Abstract
Despite the fact that technological advancements have been made in diagnosis and treatment, cardiovascular diseases (CVDs) remain the leading cause of mortality and morbidity worldwide. Early detection of atherosclerosis (AS), especially vulnerable plaques, plays a crucial role in the prevention of acute coronary syndrome (ACS). Targeting the critical cytokines and molecules that are upregulated during the biological process of AS by in vivo molecular imaging has been widely used in plaque imaging. With their three-dimensional architecture, composition, and abundant terminal functional groups, dendrimers provide a platform for multitargeting and multimodal imaging. Thus, modified dendrimers with the key molecules upregulated in AS plaques will be an innovative attempt to achieve targeted imaging of AS plaques specifically and efficiently. This review was aimed to address some recent works on imaging of AS plaques using various types of image technology and further discuss the applications of dendrimers, an innovative yet seldom used method in imaging of AS plaques due to some limitations and challenges, and we highlight the bright future of the modified dendrimers in characterizing AS plaques.
Introduction
Despite the continuous and significant advances that have been made in diagnosis and therapies over the past decades, cardiovascular diseases (CVDs), including unstable angina, stroke, acute myocardial infarction with or without ST elevation, and sudden coronary death, remain the principal cause of mortality and morbidity globally.Citation1
Most of the acute coronary syndromes (ACSs) are believed to be triggered by atheromatous plaque rupture.Citation2,Citation3 A vulnerable plaque contains a large lipid core and a thin fibrous cap with severe inflammatory cell infiltration, causing the release of matrix metalloproteinase (MMP). Released MMPs further weaken the fibrous cap, thus leading to disruption of the plaque cap, after which contents of the necrotic core, especially the collagen fibers, are exposed to the vessel lumen, resulting in thrombosis, which has been recognized as the pathological basis of many cardiovascular events. Therefore, advanced methods for early prevention as well as treatment of atherosclerosis (AS) are critically needed.Citation4
At present, commonly used imaging techniques,Citation5 including ultrasound (US), radiography, computed tomography (CT), nuclear medicine, eg, positron emission tomography (PET)Citation6–Citation8 and single photon emission CT (SPECT),Citation9,Citation10 and magnetic resonance imaging (MRI).Citation11 And MRI includes T1-weighted (T1w) and T2-weighted (T2w) MRICitation12–Citation14 or magnetic resonance angiography (MRA) scansCitation15 and also many other optical imaging methods, all play a key role in the diagnosis of AS ().Citation16,Citation17
Table 1 Commonly used imaging techniques for AS diagnosis
The development of medical imaging technology underwent three revolutionary innovation processes from structure imaging to functional imaging and to molecular imaging. Traditional intravascular invasive imaging techniques, including intravascular ultrasound (IVUS), acoustic contrast (ultrasonic contrast), intravascular MRI (IVMRI), and optical coherent tomography (OCT), are put emphasis mainly on the observation of internal structure of the vessel; however, the invasive procedures are costly and dangerous in terms of certain complications, such as wound infection, thrombosis formation, fragment detachment, and even artery rupture.Citation18–Citation21 Nowadays, with the development of imaging and advances in molecular biology, noninvasive molecular imaging becomes more and more important in disease diagnosis. Molecular imaging is a new trend in recent years, which combines molecular biology and in vivo imaging together to make cellular functions visualized, and traces molecular process in vivo as a noninvasive evaluating method.Citation20 Usually, disease-related biomarkers, which are upregulated during the pathological process of the diseases, are needed in molecular imaging to target specific cells or molecules.Citation5,Citation22–Citation24 For AS diagnosis, molecular imaging with target-specific probes has shown great promise for noninvasive in vivo monitoring of biological processes of atheromatous plaques at molecular and cellular levels in both animals and humans. By targeting the specific molecules, the contrast agents can be located to atherosclerotic lesions accurately and signal intensity of different imaging techniques can thus be increased.
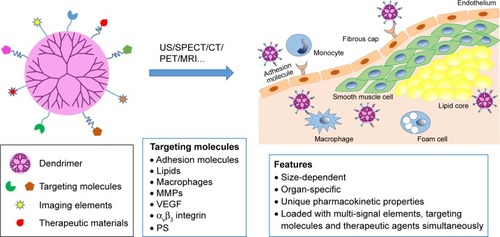
Dendrimers are a somewhat highly branched, mono-dispersed, and artificially synthetic macromolecules. Their unique well-defined three-dimensional architecture, composition, and abundant terminal functional groups make them potential carriers for imaging agents and drugs ().Citation25 For imaging, dendrimers can be modified by two or more molecules or imaging agents and have been applied for targeted imaging of many diseases such as cancer for many years.Citation26 For example, some researchers have been studying dendrimers labeled with gold (Au) or gadolinium (Gd) in imaging of AS plaques using CT or magnetic resonance (MR), which would allow researchers or surgeons to locate nidi accurately and therefore treat the diseases efficiently. Here, we give a review on the present progress of AS plaque imaging and further discuss the applications and prospects of dendrimers in characterization of the AS plaques.
Figure 1 Dendrimer-facilitated molecular imaging of atherosclerosis plaque.
Note: Dendrimer modified with multi-signal elements or therapeutic agents can target at a series of molecules that are upregulated in plaque area during AS, and can function as contrast agents or therapeutic agents there.
Abbreviations: AS, atherosclerosis; US, ultrasound; CT, computed tomography; SPECT, single photon emission CT; PET, positron emission tomography; MRI, magnetic resonance imaging; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor; PS, phosphatidylserine.
Pathophysiology of AS and biomarkers for molecular target imaging
Molecular imaging provides great potential for noninvasive visualization of the cellular and molecular components involved in the development of atheromatous plaques (). We first discuss some key molecular imaging modalities and approaches for noninvasive detection of biological processes of atherogenesis, including inflammatory infiltration (described as upregulation of endothelial adhesion molecules and infiltration of macrophages), fibrotic responses, and eventually the formation of vulnerable plaques.
Table 2 AS biomarkers used in molecular imaging
The formation of plaques
In the early stage of atherogenesis, hypercholesterolemia accounts for the vascular endothelial dysfunction, which is characterized by induced endothelial activation and increased endothelial permeability, arterial infiltration of leukocyte, platelet activation, and proliferation of smooth muscle cells.Citation27 The increased endothelial permeability allows lipids to penetrate across the arterial wall, especially oxidized low-density lipoprotein (oxLDL), which can impair endothelial cell (EC) and induce expression of growth factors by the damaged EC, including monocyte chemotactic protein-1 (MCP-1), platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and P-selectin and E-selectin expressed on the activated endothelial surface. This in turn promotes recruitment of monocytes to the vessel wall where monocytes roll along the activated endothelium and continuously absorb oxLDL through surface receptors, finally forming foam cells. These molecules further impair the function of EC. Therefore, endothelial dysfunction and lipid accumulation can be imaging biomarkers for early detection of AS.Citation1
Some researchers have developed certain peptides that can target VCAM-1, which has shown high affinity and specificity for characterizing VCAM-1 both in vitro and in vivo.Citation28–Citation30 Jansen et al imaged lipids in atheromatous plaques using spectroscopic intravascular photoacoustics (sIVPA) to understand the progress of AS.Citation31
Low-density lipoprotein (LDL) oxidation is a vital step for the initiation of atherosclerotic plaque formation, and a variety of oxidation-specific epitopes (OSEs) can generate by oxLDL. OSEs, which are not only expressed on oxLDL but also appeared on apoptotic cells and proteins in the extracellular matrix of atherosclerotic vessel wall, are proved immunogenic, proinflammatory, and proatherogenic. OSEs are usually recognized by innate pattern recognition receptors (PRRs), including scavenger receptors (SRs), which are generally present on the surface of dendritic cells and monocyte/macrophages, and also by some innate proteins, and these receptors or proteins further activate the innate immune responses, which normally are protective but can be proatherogenic if overactivated.Citation32–Citation34 Thus, OSEs have been developed as targets of noninvasive imaging of AS plaques. Briley-Saebo et alCitation35 generated manganese (Mn(II)) molecular imaging probes targeting to OSEs with oxidation-specific antibodies MDA2 and IK17. After administration of the Mn-micelles for 48–72 h, strong MR signal enhancement was observed, with localization of the Mn-micelles within intraplaque macrophages. The results demonstrated that OSEs have the potential to be biomarkers of AS plaques and could be used in its molecular imaging in the future.
Inflammation (inflammatory infiltration)
AS is a chronic inflammatory progression, which is regulated by innate and adaptive immune responses.Citation36 Innate immunity usually based on the PRR on surface of macrophages and dendritic cells to identify various pathogens. PRRs are diverse, and SRs as well as Toll-like receptors (TLRs) are playing an important role in the formation of AS lesions. The initiation of adaptive immunity also can produce a large number of T cells, B cells, and immunoglobulin into AS lesion. Pathogenesis of AS lesions initially resulted from endothelial dysfunction, which then initiates T cells and macrophages to blood vessel walls and causes local infiltration of them, T cells then recognize oxLDL cholesterol, heat shock protein (HSP), and microbe antigen through molecular PRRs and induce the release of proinflammatory cytokines, causing complex interplay between different circulating factors and various cell types in the vessel wall.Citation36–Citation38 Then, it leads to the accumulation of lipids in the sub-endothelial space and a complex process of chronic inflammation, followed by migration of monocytes to endothelium and conversion of it to macrophages, which highly express a large number of SRs.Citation39 When the lipids in the blood vessel are taken up by the macrophages through SRs and oxidized, the macrophages will change into foam cells with accumulation of esterified cholesterol. oxLDL can induce ECs to secrete MCP-1 as well as VCAM-1, which induce migration of macrophages to the lesion area. Macrophages in subcutaneous space will secrete various kinds of inflammatory cytokines that stimulate proliferation and migration of smooth muscle cells and proteolytic enzymes (eg, MMPs) that can degrade collagen and elastin, resulting in the thinning of the cap of the growing plaque and making the plaque susceptible to rupture. There is no doubt that the activated macrophages can be an important target for AS imaging, as they play such a key role in all stages of AS progression. Thus, targeting imaging of AS in many studies was also based on the accumulation of imaging particles in macrophages.Citation40–Citation44
Activated macrophages express some receptors and molecules on their surface, which can be targeted for plaque imaging. SRs such as SRA and SRB1, CD36, CD68, and TLRsCitation2 are significantly overexpressed on atherosclerotic macrophages and foam cells. Amirbekian et alCitation40 used anti-CD204 monoclonal IgG to target CD 204, a macrophage SR (MSR) for assessing AS in ApoE−/− mice by MRI, and found that macrophage-targeted immunomicelles significantly enhanced the uptake of imaging agent (by 79%) compared to the untargeted immunomicelles (by 34%). For the first time, it was discovered that MSR, a trimeric integral membrane glycoprotein that can bind to some proteins and molecules, could be detected due to its unusually broad ligand specificity and macrophages could be targeted in vivo with a positive contrast molecular construct designed for specific and sensitive assessment of AS. In addition, a large variety of polyanionic macromolecules, such as maleylated bovine serum albumin, have broad ligand specificity for several SRs overexpressed on the macrophages of the plaque and, therefore, can be applied as potential macrophage-targeting carriers.Citation44
Lectin-like oxidized LDL receptor-1 (LOX-1, a kind of SR) is an important receptor expressed on macrophages as well as some ECs and VSMCs under pathological conditions. oxLDL binding to LOX-1 will induce apoptosis as well as expression of adhesion molecules and MMP and activate the inflammatory pathway, all of which eventually contribute to the rupture of the plaques.Citation45–Citation48 In view of high expression of LOX on more than one kind of cells concerned in AS, LOX-1 will have great potential to be a target for molecular imaging of AS plaques. At present, imaging probes targeted at LOX-1 have been developed to improve sensitivity, specificity, and biocompatibility compared to the nontargeted ones.Citation49,Citation50 LOX-1 antibody-conjugated ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles could be taken in vulnerable plaques in vivo, which could be verified by colocalization of the nanoparticles with vulnerable plaque biomarkers, such as macrophages and MMP-9.Citation50 Li et alCitation47 prepared an imaging probe consisting of liposomes loaded with LOX-1 antibody or nonspecific immunoglobulin G and tested their imaging effects using SPECT/CT and magnetic resonance. The LOX-1 targeted probes could bind to atherosclerotic plaques, especially to the shoulder areas of the plaque, a region with features of vulnerable plaque and LOX-1 expresses extensively, while nontargeted ones could not. The results were confirmed by observing no signal enhancement in LDLR−/−/LOX-1−/− mice.
Moreover, inflammatory response is a complex process with many cytokines and receptors expressed in the progression of AS. Using a novel CD80-specific probe, Muller et alCitation51 discriminated the stable atherosclerotic plaques from the vulnerable ones in vitro. Neutrophil gelatinase-associated lipocalin (NGAL), which is highly expressed in plaques and associated with increased MMP-9 activity, may be developed as a new imaging target for the diagnosis of high-risk plaques.Citation52 In addition, chemokine receptors as well as IL-10 and CD40 can serve as targets for noninvasive imaging of atherosclerotic plaques.Citation53–Citation55
Fibroatheromas
Fibroatheromas consist of a necrotic core and a thick fibrous cap. The necrotic core is a result of macrophage infiltration with the release of MMP and macrophage apoptosis. The thick fibrous cap is composed of collagen, proteoglycans, and smooth muscle cells. The fibrous cap can be thinned by MMPs as well as apoptotic smooth muscle cells, leading to the rupture of the plaque cap.Citation2
MMPs are a complex family of zinc-dependent endopeptidases that are usually characterized as secreted proteases responsible for degrading extracellular matrix components.Citation56,Citation57 MMP activity in the vessel wall is based on its expression level, activation state, and presence of its inhibitors.Citation3 Overexpression of MMPs is a main factor in the transformation of plaques from stable to vulnerable. Thus, MMP-targeted imaging may have bright future in diagnosing vulnerable plaques. To characterize MMPs in plaque, some radiolabeled MMP inhibitors (MPIs) have been synthesized and employed. High radio signal could be detected in the segment with higher MMP expression for MPI targeting.Citation58 RP805, a kind of MPI labeled with 99mTc, could be used as an MMP-targeted tracer for microSPECT imaging.Citation59 Schafers et alCitation60 used another broad-spectrum MMP inhibitor HO-CGS 27023A, labeled with 123I or 125I, to image MMP activity in vivo in ApoE−/− mice using scintigraphy. Compared to the blockade group, there was ~1.5-fold increase in accumulation of the imaging agent caused by the specific uptake of the inhibitor HO-CGS 27023A in the plaque. The molecular imaging of MMP activity in lesions of AS may help predict and prevent against coronary events caused by plaque rupture.
The formation of vulnerable plaques
With the release of MMPs and the apoptosis of SMCs, the fibroatheromas transform into vulnerable plaques, which have a larger necrotic core and a thinner fibrous cap.Citation3,Citation61 Other features of vulnerable plaques include multiplication of inflammatory cells, especially in the fibrous cap, and large number of apoptotic cells (originally in the form of monocytes/macrophages and VSMCs). The infiltration of the inflammatory cells causes compensatory angiogenesis to resist hypoxia circumstances. However, neovessels will further enhance infiltration of inflammatory cells, leading to the release of proteolytic enzymes, causing excessive extracellular matrix degradation, which in turn induces matrix remodeling.Citation3 Moreover, fragile neovessels are prone to rupture, leading to intraplaque hemorrhageCitation62,Citation63 Furthermore, all the pathological process of AS mentioned earlier including hemodynamics, endothelial dysfunction, and inflammation would lead to vascular remodeling,Citation64 which usually involved in cell growth, apoptosis, migration, and extracellular matrix synthesis, degradation as well as rearrangement, to increase plaque vulnerability.Citation65 While plaque formation or intimal hyperplasia has been recognized as a major determinant of AS luminal stenosis for many years, increasing numbers of studies on AS showed that vascular remodeling may be the another key factor of narrowing lumen size, aggravating the formation and rupture of unstable plaque.Citation66,Citation67 Therefore, all these characteristics possessed by the vulnerable plaques are potential targets for molecular imaging.
Phosphatidylserine (PS), a protein usually expressed on the outer surface of apoptotic cell membranes, also contributes to the rupture of vulnerable plaques. Annexin A5 is a protein, which possesses a nanomolar binding affinity to PS. Thus, researchers have utilized Annexin A5 to target PS for noninvasive imaging of atherosclerotic plaques.Citation68–Citation70 For example, 99mTc-annexin A5 was injected to animals for non-invasive in vivo SPECT/CT imaging and the lesions were clearly visualized. The 99mTc-annexin A5 was highly taken up in the plaques rich in macrophages. Through histological and immunohistochemical evaluations, a significant correlation between radiotracer uptake and both macrophage infiltration and the extent of apoptosis was revealed.Citation71 As is well known to all, exteriorized PS residues expressed on apoptosis cells accelerate their recognition by macrophages via CD36, which triggers rapid phagocytosis.Citation72–Citation74 Maiseyeu et alCitation74 mimicked apoptosis using liposomes containing PS and looked into the utility of paramagnetic Gd liposomes enriched with PS (Gd-PS) using fluorescence plate reader as well as MRI scanner in imagining AS plaques. They found rapid and significant enhancement of the aortic wall both in vivo and in vitro, thus such Gd-PS liposomes can provide a new targeting approach of contrast agent for imaging macrophage. In addition, caspase is a promising target for imaging apoptosis. Hight et alCitation75 have successfully utilized a peptide-based caspase inhibitor for the quantification of caspase activity by PET.
αvβ3 integrin can be targeted to image neovascularization, as it is only expressed by ECs of neovascellum, proliferating VSMCs and activated macrophages.Citation76 For imaging matrix remodeling, Phinikaridou et alCitation77 successfully assessed vascular remodeling for the prediction of vulnerable plaques with an elastin-specific contrast agent. In addition, vascular endothelial growth factor (VEGF) plays a key role in vascular remodeling. Imaging methods targeted at VEGF receptor have a promising future for detecting vascular remodeling.Citation78
Limitations for current contrast agents
Although plenty of approaches have been evaluated to image AS plaques and showed great prospects for further basic and clinical studies, there remain certain limitations and challenges for plaque-targeted imaging.
One of these limitations is the low sensitivity of some imaging modalities (MRI), which only target at a few biological molecules with high expression in AS plaques.Citation14,Citation79 For smaller coronary imaging, high temporal and spatial resolution are necessary, while for nonnuclear techniques, higher payloads of labeled molecules for imaging are required. Moreover, each imaging modality (CT, PET, or MR) has its particular strengths or weaknesses in terms of resolution, applicability to particular problems, and cost and requirement for equipment and external agents; thus, combination of different imaging techniques will be a trend for plaque imaging in the future.Citation80
Besides, biological activity of targeted tracers also needs to be taken into considerations. Above all, substantial improvements have to be achieved for making contrast agents available. As we all known, the molecules overexpressed in the plaques vary in the different stage of AS progress. Although the previous studies have shown efficient plaque imaging by targeting one molecular as is mentioned earlier, single target imaging is hard to provide overall structural, functional, and molecular information of the targeted lesion area; therefore, multimodified target imaging agents will have great research value. Faced with these challenges, development of new tracers as contrast agents is of great significance, especially nanoparticles such as iron oxides, Au, dendrimers, liposomes, micelles, and biodegradable polymers.Citation17,Citation81 Combination of specific targeting probes with imaging modalities for vulnerable plaque imaging is a research area of strong interest, especially for dendrimers.Citation82,Citation83
Dendrimers as imaging agents
In in vivo, the degradation and excretion of macromolecules are slower than those of small molecules, which resulted in increased relaxivity (remained in plasma for at least 1 h), lengthened circulation clearance, and prolonged retention in blood pool. At the same time, due to the slow rotation of the molecular volume ambassador, the rotation correlation time is prolonged and the relaxation rate of the water quality can be remarkably increased. Therefore, the use of macromolecular contrast agents can reduce the dosage and enhance the examination of multiple parts of the body.Citation84 Since early 1980s, dendrimers have been comprehensively studied for their various medical applications.Citation85,Citation86 Typically, a dendrimer molecule is composed of the following three elements: an initiator core, interior layers, which are attached to the initiator core and are built of repeating units (number of the layers are expressed as generations), and multiple terminal functional groups.Citation87 Due to their unique characteristics, utilization of dendrimers in nanobiotechnology, such as bio-mimicry, diagnostics, and therapeutics, has attracted great attention of researchers over the past decade.
Previous studies have revealed many advantages of dendrimers in molecular imaging ().
Table 3 Dendrimers based molecular imaging agents
First, dendrimer-based imaging agents exhibit size-dependent, organ-specific, and unique pharmacokinetic properties due to the characteristics of dendrimers, such as nanospherical size, cores, and interiors, as well as chemical properties of exterior shell, which are superior to other nanoparticles. Compared to low-molecular weight vehicle, which is likely to be degraded and excreted, polyamidoamine (PAMAM) dendrimers are easy to “leak” across vascular wall causing rapid perfusion throughout the body.Citation79,Citation88–Citation90
Second, as macromolecular imaging agents, dendrimers, especially those modified with polyethylene glycol (PEG), can overcome some disadvantages of the traditional small molecular imaging agents, such as short half-life, nonspecificity, and renal toxicity at high concentrations. The potential toxicity of dendrimers can also be reduced by changing the types of central core or modified with anionic, neutral, and ligand molecules.Citation17,Citation25,Citation79,Citation91
In addition, dendrimers can be labeled with a variety of imaging “beacons” and such combination of dendrimers and beacons can be used as hybrid contrast agent probe, which lengthens circulation clearance and prolongs retention in blood pool but can be cleared away from major organs in short time in different imaging methods.Citation25,Citation79,Citation90,Citation92
More importantly, multiple surface functional groups of dendrimers can be labeled with multisignal elements, targeting molecules and therapeutic agents.Citation92,Citation93 Therefore, dendrimers can provide a wide range of space and binding sites for functional molecules and elements, which enables them to have more abilities and advantages for targeted molecular imaging than other nanoparticles.
Up to now, more and more researchers have been concentrating on the utilization of dendrimers as imaging agents. In the early days, dendrimers were only used as vectors of some imaging elements; for example, Mn(II) or Gd(III) chelating dendrimers were prepared as contrast agents for MRI applications;Citation88,Citation91,Citation94 dye-conjugated dendrimers were used for fluorescence imaging;Citation95,Citation96 and dendrimer-entrapped Au nanoparticles (Au DENPs) or iodinated-compounds conjugated dendrimers were applied to CT.Citation97–Citation100
With multi-signal elements and targeting molecules in one dendrimer molecule, dendrimers can easily target to the intended imagining organs. Dendrimers conjugated with folic acid (FA) and Cu2+ were studied as bioprobes to target and image human cancers using synchrotron X-ray fluorescence analysis, and the results showed that the targeting probes increased the intensity of fluorescence signal compared to the negative control.Citation93 Likewise, folate receptor-targeted dendrimer nanoclusters (DNCs) labeled with Gd can be used for MRI of cancers with high expression of folate-receptor. Cheng et alCitation26 found that when KB cells, a cell line that can be induced to highly express folate receptor, were incubated with folate receptor-targeted DNCs, there was a significant enhancement in MR signal intensity compared to other cell lines with lower or normal expression of folate receptor.
In addition, dendrimers serve as vehicles for hybrid modality imaging due to their unique structures.Citation79 It is known that Au nanoparticles are used for CT imaging while Gd(III) is for MRI. Combination of CT with MR was proved to possess advantages of both two imaging modalities. What have been confirmed in many researches is that dendrimers have superiority over other nanoparticles in loading contrast agents that have both CT and MRI elements.Citation101,Citation102 For instance, GD-loaded Au DENPs were synthetized for dual-modal CT/MRI applications. The new contrast agents possessed water solubility with stable colloid properties and were noncytotoxic within the given concentration range, which enabled efficient dual-modal CT/MRI of some major organs of rats and mice.Citation25 Li et alCitation103 utilized the multifunctional dendrimer-based nanoparticles to image the breast cancer cells in vitro and in vivo by dual-modal CT/MR and proved effectiveness of the nanoparticles. Furthermore, Chen et alCitation104 prepared FA-modified multifunctional Au DENPs loaded with Gd for target imaging of tumors by dual mode CT/MR and found that the probes showed both CT and MR enhancements in contrast with nontargeted Gd–Au DENPs. In addition, dendrimers have been applied for other hybrids such as dual-modal MR and fluorescence molecular imaging, MRI and other optical imaging, combination of molecular imaging and therapeutic agents, and multimodal contrast agents.Citation79
And more notably, dendrimers have also been used as vehicles for both imaging agents and therapeutic materials.Citation105 For example, Luong et alCitation106 designed a theranostic nanocarrier consisting of superparamagnetic iron oxide nanoparticle (SPION) core and FA-PAMAM dendrimer surface. Further to increase its therapeutic potentials, an anticancer agent 3,4-difluorobenzylidene-curcumin (CDF) was loaded in the FA-PAMAM. The results showed that the SPIONs@FA-PAMAM–CDF nanocarrier exhibited high MR contrast and a good anticancer activity simultaneously in vitro. This study indicates that multifunctions of targeting molecules for diagnosis and treatment can be combined within one molecule of dendrimers.
Most applications and attempts about dendrimers as contrast agents have focused on cancers, and researches about developing dendrimers for imaging of atherosclerotic plaques are very limited so far (). Ye et alCitation107 used PEG-modified Au DENPs to image macrophages in atherosclerotic plaques using CT and proved that the PEGylated Au DENPs had great biocompatibility and were noncytotoxic even at high concentrations, and the Au DENPs were efficiently taken up by macrophages. These results were also confirmed by another study, in which Au DENPs were labeled with PEG and fluorescein isothiocyanate (FI).Citation108 These achievements suggest that PEGylated Au DENPs could be an ideal contrast agent for CT imaging of AS.
About molecular target imaging, Seo et alCitation109 modified dendrimers with LyP-1, which can bind to p32 proteins expressed on activated macrophages and labeled the dendrimers with 64Cu for PET–CT studies. The (LyP-1)4-dendrimer-64Cu was proved to be taken up in plaques significantly higher than nontargeted control, leading to the enhancement of signal intensity. Another element, Mn, is also suitable for imaging atherosclerotic plaques by the detection of OSEs in macrophage-rich atherosclerotic plaques using biocompatible Mn(II) molecular magnetic imaging probes. Mn can replace Gd to avoid potential cell toxicity of Gd, but high payloads of Mn are required for optimizing image technology. PAMAM dendrimers composed of repeating β-alanine subunits are able to load a large number of Mn. Nguyen et alCitation110 proved that G8 dendrimers modified by Mn and antibody MDA2 allowed a high Mn payload and targeted at OSE, which may have potentials in in vivo imaging of atherosclerotic lesions.
Future perspectives
Over the past few years, great efforts have been made to explore possible imaging agents to target different molecules overexpressed in AS plaques in order to achieve accurate diagnosis as well as efficiently deal with vulnerable plaques in primetime, but there lacks exciting progress.
At present, although applications of dendrimer for AS plaque imaging are still limited in quantity, dendrimers being studied as vehicles of contrast agents have shown great promise for molecular target imaging, dual- or multimodality imaging as well as targeted theranostics. If synthetic dendrimers are not only conjugated with one of the key molecules upregulated in AS plaques but also labeled by one or more imaging agents of diverse imaging modes, it will be very helpful for obtaining sufficient and accurate information about AS plaques. Furthermore, therapeutic agents can also be conjugated to dendrimer molecules to accomplish efficient treatment and diagnosis simultaneously.
Although they have extensive application value, dendrimers themselves still have some disadvantages to overcome. For example, the artificial synthesis of the high generations of dendrimers (G8–10) is expensive and complex, which make dendrimers have some difficulties in industrial production. Besides, due to their high molecular weights, the high generations of dendrimers generally have low diffusion coefficient and slow degradation and excretion, which may benefit for the dendrimers as contrast agents by prolonging retention of in blood pool, but also bring hemolysis and cellular toxicities simultaneously,Citation84,Citation111 especially for the cationic dendrimers because of their high density of positive charges.Citation112,Citation113
Therefore, optimized synthesis and purification methods will be encouraged to guarantee mass production of the dendrimers and modification of the dendrimers with PEG or anionic, neutral, and ligand molecules will not only effectively reduce their in vivo toxicities but also significantly increase their targeting capabilities, by which dendrimers would be applied widely as contrast agents in clinic for diagnosing and treating vulnerable plaques of AS in the near future.
Executive summary
Pathophysiology of AS and biomarkers for molecular target imaging
Molecular imaging provides great potential for noninvasive visualization of the cellular and molecular components involved in the development of atheromatous plaques.
Some key molecular imaging modalities and approaches were used for noninvasive detection of biological processes of atherogenesis, including inflammatory infiltration (described as upregulation of endothelial adhesion molecules and infiltration of macrophages), fibrotic responses, and eventually the formation of vulnerable plaques.
Limitations for current contrast agents
Each imaging modality (CT, PET, or MR) has its particular strengths or weaknesses in terms of resolution, applicability to particular problems, and cost and requirement for equipment and external agents; thus, combination of different imaging techniques will be a trend for plaque imaging in the future.
Single target imaging is hard to provide overall structural, functional, and molecular information of the targeted lesion area; therefore, multi-modified target imaging agents will have great research value.
Dendrimers as imaging agents
Due to their unique characteristics, utilization of dendrimers in nanobiotechnology, such as biomimicry, diagnostics, and therapeutics, has attracted great attention of researchers over the past decade.
With their three-dimensional architecture, composition, and abundant terminal functional groups, dendrimers provide a platform for multitargeting and multimodal imaging.
In addition, dendrimers have been used as vehicles for both imaging agents and therapeutic materials.
Most applications and attempts about dendrimers as contrast agents have focused on cancers, and only a few attempts have focused on atherosclerotic plaques.
Modified dendrimers are supposed to have great influence on imaging and treating vulnerable plaques, and it may be applied for clinical use in AS diagnosis and therapy in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no 81270368).
Disclosure
The authors report no conflicts of interest in this work.
References
- PhinikaridouAAndiaMELacerdaSLorrioSMakowskiMRBotnarRMMolecular MRI of atherosclerosisMolecules20131811140421406924232739
- SakakuraKNakanoMOtsukaFLadichEKolodgieFDVirmaniRPathophysiology of atherosclerosis plaque progressionHeart Lung Circ201322639941123541627
- TavakoliSVashistASadeghiMMMolecular imaging of plaque vulnerabilityJ Nucl Cardiol201421611121128 quiz 112925124827
- KaragkiozakiVLogothetidisSPappaAMNanomedicine for atherosclerosis: molecular imaging and treatmentJ Biomed Nanotechnol201511219121026349296
- AdamsonPDNewbyDEDweckMRTranslational coronary atherosclerosis imaging with PETCardiol Clin201634117918626590788
- CatanaCWuYJudenhoferMSQiJPichlerBJCherrySRSimultaneous acquisition of multislice PET and MR images: initial results with a MR-compatible PET scannerJ Nucl Med200647121968197617138739
- AmetameySMHonerMSchubigerPAMolecular imaging with PETChem Rev200810851501151618426240
- MankoffDALeeJHEubankWBBreast cancer imaging with novel PET tracersPET Clin20094437138027157306
- EllPJSingle photon emission computed tomography (SPET) of the brainJ Neurosci Methods1990341–32072172259243
- ChengDWangYLiuXComparison of 18F PET and 99mTc SPECT imaging in phantoms and in tumored miceBioconjug Chem20102181565157020681508
- HuangYComanDHyderFAliMMDendrimer-based responsive MRI contrast agents (G1-G4) for biosensor imaging of redundant deviation in shifts (BIRDS)Bioconjug Chem201526122315232326497087
- NoguchiTKawasakiTTanakaAHigh-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary eventsJ Am Coll Cardiol2014631098999924345595
- MatsumotoKEharaSHasegawaTLocalization of coronary high-intensity signals on T1-weighted MR imaging: relation to plaque morphology and clinical severity of angina pectorisJACC Cardiovasc Imaging20158101143115226363839
- XieYKimYJPangJCoronary atherosclerosis T1-weighed characterization with integrated anatomical reference: comparison with high-risk plaque features detected by invasive coronary imagingJACC Cardiovasc Imaging201710663764827743950
- PangJSharifBArsanjaniRAccelerated whole-heart coronary MRA using motion-corrected sensitivity encoding with three-dimensional projection reconstructionMagn Reson Med201573128429124435956
- SaamTHatsukamiTSTakayaNThe vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessmentRadiology20072441647717581895
- RyvolovaMChomouckaJDrbohlavovaJModern micro and nanoparticle-based imaging techniquesSensors20121211147921482023202187
- DashDOptical coherence tomography is a kid on the block: I would choose intravascular ultrasoundIndian Heart J201769340741028648442
- AlsidawiSEffatMRahmanSAbdallahMLeesarMThe role of vascular imaging in guiding routine percutaneous coronary interventions: a meta-analysis of bare metal stent and drug-eluting stent trialsCardiovasc Ther201533636036626363283
- HerschmanHRMolecular imaging: looking at problems, seeing solutionsScience2003302564560560814576425
- PatelKTarkinJSerruysPWInvasive or non-invasive imaging for detecting high-risk coronary lesions?Expert Rev Cardiovasc Ther201715316517928256179
- LibbyPDiCarliMWeisslederRThe vascular biology of atherosclerosis and imaging targetsJ Nucl Med201051suppl 133S37S20395349
- MajmudarMDNahrendorfMCardiovascular molecular imaging: the road aheadJ Nucl Med201253567367622492729
- WangYXChoiYChenZLaurentSGibbsSLMolecular imaging: from bench to clinicBiomed Res Int2014201435725825610862
- WenSLiKCaiHMultifunctional dendrimer-entrapped gold nanoparticles for dual mode CT/MR imaging applicationsBiomaterials20133451570158023199745
- ChengZThorekDLTsourkasAGadolinium-conjugated dendrimer nanoclusters as a tumor-targeted T1 magnetic resonance imaging contrast agentAngew Chem Int Ed Engl201049234635019967688
- ProfumoEButtariBD’ArcangeloDThe nutraceutical dehydrozingerone and its dimer counteract inflammation- and oxidative stress-induced dysfunction of in vitro cultured human endothelial cells: a novel perspective for the prevention and therapy of atherosclerosisOxid Med Cell Longev20162016124648528050226
- NahrendorfMKeliherEPanizziP18F-4V for PET–CT imaging of VCAM-1 expression in atherosclerosisJACC Cardiovasc Imaging20092101213122219833312
- MichalskaMMachtoubLMantheyHDVisualization of vascular inflammation in the atherosclerotic mouse by ultrasmall superparamagnetic iron oxide vascular cell adhesion molecule-1-specific nanoparticlesArterioscler Thromb Vasc Biol201232102350235722879583
- DimastromatteoJBroisatAPerretPIn vivo molecular imaging of atherosclerotic lesions in ApoE−/− mice using VCAM-1-specific, 99mTc-labeled peptidic sequencesJ Nucl Med20135481442144923719858
- JansenKvan der SteenAFWuMSpectroscopic intravascular photoacoustic imaging of lipids in atherosclerosisJ Biomed Opt201419202600624522806
- PurushothamanKRPurushothamanMLevyAPIncreased expression of oxidation-specific epitopes and apoptosis are associated with haptoglobin genotype: possible implications for plaque progression in human atherosclerosisJ Am Coll Cardiol201260211211922766337
- van DijkRAKolodgieFRavandiADifferential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesionsJ Lipid Res201253122773279022969153
- LeibundgutGWitztumJLTsimikasSOxidation-specific epitopes and immunological responses: translational biotheranostic implications for atherosclerosisCurr Opin Pharmacol201313216817923541680
- Briley-SaeboKCNguyenTHSaeboeAMIn vivo detection of oxidation-specific epitopes in atherosclerotic lesions using biocompatible manganese molecular magnetic imaging probesJ Am Coll Cardiol201259661662622300697
- AzzamKMFesslerMBCrosstalk between reverse cholesterol transport and innate immunityTrends Endocrinol Metab201223416917822406271
- DingYHQianLYPangJThe regulation of immune cells by lactobacilli: a potential therapeutic target for anti-atherosclerosis therapyOncotarget20178599155992828938693
- ChistiakovDAOrekhovANBobryshevYVImmune-inflammatory responses in atherosclerosis: role of an adaptive immunity mainly driven by T and B cellsImmunobiology201622191014103327262513
- BadimonLSuadesRFuentesEPalomoIPadroTRole of platelet-derived microvesicles as crosstalk mediators in atherothrombosis and future pharmacology targets: a link between inflammation, atherosclerosis, and thrombosisFront Pharmacol2016729327630570
- AmirbekianVLipinskiMJBriley-SaeboKCDetecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRIProc Natl Acad Sci U S A2007104396196617215360
- NahrendorfMZhangHHembradorSNanoparticle PET–CT imaging of macrophages in inflammatory atherosclerosisCirculation2008117337938718158358
- NahrendorfMKeliherEMarinelliBDetection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomographyArterioscler Thromb Vasc Biol201131475075721252070
- TarkinJMJoshiFRRuddJHPET imaging of inflammation in atherosclerosisNat Rev Cardiol201411844345724913061
- GustafssonBHedinUCaidahlKGlycolaldehyde and maleyl conjugated human serum albumin as potential macrophage-targeting carriers for molecular imaging purposesContrast Media Mol Imaging2015101374224753457
- DominguezJHMehtaJLLiDAnti-LOX-1 therapy in rats with diabetes and dyslipidemia: ablation of renal vascular and epithelial manifestationsAm J Physiol Renal Physiol20082941F110F11917989113
- LiDMehtaJLIntracellular signaling of LOX-1 in endothelial cell apoptosisCirc Res2009104556656819286611
- LiDPatelARKlibanovALMolecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonanceCirc Cardiovasc Imaging20103446447220442371
- DingZLiuSWangXLOX-1, oxidant stress, mtDNA damage, autophagy, and immune response in atherosclerosisCan J Physiol Pharmacol201492752453024959993
- De VosJMathijsIXavierCSpecific targeting of atherosclerotic plaques in ApoE(−/−) mice using a new Camelid sdAb binding the vulnerable plaque marker LOX-1Mol Imaging Biol201416569069824687730
- WenSLiuDFCuiYIn vivo MRI detection of carotid atherosclerotic lesions and kidney inflammation in ApoE-deficient mice by using LOX-1 targeted iron nanoparticlesNanomedicine201410363964924103305
- MullerAMuLMelettaRTowards non-invasive imaging of vulnerable atherosclerotic plaques by targeting co-stimulatory moleculesInt J Cardiol2014174350351524834996
- te BoekhorstBCBovensSMHellingsWEMolecular MRI of murine atherosclerotic plaque targeting NGAL: a protein associated with unstable human plaque characteristicsCardiovasc Res201189368068821030534
- AlmerGFrascioneDPali-SchollIInterleukin-10: an anti-inflammatory marker to target atherosclerotic lesions via PEGylated liposomesMol Pharm201310117518623176185
- LiuYPierceRLuehmannHPSharpTLWelchMJPET imaging of chemokine receptors in vascular injury-accelerated atherosclerosisJ Nucl Med20135471135114123658218
- YuHSegersFSliedregt-BolKIdentification of a novel CD40 ligand for targeted imaging of inflammatory plaques by phage displayFASEB J201327104136414623896727
- BackMKetelhuthDFAgewallSMatrix metalloproteinases in atherothrombosisProg Cardiovasc Dis201052541042820226959
- SmallCDCrawfordBDMatrix metalloproteinases in neural development: a phylogenetically diverse perspectiveNeural Regen Res201611335736227127457
- HartungDSchafersMFujimotoSTargeting of matrix metalloproteinase activation for noninvasive detection of vulnerable atherosclerotic lesionsEur J Nucl Med Mol Imaging200734suppl 1S1S817497106
- RazavianMNieLChallaALipid lowering and imaging protease activation in atherosclerosisJ Nucl Cardiol201421231932824368425
- SchafersMRiemannBKopkaKScintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivoCirculation2004109212554255915123523
- MojtahediAAlaviAThamakeSAssessment of vulnerable atherosclerotic and fibrotic plaques in coronary arteries using (68) Ga-DOTATATE PET/CTAm J Nucl Med Mol Imaging201551657125625028
- MichelJBMartin-VenturaJLNicolettiAHo-Tin-NoeBPathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhagesAtherosclerosis2014234231131924726899
- LuJDuanWQiaoAFinite element analysis of mechanics of neovessels with intraplaque hemorrhage in carotid atherosclerosisBiomed Eng Online201514suppl 1S3
- HumphreyJDVascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levelsCell Biochem Biophys2008502537818209957
- KorshunovVASchwartzSMBerkBCVascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenonArterioscler Thromb Vasc Biol20072781722172817541029
- ZhaoXKongJZhaoYGene silencing of TACE enhances plaque stability and improves vascular remodeling in a rabbit model of atherosclerosisSci Rep201551793926655882
- YounSWParkKKSmall-nucleic-acid-based therapeutic strategy targeting the transcription factors regulating the vascular inflammation, remodeling and fibrosis in atherosclerosisInt J Mol Sci2015165118041183326006249
- KietselaerBLReutelingspergerCPHeidendalGANoninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosisN Engl J Med20043501414721473
- ZhaoYKugeYZhaoSComparison of 99mTc-annexin A5 with 18F-FDG for the detection of atherosclerosis in ApoE−/− miceEur J Nucl Med Mol Imaging200734111747175517437104
- ZhaoYWatanabeAZhaoSSuppressive effects of irbesartan on inflammation and apoptosis in atherosclerotic plaques of ApoE−/− mice: molecular imaging with 14C-FDG and 99mTc-annexin A5PLoS One201492e8933824586699
- IsobeSTsimikasSZhouJNoninvasive imaging of atherosclerotic lesions in apolipoprotein E-deficient and low-density-lipoprotein receptor-deficient mice with annexin A5J Nucl Med20064791497150516954559
- TaitJFSmithCPhosphatidylserine receptors: role of CD36 in binding of anionic phospholipid vesicles to monocytic cellsJ Biol Chem19992745304830549915844
- HensonPMBrattonDLFadokVAApoptotic cell removalCurr Biol20011119R795R80511591341
- MaiseyeuAMihaiGKampfrathTGadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosisJ Lipid Res200950112157216319017616
- HightMRCheungYYNickelsMLA peptide-based positron emission tomography probe for in vivo detection of caspase activity in apoptotic cellsClin Cancer Res20142082126213524573549
- WinterPMNeubauerAMCaruthersSDEndothelial alpha(v) beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosisArterioscler Thromb Vasc Biol20062692103210916825592
- PhinikaridouAAndiaMEIndermuehleAVascular remodeling and plaque vulnerability in a rabbit model of atherosclerosis: comparison of delayed-enhancement MR imaging with an elastin-specific contrast agent and unenhanced black-blood MR imagingRadiology2014271239039924475852
- ZhangJRazavianMTavakoliSMolecular imaging of vascular endothelial growth factor receptors in graft arteriosclerosisArterioscler Thromb Vasc Biol20123281849185522723442
- LongmireMChoykePLKobayashiHDendrimer-based contrast agents for molecular imagingCurr Top Med Chem20088141180118618855704
- Thorp-GreenwoodFLCooganMPMultimodal radio-(PET/SPECT) and fluorescence imaging agents based on metallo-radioisotopes: current applications and prospects for development of new agentsDalton Trans201140236129614321225080
- ParveenSMisraRSahooSKNanoparticles: a boon to drug delivery, therapeutics, diagnostics and imagingNanomedicine20128214716621703993
- XingHBuWZhangSMultifunctional nanoprobes for upconversion fluorescence, MR and CT trimodal imagingBiomaterials20123341079108922061493
- ChengDLiXZhangCDetection of vulnerable atherosclerosis plaques with a dual-modal single-photon-emission computed tomography/magnetic resonance imaging probe targeting apoptotic macrophagesACS Appl Mater Interfaces2015742847285525569777
- HegdeARRewatkarPVManikkathJTupallyKParekhHSMutalikSPeptide dendrimer-conjugates of ketoprofen: synthesis and ex vivo and in vivo evaluations of passive diffusion, sonophoresis and iontophoresis for skin deliveryEur J Pharm Sci201710223724928285173
- Jatczak-PawlikIGorzkiewiczMStudzianMSugar-modified poly(propylene imine) dendrimers stimulate the NF-kappaB pathway in a myeloid cell linePharm Res201734113614727766462
- YuMJieXXuLRecent advances in dendrimer research for cardiovascular diseasesBiomacromolecules20151692588259826310544
- MarkowiczMSzymanskiPCiszewskiMKlysAMikiciuk-OlasikEEvaluation of poly(amidoamine) dendrimers as potential carriers of iminodiacetic derivatives using solubility studies and 2D-NOESY NMR spectroscopyJ Biol Phys201238463765623144513
- KobayashiHBrechbielMWNano-sized MRI contrast agents with dendrimer coresAdv Drug Deliv Rev200557152271228616290152
- MenjogeARKannanRMTomaliaDADendrimer-based drug and imaging conjugates: design considerations for nanomedical applicationsDrug Discov Today2010155–617118520116448
- LongmireMROgawaMChoykePLKobayashiHDendrimers as high relaxivity MR contrast agentsWiley Interdiscip Rev Nanomed Nanobiotechnol20146215516224155241
- KojimaCTurkbeyBOgawaMDendrimer-based MRI contrast agents: the effects of PEGylation on relaxivity and pharmacokineticsNanomedicine2011761001100821515406
- KuilJBuckleTOldenburgJHybrid peptide dendrimers for imaging of chemokine receptor 4 (CXCR4) expressionMol Pharm2011862444245322085282
- ZhangYXuXWangLDendrimer-folate-copper conjugates as bioprobes for synchrotron X-ray fluorescence imagingChem Commun201349881038810390
- KlemmPJFloydWC3rdAndolinaCMFrechetJMRaymondKNConjugation to biocompatible dendrimers increases lanthanide T2 relaxivity of hydroxypyridinone (HOPO) complexes for magnetic resonance imaging (MRI)Eur J Inorg Chem20122012122108211423539072
- DenoraNLaquintanaVLopalcoAIn vitro targeting and imaging the translocator protein TSPO 18-kDa through G(4)-PAMAM-FITC labeled dendrimerJ Control Release201317231111112524096015
- KimYKimSHTanyeriMKatzenellenbogenJASchroederCMDendrimer probes for enhanced photostability and localization in fluorescence imagingBiophys J201310471566157523561533
- PengCZhengLChenQPEGylated dendrimer-entrapped gold nanoparticles for in vivo blood pool and tumor imaging by computed tomographyBiomaterials20123341107111922061490
- LiuHWangHXuYLactobionic acid-modified dendrimer-entrapped gold nanoparticles for targeted computed tomography imaging of human hepatocellular carcinomaACS Appl Mater Interfaces2014696944695324712914
- LiuHWangHXuYSynthesis of PEGylated low generation dendrimer-entrapped gold nanoparticles for CT imaging applicationsNanoscale2014694521452624647803
- LiKZhangZZhengLArg-Gly-Asp-D-Phe-Lys peptide-modified PEGylated dendrimer-entrapped gold nanoparticles for targeted computed tomography imaging of breast carcinomaNanomedicine (Lond)201510142185219726214356
- CaiHLiKLiJDendrimer-assisted formation of Fe3O4/Au nanocomposite particles for targeted dual mode CT/MR imaging of tumorsSmall201511354584459326061810
- ChenQWangHLiuHMultifunctional dendrimer-entrapped gold nanoparticles modified with RGD peptide for targeted computed tomography/magnetic resonance dual-modal imaging of tumorsAnal Chem20158773949395625768040
- LiKWenSLarsonACMultifunctional dendrimer-based nanoparticles for in vivo MR/CT dual-modal molecular imaging of breast cancerInt J Nanomedicine201382589260023888113
- ChenQLiKWenSTargeted CT/MR dual mode imaging of tumors using multifunctional dendrimer-entrapped gold nanoparticlesBiomaterials201334215200520923583039
- AbboudDHansonJChemokine neutralization as an innovative therapeutic strategy for atopic dermatitisDrug Discov Today201722470271127956056
- LuongDSauSKesharwaniPIyerAKPolyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targetingBiomacromolecules20171841197120928245646
- YeKQinJPengZPolyethylene glycol-modified dendrimer-entrapped gold nanoparticles enhance CT imaging of blood pool in atherosclerotic miceNanoscale Res Lett20149152925288918
- QinJPengCZhaoBNoninvasive detection of macrophages in atherosclerotic lesions by computed tomography enhanced with PEGylated gold nanoparticlesInt J Nanomedicine201495575559025506213
- SeoJWBaekHMahakianLM(64)Cu-labeled LyP-1-dendrimer for PET–CT imaging of atherosclerotic plaqueBioconjug Chem201425223123924433095
- NguyenTHBryantHShapsaAManganese G8 dendrim-ers targeted to oxidation-specific epitopes: in vivo MR imaging of atherosclerosisJ Magn Reson Imaging201541379780524610640
- ChoudharySGuptaLRaniSDaveKGuptaUImpact of dendrimers on solubility of hydrophobic drug moleculesFront Pharmacol2017826128559844
- WilczewskaAZNiemirowiczKMarkiewiczKHCarHNanoparticles as drug delivery systemsPharmacol Rep20126451020103723238461
- BahadirEBSezginturkMKPoly(amidoamine) (PAMAM): an emerging material for electrochemical bio(sensing) applicationsTalanta201614842743826653469
- ChanJMSMonacoCWylezinska-ArridgeMImaging vulnerable plaques by targeting inflammation in atherosclerosis using fluorescent-labeled dual-ligand microparticles of iron oxide and magnetic resonance imagingJ Vasc Surg Epub2017622
