Abstract
Nanotechnology offers new tools for developing therapies to prevent and treat oral infections, particularly biofilm-dependent disorders, such as dental plaques and endodontic and periodontal diseases. Chlorhexidine (CHX) is a well-characterized antiseptic agent used in dentistry with broad spectrum activity. However, its application is limited due to inactivation in body fluid and cytotoxicity toward human cells, particularly at high concentrations. To overcome these limitations, we synthesized nanosystems composed of aminosilane-coated magnetic nanoparticles functionalized with chlorhexidine (MNP@CHX). In the presence of human saliva, MNPs@CHX displayed significantly greater bactericidal and fungicidal activity against planktonic and biofilm-forming microorganisms than free CHX. In addition, CHX attached to MNPs has an increased ability to restrict the growth of mixed-species biofilms compared to free CHX. The observed depolarization of mitochondria in fungal cells treated with MNP@CHX suggests that induction of oxidative stress and oxidation of fungal structures may be a part of the mechanism responsible for pathogen killing. Nanoparticles functionalized by CHX did not affect host cell proliferation or their ability to release the proinflammatory cytokine, IL-8. The use of MNPs as a carrier of CHX has great potential for the development of antiseptic nanosystems.
Introduction
Chlorhexidine (CHX) is a biguanide derivative with broad antimicrobial activity against bacteria, fungi and viruses.Citation1–Citation3 It targets compounds such as lipopolysaccharide and lipoteichoic acid on the microorganism membrane,Citation4,Citation5 and at low concentrations CHX affects membrane integrity, while at higher concentrations it causes the cytoplasm to congeal. Previously published data indicate that CHX may be toxic to mammalian cells.Citation6 CHX is one of the most frequently used antiseptics in medicine, particularly in dentistry and surgery.Citation7–Citation9 It is used for the treatment and prevention of gingivitis and periodontitis, and as agents for the treatment of wounds or burns.Citation10–Citation13 The antimicrobial activity of CHX is pH sensitive and the optimal pH value is ~7.0.Citation14 Furthermore, its antimicrobial activity is reduced in the presence of serum, plasma, pus and other organic material, such as surfactants and anionic polyelectrolytes.Citation1,Citation14,Citation15 Interestingly, recent data suggest that certain Gram-negative bacterial isolates are able to grow in the presence of 1% CHX.Citation16 It has also been noted that CHX is responsible for the induction of VanA-type vancomycin resistance genes in Enterococcus species.Citation17 In addition, Liu et al demonstrated the increasing prevalence of CHX-tolerant high-level mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MRSA) strains.Citation18 Taken together, these observations suggest that the use of CHX may be limited in certain settings and may require higher doses to obtain the desired therapeutic effects. An emerging therapeutic strategy is the use of nanoparticles as drug carriers.Citation19,Citation20 Studies show that the immobilization/encapsulation process improves the overall biological effectiveness of active agents including pharmacokinetic parameters (bioavailability, clearance) and reduces the side effects.Citation21–Citation23 In addition, it has been noted that the antibiotic resistance of drug-resistant pathogens may be overcome by using nanoparticles as antibiotic carriers.Citation24 Among the many types of nanostructures, core–shell magnetic iron oxide nanoparticles are the most commonly used drug carriers in biomedical studies.Citation20,Citation25 Due to their unique properties (size, magnetic moment, surface properties), the use of magnetic nanomaterials (MNPs) may represent a new approach for the treatment of infections caused by drug-resistant pathogens. These nanoparticles are able to interact with planktonic bacteria as well as those embedded in a biofilm matrix.Citation26,Citation27 The general mechanism of nanoparticle toxicity against pathogens involves binding to the cell wall and subsequent disruption of the membrane through either direct interaction and/or oxidation of macromolecules.Citation28 Interestingly, recent reports indicate that metal-oxide nanoparticles are nontoxic to mammalian cells due to their ability to phagocytose and degrade nanoparticles through lysosomal fusion.Citation28 These properties allow treatment with nanoparticles to maintain normal cell function while also restricting the processes that promote pathogen growth and infection development. These properties govern nanomaterial selectivity to enhance tissue-forming cell function, while also restricting pathogen growth and viability that are responsible for infection development. Our previous studies indicate that the immobilization of ceragenin on the MNP surface increases its antibacterial activity in the presence of body fluids.Citation22 In addition, recent work suggests that the application of nanosystems composed of MNPs and polyene antibiotic may be used for the treatment of infections caused by polyene-resistant fungal strain.Citation29 In the present study, we evaluate the activity of magnetic nanosystems functionalized with chlorhexidine against clinical isolates of pathogens from different groups including representatives of Gram-positive and Gram-negative bacteria and fungal cells. We demonstrate that covalent attachment of CHX on the magnetic carrier improves their antimicrobial activity compared to a nonimmobilized agent in conditions that mimic the oral cavity environment. Importantly, these nanosystems are characterized by lower cytotoxic effects on dental osteoblasts indicating enhanced biocompatibility. Furthermore, CHX-functionalized nanoparticles did not affect cell proliferation or the ability to release IL-8 from human osteoblast cells.
Materials and methods
Synthesis of magnetic nanoparticles functionalized by CHX
The magnetic core was synthesized using a well-known modification of the Massart method, which is based on the coprecipitation of hydrated iron chloride salts under treatment by ammonium hydroxide (25%).Citation25,Citation30 Stöber methods were used to obtain core–shell magnetic nanostructures with terminal propylamine groups.Citation26,Citation31 Immobilization of CHX onto the surface of nanoparticles was achieved by interaction between the primary amine group of nanoparticles and the chloride group from CHX. After anchoring, the precipitate was magnetically collected, washed with ethanol and phosphate-buffered saline (PBS) and dried at 50°C.
Nanoparticle characterization
Fourier transform infrared spectroscopy (FTIR) spectra were recorded using the Thermo Fisher Scientific Nicolet iN10 MX FTIR microscope. A 10 μL sample (1 mg/mL) was dropped on the surface of a glossy metal plate, and the solvent was left to evaporate. All spectra were collected in the 4,000–800 cm−1 range by co-adding 64 scans with a resolution of 4 cm−1. Data analysis was performed using OMNIC software (Thermo Fisher Scientific). UV–VIS spectra were recorded using Thermo Fisher Scientific Varioskan LUX. The absorbance spectra (200–400 nm) of a 100 μL sample (1 mg/mL) were registered. A METTLER TOLEDO DSC 1 STAR system was used to perform differential scanning calorimetry (DSC). Nitrogen was used as a purge gas (10 mL/min). Samples (2 mg) were placed in aluminum pans and heated from 25°C to 450°C with a heating rate of 20°C/min. Thermogravimetric analysis (TGA) was recorded using a Mettler Toledo Star TGA/DSC unit. Nanoparticles (2 mg) were placed in the aluminum pans (40 μL) and heated from 50°C to 900°C with a heating rate of 10°C/min. Magnetic properties of the tested nanoparticles were determined using a Quantum Design MPMS 5XL SQUID-type magnetometer; the samples were prepared as described previously.Citation32 The morphology of MNP@CHX was studied using transmission electron microscopy (TEM; Tecnai G2 X-TWIN). Samples for TEM were prepared by solvent evaporation from nanoparticle dispersion on holey carbon copper grids.
Determination of minimum inhibitory concentration (MIC)/minimal fungicidal concentration (MFC)/minimal biofilm inhibitory concentration (MBIC) values
The MIC and minimal bactericidal concentration (MBC) or MFC were determined using pathogens at the logarithmic phase of growth. All experiments were performed using CHX and MNP@CHX at a concentration range of 32–0.125 μg/mL. The activity of the tested agents was evaluated against representatives of Gram-positive bacteria such as MRSA, methicillin-sensitive Staphylococcus aureus (MSSA) and Enterococcus faecalis, and Gram-negative bacteria such as Escherichia coli (ESBL) and Pseudomonas aeruginosa Xen 5 (MDR) and fungal Candida sp. laboratory strains and clinical isolates (~103 CFU/mL). The microbial identification was performed using VITEK® 2 SYSTEM (bioMérieux). The MIC/MBC or MFC values were determined using the microdilution method. An MTT assay was performed to evaluate the MBIC.Citation33 The MIC values were determined visually as the lowest concentration of tested antimicrobial agents that inhibit pathogen growth. MBC or MFC was performed by spotting treated samples of microbial cells on Luria–Bertani (LB) agar or Sabouraud dextrose agar plates. To evaluate the impact of saliva on the antimicrobial activity of CHX and MNP@CHX, the MIC/MFC/MBIC values were determined in the presence of 50% saliva. The chemiluminescent intensity of P. aeruginosa Xen5 (OD600 =0.5) following treatment with CHX/MNP@CHX in the presence or absence of saliva was measured as an additional method to assess bacterial viability.
Anti-biofilm assay
To evaluate the inhibitory activity of tested agents against biofilm formation, cells (~106 CFU/mL) were grown for 48 h at 37°C in the presence or absence of CHX or MNP@CHX in the concentration range of 0.5–50 μg/mL in LB liquid medium. After incubation, the biofilm was washed four times with 100 μL PBS to remove planktonic nonadherent cells. Crystal violet (CV) staining (0.1%) was used to determine the total biofilm mass. The viability of cells embedded in the biofilm matrix was performed using an MTT assay and by CFU determination via spotting on an agar plate. Measurement of OD to evaluate biofilm mass (CV) and cell viability (MTT) was performed using Varioskan LUX. The positive control was pathogen growth without the presence of tested agents, while the negative control was empty well without bacteria/fungal cells.Citation34,Citation35
Determination of oxidative stress
Oxidative stress was analyzed using a Mitochondrial Potential Kit and the NucleoCounter® NC-3000™ system following the manufacturer’s instructions. Fungal cells stained with JC-1/DAPI were immediately loaded into an NCSlide. Mitochondrial depolarization was calculated as a decrease in the red/green fluorescence intensity ratio. Necrotic and late apoptotic cells were detected as blue fluorescent DAPI stained cells.
Cell culture
Human osteoblast cells hFOB 1.19 were purchased from American Type Culture Collection (Manassas, Virginia, USA) and maintained in a 1:1 mixture of Ham’s F12 Medium Dulbecco’s Modified Eagle’s Medium, with 2.5 mM L-glutamine, without phenol red, supplemented with 10% fetal bovine serum, 50 U/mL penicillin, 50 mg/mL streptomycin and 0.3 mg/mL G418 in a humidified incubator containing 5% CO2 in air at 34°C.
Evaluation of IL-8 concentration and cytotoxicity using hFOB 1.19 cell line
To evaluate interleukin-8 (IL-8) release and cytotoxicity after CHX and MNP@CHX addition, cells were seeded at a density of 2×104 cells per well in 1 mL of growth medium in 24-well plates. After 24 h of culture, cells were treated with the tested agents at a concentration range of 1–150 μg/mL. After 24 h incubation, an IL-8 and LDH assay was performed. The average of all the experiments is shown as the percentage of cell viability compared to control, while cells treated with lytic solution were considered as 100% LDH release. IL-8 concentration was determined using an IL-8 ELISA kit (Invitrogen).
Proliferation assay
To assess the effect of free CHX or immobilized on the magnetic nanoparticles, hFOB 1.19 cells proliferation was assessed. Cells were seeded in 96-well plates at 2×104 cells/well with 100 μL of growth medium. Then the tested agents were added to subconfluent cells at a concentration range of 5–250 μg/mL. After 24 h incubation at 34°C, resazurin (5 μg/mL) was added and fluorescence was recorded for 6 h with excitation/emission wavelengths of 520/590 nm.
Ethics statement
To perform the evaluation of tested agents in the presence of saliva, the materials were collected under IRB protocol: R-I-002/575/2013. This study was approved by the institutional review board (IRB) of the Medical University of Białystok. For both studies, all subjects provided informed written consent and collected samples were anonymous.
Results
Nanoparticle characterization
The synthesized chlorhexidine-coated magnetic nanoparticles (MNP@CHX) were characterized by FTIR spectroscopy. shows FTIR spectra for CHX in free (CHX) and immobilized form – MNP@CHX. The presence of the aminosilane shell around the magnetic core is indicated by a broad band at ~1,038–1,249 cm−1 corresponding to Si–O, Si–O–Si and Fe–O–Si stretching vibrations. In both samples, the bands at 3,355 and 3,393 cm−1 correspond to N–H bending and stretching vibrations, respectively. Bonds registered at 1,530–1,581 cm−1 have been assigned for the NH2 in-plane deformation vibration. The weak signals observed at 868 cm−1 for CHX and at 847 cm−1 from MNP@CHX are due to NH wagging. Strong adsorption bonds around 1,600 cm−1 might be ascribed to C=N stretching vibration which is characteristic for biguanide derivatives. After functionalization with CHX, a stretching vibration of the C=O bond around 1,734 cm−1 was recorded. The occurrence of C–H stretching bonds around 2,900 cm−1 was associated with symmetric and asymmetric ethyl and alkyl groups, which confirm the existence of an aminosilane shell on the MNP surface and the presence of CHX. UV–VIS spectroscopy was used to confirm successful functionalization of the nanoparticle surface. The absorbance spectra were obtained for aminosilane-coated MNPs (filled triangles) before functionalization. The similarity between the CHX curves (empty squares) and CHX functionalized nanocarriers MNP@CHX (filled squares) registered at specific for biguanide region (255–275 nm) strongly supports our results that CHX was successfully attached to the nanoparticle surface ().
Figure 1 Physicochemical properties of chlorhexidine-coated magnetic nanoparticles.
Notes: ATR FTIR spectra of the CHX and MNP@CHX (A). The UV–VIS spectra of uncoated MNP, MNP@CHX and free CHX (B). The DSC thermographs of CHX and MNP@CHX (C). Thermogravimetric analysis curves of magnetic nanoparticles coated by aminosilane and MNP@CHX (D). Transmission electron microscopy images (E) and size distribution (F) of MNP@CHX.
Abbreviations: ATR, attenuated total reflectance; CHX, chlorhexidine; DSC, differential scan ning calorimetry; FTIR, Fourier transform infrared spectroscopy; MNP, magnetic nanoparticles; MNP@CHX, MNP functionalized by chlorhexidine; TG, thermogravimetric; UV-VIS, ultraviolet-visible.
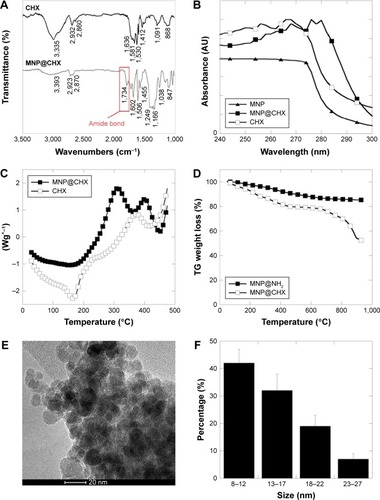
The thermal properties of synthesized nanoparticles were characterized by DSC (). The heating curves of free CHX (empty squares) and MNP@CHX (filled squares) indicate similarity in the chemical nature of the tested compounds. The decomposition of the organic skeleton at temperatures higher than 150°C was observed in the DSC curve of both agents. However, a weaker effect was observed for MNP@CHX. In both cases, large exothermic peaks were found around 370°C–400°C. shows the thermogravimetric analysis of the aminosilane- and CHX-decorated MNPs, respectively. In accordance with previously published results, a 6%–8% weight loss was observed for bare MNPs in the temperature range of 50°C–800°C, which is ascribed to the removal of adsorbed water and decomposition of hydroxyl surface groups.Citation36 The thermogravimetric analysis curves for aminosilane-coated MNPs (filled squares) show that the weight loss is about 9% (). The thermogravimetric analysis of CHX-functionalized MNPs (empty squares) shows a weight loss around 40% compared to the weight loss recorded for bare and aminosilane-coated MNPs. This indicates successful CHX immobilization on the MNP surface, with ~40% yield.
The analysis of saturation magnetization value of tested nanoparticles indicated a value of 68.7 emu/g for MNP@CHX. A relatively narrow size distribution of magnetic nanoparticles functionalized with CHX showed that MNP@CHX was characterized by spherical shape with a diameter of 12±2 nm as determined from the TEM image (the size was calculated using 100 randomly selected particles; ). In comparison with previously characterized aminosilane-coated MNPs, the obtained size of CHX-decorated MNPs did not significantly differ.Citation37
Covalent immobilization of CHX increases antimicrobial properties and enhances activity in the presence of saliva
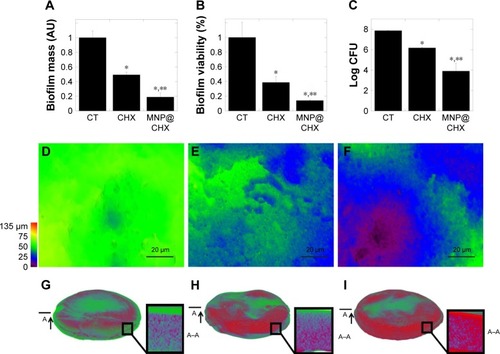
In order to evaluate the antimicrobial potential of the synthesized derivative, MIC/MBC or MFC/MBIC values of CHX and MNP@CHX against bacterial and fungal pathogens were assessed. As shown in , the functionalization of CHX onto the MNP surface strongly enhances the killing effect of CHX against all the tested strains. We observed a significant decrease in MIC/MBC or MFC/MBIC concentrations from 2 to 32 times. In the case of activity of magnetic nanocarriers, their activity against all tested strains was >32 μg/mL. Importantly, this trend is preserved in the presence of human saliva (). It is noteworthy that covalent immobilization of CHX on the MNPs not only increases the antimicrobial properties of CHX against individual bacterial and fungal strains but is also effective against samples containing mixed bacterial and fungal pathogens. Reduction of biofilm formation, particularly those formed by mixed species in conditions mimicking the oral cavity, is important for the creation of multimode antimicrobial agents. The preventive activities of CHX and MNP@CHX against biofilms representative of Gram-positive and Gram-negative bacteria and fungi are shown in . Functionalization of CHX to the MNP surface enhanced bactericidal activity two times in dosage range from 0.5 to 20 μg/mL for S. aureus, E. coli and C. albicans (). Furthermore, compared to CHX, MNP@CHX restricted biofilm formation by >50% (). Functionalization of CHX does not improve efficacy against all tested strains; similar activity of CHX and MNP@CHX were observed against E. faecalis biofilm formation (). We then tested the activity of CHX and MNP@CHX in the presence of human saliva, conditions designed to mimic the oral cavity. For all tested species, human saliva impaired the activity of free CHX (). However, this effect was not observed for MNP@CHX. We observed two- to sixfold enhanced inhibition of biofilm formation after addition of MNP@CHX at concentrations from 1 to 50 μg/mL (). Additionally, data in suggest that MNP@CHX has an increased ability to restrict growth of mixed-species biofilms compared to nonimmobilized agents. When CHX is covalently immobilized on the surface of MNPs, the growth of pathogenic cells is inhibited two times in all conditions (). The ability of the tested agents to inhibit fungal biofilm formation was further demonstrated in the presence of human saliva (). Biofilm mass as well as biofilm viability on the surface of dental fillings were reduced by twofold after the addition of CHX and fourfold following the addition of MNP@CHX. The aforementioned results were further confirmed by microtomography (). A similar result was obtained in the case of acryl substrate (data not shown).
Table 1 MIC (μg/mL) and MBC/MFC (μg/mL) of CHX and MNP@CHX against different pathogens including different clinical isolated from oral cavity/throatTable Footnote*
Table 2 MIC (μg/mL) and MBC/MFC (μg/mL) of CHX and MNP@CHX against different pathogens in the presence of human saliva
Figure 2 Anti-biofilm activity of chlorhexidine-coated magnetic nanoparticles against different biofilm-forming pathogens.
Notes: (A–E) Preventive effect of free CHX and MNP@CHX against biofilm-forming representative of Gram-positive and Gram-negative bacteria and fungi. *Statistically significant (P≤0.05) activity of MNP@CHX compared to CHX.
Abbreviations: CHX, chlorhexidine; MNP@CHX, magnetic nanoparticles functionalized by chlorhexidine; MRSA, methicillin-resistant Staphylococcus aureus.
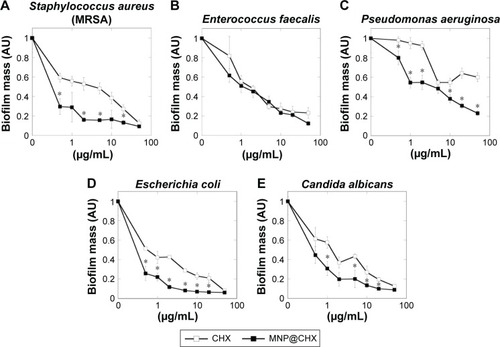
Figure 3 Magnetic nanoparticles increase anti-biofilm activity of chlorhexidine in the presence of human saliva.
Notes: Preventive activity of free CHX and MNP@CHX against biofilm-forming pathogens in the presence of human saliva (A–D). Effective inhibition of mixed-species biofilm formation in the presence of human saliva (E–G). *Statistically significant (P≤0.05) activity of magnetic nanoparticles coated by chlorhexidine compared to nonimmobilized drugs.
Abbreviations: CHX, chlorhexidine; MNP@CHX, magnetic nanoparticles functionalized by chlorhexidine; MRSA, methicillin-resistant Staphylococcus aureus.
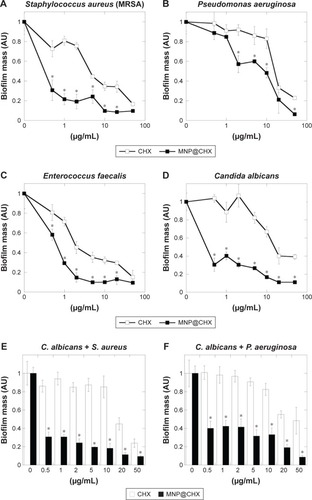
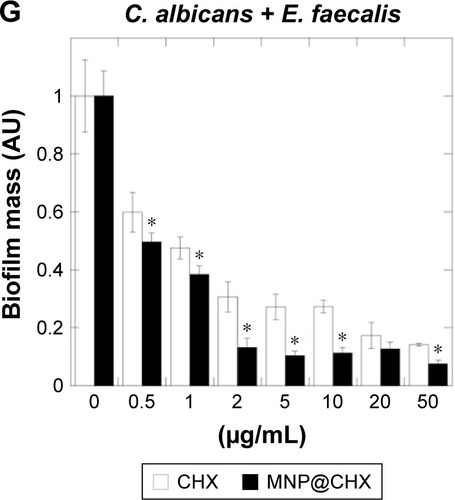
Figure 4 Chlorhexidine in free and immobilized form activity against fungal biofilm formation on the surface of dental filling.
Notes: Reduction of biofilm mass (A) and cell viability (B, C) after addition of CHX and MNP@CHX at 20 μg/mL. Panels (E and F) represent effective restriction of biofilm formation during 72 h on the surface of dental fillings after addition of chlorhexidine and MNP@CHX at 20 μg/mL compared to control (D). X-ray microtomography 3D images and cross sections of dental fillings with mature fungal biofilm before (G) and after treatment by chlorhexidine (H) and MNP@CHX (I) at 20 μg/mL. *Statistically significant (P≤0.05) activity tested agents compared to untreated control (CT). **Statistically significant (P≤0.05) activity of magnetic nanoparticles coated by chlorhexidine compared to nonimmobilized drugs. Red color indicates dental filling; green color shows the presence of fungal biofilm. The “A”s, arrows, and line segments indicate the place of cutting of material (technical drawing); the “A–A”s indicate cross-section of material.
Abbreviations: CFU, colony forming units; CHX, chlorhexidine; MNP@CHX, magnetic nanoparticles functionalized by chlorhexidine.
Magnetic nanoparticles modify CHX mode of action
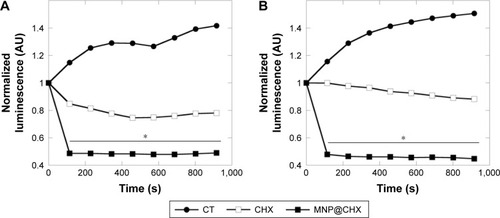
As shown in , the treatment of Candida cells by magnetic derivatives of CHX caused ~15% increase in mitochondrial damage compared to free drug (). The level of oxidative stress induced by MNP@CHX is comparable to that caused by hydrogen peroxide (). Moreover, luminomeric analysis indicates that MNP@CHX inhibit the metabolic activity of bacterial cells by twofold (). In addition, data in show that the presence of human saliva significantly decreases CHX metabolic effects while the effects of MNP@CHX remain unchanged.
Figure 5 Magnetic nanoparticles induce oxidative stress in Candida cells.
Notes: Flow cytometry analysis of the oxidative damage of untreated (A) and treated by 3% H2O2 (B) Candida albicans cells compared to cells treated by 10 μg/mL chlorhexidine (C) and chlorhexidine-coated magnetic nanoparticles (D) after 30 min incubation.
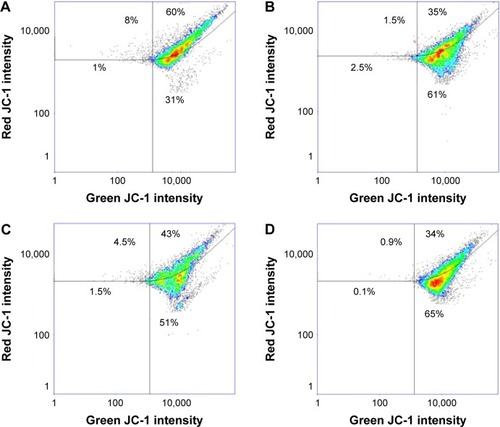
Figure 6 Magnetic nanoparticles accelerate chlorhexidine antibacterial action.
Notes: Reduction of Pseudomonas aeruginosa Xen 5 chemiluminescence signal after addition of chlorhexidine in free and immobilized forms (A) and in the presence of human saliva (B). *Statistically significant (P<0.05) compared to CHX in free form.
Abbreviations: CHX, chlorhexidine; CT, untreated control; MNP@CHX, magnetic nanoparticles functionalized by chlorhexidine.
Magnetic nanoparticles increase biocompatibility of CHX toward human osteoblast cells
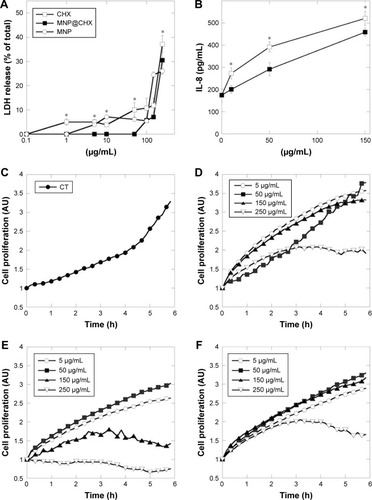
The lytic activities of the pure agents, bare magnetic nanocarrier and tested nanosystem on hFOB 1.19 cells are shown in . Application of nanosystems at concentrations from 1 to 50 μg/mL did not disrupt osteoblast membranes. In comparison with MNP@CHX, bare nanocarriers and free CHX induced significantly toxic effects. As shown in , the tested agents caused IL-8 release from human osteoblast cells at concentration ranges similar to those required for effective pathogen killing and prevention of biofilm formation. Data in demonstrate that compared to control CHX exerts high inhibition effect on hFOB 1.19 cell proliferation. The twofold decrease of cell proliferation was also observed after the addition of bare and CHX-decorated carriers at 250 μg/mL. Notably, treatment of cells with MNP@CHX did not affect cell proliferation at the concentration range of 5–150 μg/mL.
Figure 7 Effect of chlorhexidine in free and immobilized forms on human osteoblast cell integrity, interleukin release and their growth.
Notes: Panels (A, B) show the release of LDH and IL-8 from human osteoblast after 24 h of treatment. The growth curves of untreated osteoblast cells (C) and after addition of magnetic nanoparticles (D), chlorhexidine (E) and MNP@CHX (F) from 5 to 250 μg/mL. *Statistically significant (P<0.05) compared to CHX in immobilized form.
Abbreviations: CHX, chlorhexidine; CT, untreated control; MNP@CHX, magnetic nanoparticles functionalized by chlorhexidine.
Discussion
In recent years, inorganic metallic-based core–shell nanomaterials, especially those composed of an iron oxide core, have emerged as drug delivery systems with unique properties, such as the easier synthesis, the ability for drug loading, release of their cargo in a specified manner as well as improved biocompatibility. In addition, iron oxide magnetic nanomaterials are able to disrupt pathogen membranes and generate reactive oxygen species, which cause mitochondrial damage. These beneficial features support the application of magnetic nanomaterials as a drug carrier in modern therapies. The number of infections caused by drug-resistant pathogens is constantly growing; it is estimated that patients with MDR strains have >60% mortality compared to those with nonresistant infections. A consequence of increasing drug resistance is persistence of infection with increased risk of spread. Development of resistance is directly associated with the ability of pathogens to form biofilms. Biofilm formation usually exhibits resistance to classical antibiotic treatment, which is conditioned by an increase of total macromolecules contents of the biofilm matrix.Citation38,Citation39 In effect, nanotechnology-based therapies may be promising in the eradication of bacterial and fungal pathogens, including those resistant to conventional antibiotic therapy.Citation19
In our study, the antimicrobial properties of magnetic nanoparticles functionalized by the well-known antiseptic, CHX, were thoroughly analyzed. CHX is a biguanide derivative and, due to both hydrophilic amine groups and hydrophobic structure, causes disruptions to membrane permeability at low doses. This is additionally facilitated by the positive charge of CHX, which allows for binding of antimicrobial agents to anionic molecules present on the pathogen cell wall.Citation1 Importantly, it has been shown that this phenomenon is more likely than the inhibition of cell membrane ATPases and that destabilization of pathogen membranes is the lethal event in the CHX mechanism of action.Citation40 At higher concentrations, CHX attacks the bacterial cytoplasmic membrane and denatures microbial proteins. CHX has both a rapid onset of bactericidal action and prolonged antimicrobial efficacy through residual effects.Citation1 It is established that CHX possess broad spectrum of activity against Gram-positive and Gram-negative bacteria and fungi. However, in that case, interestingly, Barrett-Bee et al demonstrated the treatment of Gram-negative bacteria with CHX leads to the release of periplasmic enzymes, but not the disruption of inner membranes.Citation41 Moreover, Audus et al showed that CHX strongly inhibits adherence of C. albicans to human buccal epithelial cells and altered epithelial-cell membrane-lipid packing order, which is likely involved in the antifungal actions of the drug.Citation42 According to the data shown here, immobilization of CHX on magnetic nanocarriers modulates its mechanism of action, resulting in depolarization of mitochondria in treated fungal cells. Taken together, we propose that induction of oxidative stress and oxidation of fungal structures are additional mechanisms responsible for pathogen killing in the presence of MNP@CHX.
Development of modern dentistry using nanotechnology approaches is focusing on esthetics by making the materials more translucent, modify the wear properties as well as prevent rather than treat biofilm-dependent oral diseases including formation of dental caries and endodontic and periodontal diseases.Citation43 To data, several nanomaterials such as metallic (silver, zinc oxide) and polymeric (mesoporous silica, polyethylenimine) have been used for dental composite or dental adhesive creation to inhibit pathogen growth. This is due to the generation of reactive oxygen species, disruption of the cell membrane, inhibition metabolic processes, displacement of magnesium ions required for the enzymatic activity of oral biofilms, disturbance of the electron transportation, oxidation of macromolecules and prevention of DNA replication.Citation44 These nanoparticles are able to reduce the bacterial dental plaque biofilm formation in an in vitro setting.Citation45 It has also been shown that coating teeth with antibacterial nanomaterials is effective in restricting bacteria growth as well as inhibiting bacterial adhesion and preserving its integrity in the presence of saliva.Citation46 In our study, we report, for the first time, the enhanced activity of CHX-functionalized magnetic nanoparticles against Candida sp. which are the most common opportunistic pathogens in oral microbiota and play a key role in failure of root canal treatment.Citation47,Citation48 We have also shown that our nanosystem is able to restrict the growth of pathogens in monomicrobial and polymicrobial conditions. Broad spectrum activity that includes activity against Gram-positive and Gram-negative bacteria and fungi is important because mixed infections are common in oral cavity causing topical and postsurgery infections.Citation49 Moreover, significant reduction of fungal biofilm growth and prevention before penetration into dental filling has been observed. Importantly, in agreement with previous reports, we confirmed that magnetic derivatives of CHX not only maintained the antimicrobial activity of CHX but also increased its killing properties in the presence of saliva. This observation is significant as previous reports demonstrate reduced antimicrobial activity of CHX against oral bacteria in human saliva.Citation50,Citation51 The neutralizing effect of saliva on CHX may be overcome by the addition of 7% ethanol due to its antimicrobial effect and its impact on salivary proteins.Citation50 Despite the desirable synergistic effect of ethanol on the antimicrobial activity of CHX, some reports indicate that the addition of this compound into mouth rinse formulations might be associated with the development of oral cancer due to DNA adduct formation and sister chromatid exchange in vitro.Citation52 However, more recent research suggests that the protumorigenic effect of ethanol is observed if concentrations above 25% are used.Citation53 The attachment of CHX onto magnetic nanoparticles considerably enhances the activity of CHX in human saliva and offers an alternative for ethanol in mouth rinse formulations. Importantly, this effect is not associated with the toxic effects initially observed for unmodified CHX. A number of studies confirm the cytotoxic effect of CHX on the viability of host cells. Balloni et al showed that CHX-containing mouth rinses reduce fibroblast and keratinocyte adhesion and considerably affects cell proliferation due to downregulation of cell adhesion receptors and altered gene expression of matrix components.Citation54 CHX is also considered as the one of the most cytotoxic of the commonly used root canal irrigants.Citation55 Among other intracanal irritants, more toxic agents such as sodium hypochlorite might be distinguished.Citation56,Citation57 In contrast, CHX does not affect significantly the viability of murine macrophages, but may have an immunosuppressive effect on exposed macrophages.Citation58 A recent study demonstrated that cytotoxicity of CHX might be reduced using modified nonequilibrium plasma. Considering these data, plasma-based modification was proposed as a safe option for root canal treatment.Citation59 Data presented in this study confirmed that immobilization of CHX on MNPs also decreases the toxic effect of CHX without affecting its killing properties. In effect, magnetic derivatives of CHX may be an interesting alternative for formulations presented by Chen et al.Citation60 Importantly, the employment of nanotechnology-based structures in dentistry provides a way for the introduction of novel therapy approaches based on structures that allow to control drug release and to improve the regenerative processes with subsequent strong antimicrobial activity, even in the presence of salivary proteins.Citation61
Conclusion
MNPs functionalized by CHX represent a novel approach for the therapy of oral pathogens. Due to the strong antimicrobial activity of magnetic derivatives, the proposed formulation is effective in the treatment of monomicrobial as well as polymicrobial infections, which is additionally intensified by the strong anti-biofilm activity of this derivative. Essentially, the enhanced killing activity of MNP@CHX in the presence of salivary proteins coupled with its low toxicity against human osteoblasts strongly indicates the potential use of this formulation in the treatment of local infections caused by oral microflora.
Acknowledgments
This work was supported by the National Science Center, Poland, under grant UMO-2014/15/D/NZ6/02665 (to KN). In 2016, KN was awarded a fellowship from the Foundation for Polish Science. This study was conducted with the use of equipment purchased by the Medical University of Białystok as part of the RPOWP 2007–2013 funding, Priority I, Axis 1.1, contract number UDA-RPPD.01.01.00-20-001/15-00, dated 26.06.2015.
Disclosure
The authors report no conflicts of interest in this work.
References
- KarpińskiTMSzkaradkiewiczAKChlorhexidine – pharmaco-biological activity and applicationEur Rev Med Pharmacol Sci20151971321132625912596
- WoodAPayneDThe action of three antiseptics/disinfectants against enveloped and non-enveloped virusesJ Hosp Infect19983842832959602977
- FathilahARHimratul-AznitaWHFatheenARSurianiKRThe antifungal properties of chlorhexidine digluconate and cetylpyrinidinium chloride on oral CandidaJ Dent201240760961522521700
- KuyyakanondTQuesnelLBThe mechanism of action of chlorhexidineFEMS Microbiol Lett19921001–32112151335944
- ZorkoMJeralaRAlexidine and chlorhexidine bind to lipopolysaccharide and lipoteichoic acid and prevent cell activation by antibioticsJ Antimicrob Chemother200862473073718635521
- BonacorsiCRaddiMSCarlosIZCytotoxicity of chlorhexidine digluconate to murine macrophages and its effect on hydrogen peroxide and nitric oxide inductionBraz J Med Biol Res200437220721214762575
- MimozOLucetJCKerforneTSkin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trialLancet2015386100082069207726388532
- CoetzeeERodeHKahnDPseudomonas aeruginosa burn wound infection in a dedicated paediatric burns unitS Afr J Surg2013512505323725892
- VaroniETarceMLodiGCarrassiAChlorhexidine (CHX) in dentistry: state of the artMinerva Stomatol201261939941922976567
- ManikandanDBalajiVRNiaziTMRohiniGKarthikeyanBJesudossPChlorhexidine varnish implemented treatment strategy for chronic periodontitis: a clinical and microbial studyJ Pharm Bioallied Sci20168Suppl 1S133S13727829764
- EdmistonCEJrBrudenBRucinskiMCHenenCGrahamMBLewisBLReducing the risk of surgical site infections: does chlorhexidine gluconate provide a risk reduction benefit?Am J Infect Control2013415 SupplS49S5523622749
- MillerLMLoderJSHansbroughJFPetersonHDMonafoWWJordanMHPatient tolerance study of topical chlorhexidine diphosphanilate: a new topical agent for burnsBurns19901632172202383364
- SupranotoSCSlotDEAddyMVan der WeijdenGAThe effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration: a systematic reviewInt J Dent Hyg2015132839225059640
- AthanassiadisBAbbottPVWalshLJThe use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodonticsAust Dent J2007521 SupplS64S8217546863
- McDonnellGRussellADAntiseptics and disinfectants: activity, action, and resistanceClin Microbiol Rev19991211471799880479
- BrooksSEWalczakMAHameedRCoonanPChlorhexidine resistance in antibiotic-resistant bacteria isolated from the surfaces of dispensers of soap containing chlorhexidineInfect Control Hosp Epidemiol2002231169269512452299
- BhardwajPZieglerEPalmerKLChlorhexidine induces vanA-type vancomycin resistance genes in enterococciAntimicrob Agents Chemother20166042209222126810654
- LiuQZhaoHHanLShuWWuQNiYFrequency of biocide-resistant genes and susceptibility to chlorhexidine in high-level mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MuH MRSA)Diagn Microbiol Infect Dis201582427828326008124
- HuhAJKwonYJ“Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant eraJ Control Release2011156212814521763369
- PiktelENiemirowiczKWątekMWollnyTDeptułaPBuckiRRecent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapyJ Nanobiotechnology20161413927229857
- NiemirowiczKProkopIWilczewskaAZMagnetic nanoparticles enhance the anticancer activity of cathelicidin LL-37 peptide against colon cancer cellsInt J Nanomedicine2015103843385326082634
- NiemirowiczKSurelUWilczewskaAZBactericidal activity and biocompatibility of ceragenin-coated magnetic nanoparticlesJ Nanobiotechnology20151313225929281
- MichalakGGluszekKPiktelEPolymeric nanoparticles – a novel solution for delivery of antimicrobial agentsStudia Medyczne20163215662
- GuHHoPLTongEWangLXuBPresenting vancomycin on nanoparticles to enhance antimicrobial activitiesNano Letters20033912611263
- NiemirowiczKMarkiewiczKHWilczewskaAZCarHMagnetic nanoparticles as new diagnostic tools in medicineAdv Med Sci201257219620723154427
- NiemirowiczKSwiecickaIWilczewskaAZGrowth arrest and rapid capture of select pathogens following magnetic nanoparticle treatmentColloids Surf B Biointerfaces2015131293825942700
- ParkHParkHJKimJAInactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticlesJ Microbiol Methods2011841414520971135
- TaylorEWebsterTJReducing infections through nanotechnology and nanoparticlesInt J Nanomedicine201161463147321796248
- NiemirowiczKDurnaśBTokajukGMagnetic nanoparticles as a drug delivery system that enhance fungicidal activity of polyene antibioticsNanomedicine20161282395240427464757
- MassartRPreparation of aqueous magnetic liquids in alkaline and acidic mediaIEEE Transactions on Magnetics198117212471248
- NiemirowiczKSwiecickaIWilczewskaAZGold-functionalized magnetic nanoparticles restrict growth of Pseudomonas aeruginosaInt J Nanomedicine201492217222424855358
- MisztalewskaIWilczewskaAZWojtasikOMarkiewiczKHKuchlewskiPMajcherAMNew acetylacetone-polymer modified nanoparticles as magnetically separable complexing agentsRSC Advances20155121100281100289
- MeletiadisJMoutonJWMeisJFComparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis [2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide], for antifungal susceptibility testing of Aspergillus speciesJ Clin Microbiol200139124256426311724829
- SabaeifardPAbdi-AliASoudiMRDinarvandROptimization of tetrazolium salt assay for Pseudomonas aeruginosa biofilm using microtiter plate methodJ Microbiol Methods201410513414025086178
- O’TooleGAMicrotiter dish biofilm formation assayJ Vis Exp201147 pii 2437
- Hussein-Al-AliSHEl ZowalatyMEHusseinMZIsmailMWebsterTJSynthesis, characterization, controlled release, and antibacterial studies of a novel streptomycin chitosan magnetic nanoantibioticInt J Nanomed20149549557
- NiemirowiczKPiktelEWilczewskaAZCore-shell magnetic nanoparticles display synergistic antibacterial effects against Pseudomonas aeruginosa and Staphylococcus aureus when combined with cathelicidin LL-37 or selected cerageninsInt J Nanomedicine2016115443545527799768
- BuckiRNiemirowiczKWnorowskaUPolyelectrolyte-mediated increase of biofilm mass formationBMC Microbiol20151511726048182
- WnorowskaUWątekMDurnaśBExtracellular DNA as an essential component and therapeutic target of microbial biofilmStudia Medyczne2015312132138
- NguyenTKDuongHTTSelvanayagamRBoyerCBarraudNIron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacySci Rep201551838526681339
- Barrett-BeeKNewboultLEdwardsSThe membrane destabilising action of the antibacterial agent chlorhexidineFEMS Microbiol Lett19941191–22492538039666
- AudusKLTavakoli-SaberiMRZhengHBoyceENChlorhexidine effects on membrane lipid domains of human buccal epithelial cellsJ Dent Res1992716129813031613179
- MantriSSMantriSPThe nano era in dentistryJ Nat Sci Biol Med201341394423633833
- Abou NeelEABozecLPerezRAKimHWKnowlesJCNanotechnology in dentistry: prevention, diagnosis, and therapyInt J Nanomedicine2015106371639426504385
- LiFWeirMDFouadAFXuHHEffect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm modelDent Mater201430218219124332270
- BesinisADe PeraltaTHandyRDInhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentineNanotoxicology20148774575423875717
- SiqueiraJFSenBHFungi in endodontic infectionsOral Surg Oral Med Oral Pathol Oral Radiol Endod200497563264115153878
- GomesCFidelSFidelRde Moura SarquisMIIsolation and tax-onomy of filamentous fungi in endodontic infectionsJ Endod201036462662920307734
- O’DonnellLEMillhouseESherryLPolymicrobial Candida biofilms: friends and foe in the oral cavityFEMS Yeast Res2015157
- AmenabarIPolySNuansingWStructural analysis and mapping of individual protein complexes by infrared nanospectroscopyNat Commun20134289024301518
- ShiYBLiuLShaoWWeiTLinGMMicrocalorimetry studies of the antimicrobial actions of alkaloidsJ Zhejiang Univ Sci B201516869069526238544
- MiyakeYTsunodaTMinagiSAkagawaYTsuruHSuginakaHAntifungal drugs affect adherence of Candida albicans to acrylic surfaces by changing the zeta-potential of fungal cellsFEMS Microbiol Lett19905732112142210332
- SardiJCScorzoniLBernardiTFusco-AlmeidaAMMendes GianniniMJCandida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic optionsJ Med Microbiol201362Pt 1102423180477
- BalloniSLocciPLumareAMarinucciLCytotoxicity of three commercial mouthrinses on extracellular matrix metabolism and human gingival cell behaviourToxicol In Vitro201634889627039991
- PoletaevABouraPThe immune system, natural autoantibodies and general homeostasis in health and diseaseHippokratia201115429529824391407
- WongDTCheungGSExtension of bactericidal effect of sodium hypochlorite into dentinal tubulesJ Endod201440682582924862710
- PeckBWWorkenehBKadikoyHAbdellatifASodium hypochlorite-induced acute kidney injurySaudi J Kidney Dis Transpl201425238138424626008
- CavaleiroEDuarteASEstevesACNovel linear polymers able to inhibit bacterial quorum sensingMacromol Biosci201515564765625626858
- GuéganSLanternierFRouzaudCDupinNLortholaryOFungal skin and soft tissue infectionsCurr Opin Infect Dis201629212413026915073
- ChenHShiQQingYYaoYCCaoYGCytotoxicity of modified nonequilibrium plasma with chlorhexidine digluconate on primary cultured human gingival fibroblastsJ Huazhong Univ Sci Technolog Med Sci201636113714126838755
- WangBWeiPWangXLouWControlled synthesis and size-dependent thermal conductivity of Fe3O4 magnetic nanofluidsDalton Trans201241389689922086086
