rev Med20126318519821888516
- SzulcJDudzikMWoyczikowskiBSznitowskaMJanickiSLiposomes – therapeutic progress and technological problemsPol Merkur Lekarski20021268164168 Polish [with English abstract]11995258
- KalepuSNekkantiVInsoluble drug delivery strategies: review of recent advances and business prospectsActa Pharm Sin B20155544245326579474
- KangDIKangHKGwakHSHanHKLimSJLiposome composition is important for retention of liposomal rhodamine in P-glycoprotein-overexpressing cancer cellsDrug Deliv200916526126719538007
- StraubingerRMPapahadjopoulosDLiposomes as carriers for intracellular delivery of nucleic acidsMethods Enzymol19831015125276193396
- SharmaAStraubingerRMOjimaIBernackiRJAntitumor efficacy of taxane liposomes on a human ovarian tumor xenograft in nude athymic miceJ Pharm Sci19958412140014048748320
- SharmaAMayhewEBolcsakLActivity of paclitaxel liposome formulations against human ovarian tumor xenograftsInt J Cancer19977111031079096672
- SukJSXuQKimNHanesJEnsignLMPEGylation as a strategy for improving nanoparticle-based drug and gene deliveryAdv Drug Deliv Rev201699Pt A285126456916
- YangTCuiFDChoiMKEnhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluationInt J Pharm20073381–231732617368984
- DhankharRVyasSPJainAKAroraSRathGGoyalAKAdvances in novel drug delivery strategies for breast cancer therapyArtif Cells Blood Substit Immobil Biotechnol201038523024920677900
- KullbergMOwensJLMannKListeriolysin O enhances cytoplasmic delivery by Her-2 targeting liposomesJ Drug Target201018431332020201742
- WongMYChiuGNSimultaneous liposomal delivery of quercetin and vincristine for enhanced estrogen-receptor-negative breast cancer treatmentAnticancer Drugs201021440141020110806
- SenguptaSEavaroneDCapilaITemporal targeting of tumour cells and neovasculature with a nanoscale delivery systemNature2005436705056857216049491
- DharSKolishettiNLippardSJFarokhzadOCTargeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivoProc Natl Acad Sci U S A201110851850185521233423
- MadaanKKumarSPooniaNLatherVPanditaDDendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issuesJ Pharm Bioallied Sci20146313915025035633
- WolinskyJBGrinstaffMWTherapeutic and diagnostic applications of dendrimers for cancer treatmentAdv Drug Deliv Rev20086091037105518448187
- KitchensKMEl-SayedMEGhandehariHTransepithelial and endothelial transport of poly (amidoamine) dendrimersAdv Drug Deliv Rev200557152163217616289433
- NorthfeltDWDezubeBJThommesJAPegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trialJ Clin Oncol1998167244524519667262
- LuHLSyuWJNishiyamaNKataokaKLaiPSDendrimer phthalocyanine-encapsulated polymeric micelle-mediated photochemical internalization extends the efficacy of photodynamic therapy and overcomes drug-resistance in vivoJ Control Release2011155345846421689700
- FuchsSKappTOttoHA surface-modified dendrimer set for potential application as drug delivery vehicles: synthesis, in vitro toxicity, and intracellular localizationChemistry20041051167119215007808
- JainSHirstDGO’SullivanJMGold nanoparticles as novel agents for cancer therapyBr J Radiol201285101010111322010024
- LiJLWangLLiuXYIn vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticlesCancer Lett2009274231932618977071
- YangPHSunXChiuJFSunHHeQYTransferrin-mediated gold nanoparticle cellular uptakeBioconjug Chem200516349449615898713
- BalakrishnanSBhatFARaja SinghPGold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancerCell Prolif201649667869727641938
- EissaSAzzazyHMMatboliMShawkySMSaidHAnousFAThe prognostic value of histidine-rich glycoprotein RNA in breast tissue using unmodified gold nanoparticles assayAppl Biochem Biotechnol2014174275176125091325
- ChenYJLeeYCHuangCHChangLSGallic acid-capped gold nanoparticles inhibit EGF-induced MMP-9 expression through suppression of p300 stabilization and NFκB/c-Jun activation in breast cancer MDA-MB-231 cellsToxicol Appl Pharmacol20163109810727634460
- GuptaAKGuptaMSynthesis and surface engineering of iron oxide nanoparticles for biomedical applicationsBiomaterials200526183995402115626447
- WangYXXuanSPortMIdeeJMRecent advances in superpara-magnetic iron oxide nanoparticles for cellular imaging and targeted therapy researchCurr Pharm Des201319376575659323621536
- AhmedMDouekMThe role of magnetic nanoparticles in the localization and treatment of breast cancerBiomed Res Int2013201328123023936784
- ThorekDLChenAKCzuprynaJTsourkasASuperparamagnetic iron oxide nanoparticle probes for molecular imagingAnn Biomed Eng2006341233816496086
- BaePKChungBHMultiplexed detection of various breast cancer cells by perfluorocarbon/quantum dot nanoemulsions conjugated with antibodiesNano Converg2014112328191403
- WolinskyJBColsonYLGrinstaffMWLocal drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafersJ Control Release20121591142622154931
- JayakumarRPrabaharanMNairSVTamuraHNovel chitin and chitosan nanofibers in biomedical applicationsBiotechnol Adv201028114215019913083
- HanHDMoraEMRohJWChitosan hydrogel for localized gene silencingCancer Biol Ther201111983984521358280
- SegoviaNPontMOlivaNRamosVBorrósSArtziNHydrogel doped with nanoparticles for local sustained release of siRNA in breast cancerAdv Healthc Mater20154227128025113263
- DooleyWCLjungBMVeronesiUDuctal lavage for detection of cellular atypia in women at high risk for breast cancerJ Natl Cancer Inst200193211624163211698566
- DaveKAverineniRSahdevPPerumalOTranspapillary drug delivery to the breastPLoS One2014912e11571225545150
- LuRMChenMSChangDKTargeted drug delivery systems mediated by a novel peptide in breast cancer therapy and imagingPLoS One201386e6612823776619
- MontemurroFAgliettaMIncorporating trastuzumab into the neoadjuvant treatment of HER2-overexpressing breast cancerClin Breast Cancer200561778015899075
- ValabregaGMontemurroFAgliettaMTrastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancerAnn Oncol200718697798417229773
- AgusDBAkitaRWFoxWDTargeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growthCancer Cell20022212713712204533
- ShimizuNBehzadianMAShimizuYGenetics of cell surface receptors for bioactive polypeptides: binding of epidermal growth factor is associated with the presence of human chromosome 7 in human-mouse cell hybridsProc Natl Acad Sci U S A1980776360036046968072
- BurnessMLGrushkoTAOlopadeOIEpidermal growth factor receptor in triple-negative and basal-like breast cancer: promising clinical target or only a marker?Cancer J2010161233220164687
- GuérinMGabillotMMathieuMCStructure and expression of c-erbB-2 and EGF receptor genes in inflammatory and non-inflammatory breast cancer: prognostic significanceInt J Cancer19894322012082563719
- MorinMJFrom oncogene to drug: development of small molecule tyrosine kinase inhibitors as anti-tumor and anti-angiogenic agentsOncogene200019566574658311426642
- KulikGKlippelAWeberMJAntiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and AktMol Cell Biol1997173159516069032287
- YoungrenJFGableKPenarandaCNordihydroguaiaretic acid (NDGA) inhibits the IGF-1 and c-erbB2/HER2/neu receptors and suppresses growth in breast cancer cellsBreast Cancer Res Treat2005941374616142439
- ArteagaCLKittenLJCoronadoEBBlockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic miceJ Clin Invest1989845141814232553774
- SurmaczEFunction of the IGF-I receptor in breast cancerJ Mammary Gland Biol Neoplasia2000519510510791772
- KhandwalaHMMcCutcheonIEFlyvbjergAFriendKEThe effects of insulin-like growth factors on tumorigenesis and neoplastic growthEndocr Rev200021321524410857553
- PragerDLiHLAsaSMelmedSDominant negative inhibition of tumorigenesis in vivo by human insulin-like growth factor I receptor mutantProc Natl Acad Sci U S A1994916218121858134369
- SachdevDLiSLHartellJSFujita-YamaguchiYMillerJSYeeDA chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-ICancer Res200363362763512566306
- FerraraNGerberHPLeCouterJThe biology of VEGF and its receptorsNat Med20039666967612778165
- KinoshitaJKitamuraKKabashimaASaekiHTanakaSSugimachiKClinical significance of vascular endothelial growth factor-C (VEGF-C) in breast cancerBreast Cancer Res Treat200166215916411437102
- PidgeonGPBarrMPHarmeyJHFoleyDABouchier-HayesDJVascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cellsBr J Cancer200185227327811461089
- ValtolaRSalvenPHeikkiläPVEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancerAm J Pathol199915451381139010329591
- ZhangJWuYOXiaoLLiKChenLLSiroisPTherapeutic potential of RNA interference against cellular targets of HIV infectionMol Biotechnol200737322523617952669