Abstract
Although nanoscale titanium dioxide (nano-TiO2) has been extensively used in industrial food applications and daily products for pregnant women, infants, and children, its potential toxicity on fetal development has been rarely studied. The main objective of this investigation was to establish the effects of maternal exposure of nano-TiO2 on developing embryos. Female imprinting control region mice were orally administered nano-TiO2 from gestational day 0 to 17. Our findings showed that Ti concentrations in maternal serum, placenta, and fetus were increased in nano-TiO2-exposed mice when compared to controls, which resulted in reductions in the contents of calcium and zinc in maternal serum, placenta, and fetus, maternal weight gain, placental weight, fetal weight, number of live fetuses, and fetal crown–rump length as well as cauda length, and caused an increase in the number of both dead fetuses and resorptions. Furthermore, maternal nano-TiO2 exposure inhibited development of the fetal skeleton, suggesting a significant absence of cartilage, reduced or absent ossification, and an increase in the number of fetuses with dysplasia, including exencephaly, spina bifida, coiled tail, scoliosis, rib absence, and sternum absence. These findings indicated that nano-TiO2 can cross the blood–fetal barrier and placental barrier, thereby delaying the development of fetal mice and inducing skeletal malformation. These factors may be associated with reductions in both calcium and zinc in maternal serum and the fetus, and both the placenta and embryos may be major targets of developmental toxicity following maternal exposure to nano-TiO2 during the prenatal period. Therefore, the application of nano-TiO2 should be carried out with caution.
Introduction
Nanoscale titanium dioxide (nano-TiO2) is widely used in various applications, including industry, medicine, and in consumer products such as cosmetics, sunscreens, food products, toothpaste, and sterilization and environmental control measures.Citation1–Citation6 Initially, nano-TiO2 was thought to exhibit relatively low toxicity as compared with other nanomaterials.Citation7–Citation10 However, numerous studies have suggested that following exposure via various routes including inhalation, injection, dermal deposition, and gastrointestinal tract absorption, nano-TiO2 can be found in various organs including the lung, liver, kidney, spleen, thymus, heart, stomach, brain, ovary, and testis potentially inducing toxic effects.Citation11–Citation13 Thus, based on a recent assessment, the International Agency for Research on Cancer has classified TiO2 as a human group 2B carcinogen.Citation14 Despite the fact that increased use and production of nano-TiO2 may result in health impacts due to its various uses, there is still a lack of knowledge regarding the adverse effects of nano-TiO2 on pregnant dams and embryo–fetal development.
It is known that the development of humans and animals, in particular developing embryos, is significantly influenced by environmental factors.Citation15,Citation16 The ability to scavenge toxins in the body is low as the developing embryo lacks the feedback protection mechanism,Citation17,Citation18 and may be more sensitive to the effects of nano-TiO2 compared with mature organisms. Recently, maternal exposure to manufactured nanoparticles (NPs) which induced toxicity during embryo development has attracted considerable attention.Citation19,Citation20 It was reported that maternal exposure to a variety of manufactured NPs was associated with harmful effects on mouse and rat embryos.Citation21–Citation25 NPs were detected in prenatally exposed offspring thereby indicating that NPs were transferred to the developing conceptus across the placenta. However, nano-CdO was detected in the placenta and maternal organs of treated mice but not in their fetuses.Citation26–Citation28 Exposure of pregnant female mice or rats to different types of NPs may result in a variety of adverse pregnancy outcomes such as embryo mortality, intrauterine growth retardation, and structural anomalies in the prenatally exposed offspring,Citation19 as developmentally toxic NPs crossed the placenta, embryos were directly exposed, and the embryotoxic effects of NPs seemed to have been secondary to maternal and/or placental toxicity.Citation24 Philbrook et al indicated that the intragastric administration of hydroxyl-modified single-walled carbon nanotubes to pregnant mice resulted in increased skeletal defects, such as forked cervical vertebrae, reduced ossification of sternebrae and phalanges, and morphological abnormalities.Citation29 It was proved that exposure to nanosilica (70 nm) led to fetal resorption and inhibition of fetal growth.Citation30 Pregnant female rats were orally treated with nano zinc oxide (ZnO) for 15 days, during gestational days (GDs) 5–19, at doses of 100, 200, and 400 mg/kg/day, and it was found that nano ZnO had no impact on embryo–fetal development in rats.Citation31 Following maternal exposure to nano-TiO2, it was demonstrated that nano-TiO2 migrated into the uterus, affected the embryo, and caused abortion; for example, 500 mg/kg led to significant decreases in the total length of limb buds, arm skin thickness, number of proliferating chondrocytes in the arm, red blood cells in the fingers, and mesenchymal cells in the palm and wrist.Citation32,Citation33 Maternal exposure to nano-TiO2 was also reported to affect the development and function of the central nervous system in mouse and rat offspring.Citation34–Citation40 Therefore, we hypothesized that maternal exposure to nano-TiO2 may lead to disorders in embryo–fetal development, especially fetal bone development.
Accordingly, we determined whether mouse embryonic development was influenced by the toxic effects of nano-TiO2 as there is evidence to suggest that fetuses are affected more than adults by a variety of environmental toxins, due to physiological immaturity. Female imprinting control region (ICR) mice were selected as the experimental model and exposed to nano-TiO2 via oral (gavage) administration from GD 0 to 17. After the 18-day gestational period, Ti, Ca, and Zn contents in maternal serum, placenta, and fetus, maternal weight gain, fetal crown–rump and cauda length, body weight (BW) and placental weight, and numbers of live fetuses, dead fetuses, and resorption sites as well as fetal bone development were examined following maternal nano-TiO2 exposure, with a view to clarifying its toxic effects on developing mouse embryos.
Methods
Chemicals
Nanoparticulate TiO2 was prepared by controlled hydrolysis of titanium tetrabutoxide. Details of the synthesis and characterization of nano-TiO2 are described in a previous report by our group.Citation41 NP characteristics were as follows: anatase phase, 6.5 nm particle size, mainly 294 nm hydrodynamic diameter, 174.8 m2/g surface area, and 7.57 mV zeta potential.Citation41
Ethics approval
All animal experiments were conducted during the light phase of the light-dark cycle and approved by the Animal Experimental Committee of Soochow University (grant 2111270). Procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Animals and treatment
Female CD-1 (ICR) mice (20±1.5 g) and male mice (23±2 g) were purchased from the Animal Center of Soochow University (China). All mice were housed in stainless steel cages in a ventilated animal room. The room temperature of the housing facility was maintained at 20°C–25°C with a relative humidity of 45%–55% and a 12-hour light/dark cycle. Distilled water and sterilized food were available ad libitum. Prior to dosing, the mice were acclimated to the environment for 5 days. Mice were kept in cages at a ratio of 2:1 (female:male) from 18:00 every evening, and the vaginal suppository of female mice was checked the next morning at 06:00–08:00. Female mice were considered pregnant when the vaginal suppository was found (day 0). Twenty pregnant mice were randomly divided into four subgroups (n=5 per group), including a control group treated with 0.5% w/v hydroxypropylmethylcellulose (Sigma-Aldrich Co., St Louis, MO, USA) and three experimental groups treated with 25, 50, and 100 mg/kg BW nano-TiO2. For appropriate dose selection, previous reports were consulted.Citation42,Citation43 A report by the World Health Organization from 1969 was also consulted, and according to this report, the lethal dose 50 of TiO2 for rats was found to be >12 g/kg BW after oral administration. In addition, US Food and Drug Administration states that the quantity of nano-TiO2 should not exceed 1% by weight of the food. Animals received 25, 50, and 100 mg/kg BW nano-TiO2 orally (gavage) from GD 0 to 17 in an isolated animal room under specific pathogen-free conditions. After the 18-day gestational period, all pregnant mice were weighed and then sacrificed after being anesthetized with ether. Blood samples were collected from the eye vein by rapidly removing the eyeball. Serum was collected by centrifuging blood at 1,200× g for 10 minutes. The uterus was quickly removed, the numbers of live fetuses, dead fetuses, and resorption sites were counted and recorded for each litter, the weight of live fetuses in each litter was recorded, and the length of the fetal crown–rump and cauda were measured. Every effort was made to minimize animal suffering. All experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Element content analysis
Two milliliters of maternal sera and ~0.1 g of the placenta and the fetuses were digested, and analyzed for element contents. Inductively coupled plasma-mass spectrometry (Thermo Elemental X7; Thermo Electron Co, Waltham, MA, USA) was used to analyze the Ti, Ca, and Zn concentrations in the samples.Citation42,Citation43 An indium concentration of 20 ng/mL was used as an internal standard, and the detection limit of Ti was 0.074 ng/mL.
Evaluation of fetal skeleton development
The skeletal development of fetuses was evaluated using Menegola’s method.Citation44 The selected fetal mice were soaked in hot water at 80°C for 10 seconds, the skin and viscera were carefully removed with eye forceps and scissors, and stained with alcian blue dye solution for 48 hours. The cartilage in the specimen appeared blue (slightly deeper than the color of the stain solution) when viewed with a stereo microscope (Olympus SZ61; Olympus Corporation, Tokyo, Japan) during the staining period; the specimen was then rinsed 2–3 times with tap water, immersed in alizarin red dye solution for 36–48 hours; the solution was renewed every 12 hours until the bone was purple, and thereafter again the specimen was gently rinsed 2–3 times with tap water. The double stained fetuses were placed in a new transparent solution containing 1% potassium hydroxide for 36–48 hours.Citation45,Citation46 The blue embryonic cartilage and purplish red skeletal ossification were observed under the stereo microscope.
Statistical analysis
Data were analyzed using Statistical Analysis Software (SAS 9.1) (Chicago, IL, USA). The significance of the differential expression between groups was assessed using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post hoc test, and the results are presented as means ± standard deviation. Differences were considered statistically significant at p<0.05.
Results
Element contents in maternal serum and fetuses
In order to confirm whether nano-TiO2 entered the blood circulation in pregnant mice and crossed the blood–fetal barrier to enter the fetus, we observed and found significant increases in the Ti contents in the maternal serum, placenta, and fetus (, p<0.05). Furthermore, to confirm the effects of maternal exposure to nano-TiO2 on embryonic development, we also examined the changes in Ca and Zn contents in maternal serum, placenta, and fetus, and found that the contents of Ca and Zn decreased significantly in the high dose nano-TiO2-treated groups (, p<0.05).
Table 1 Effects of nano-TiO2 on element contents in maternal serum, placenta, and fetus
Maternal weight gain
Pregnant mice were exposed to different concentrations of nano-TiO2 from GD 0 to 17, and the weight of pregnant mice at days 0, 3, 9, 12, 15, and 18 during pregnancy is presented in . It can be seen that maternal weight gain gradually increased with pregnancy duration in the control group and nano-TiO2 groups, whereas maternal weight gain in the nano-TiO2-exposed groups at day 18 was significantly lower than that of the control (p<0.05).
Table 2 Effects of nano-TiO2 on maternal weight (g) gain during pregnancy
Fetal growth
Pregnant mice were sacrificed at GD 18, and the cesarean section was performed to measure the crown–rump and cauda length, and fetal BW and placental weight of the live fetal mice (). Compared with the control, both fetal crown–rump and cauda length, and fetal BW and placental weight in the 100 mg/kg nano-TiO2-treated group were markedly lower than that in the control (, p<0.05).
Table 3 Effects of nano-TiO2 on fetal growth of mice
Survival and mortality of fetuses
After GD 18, the numbers of live, dead, and resorption sites were counted and are shown in . Compared with the control, the number of live fetuses in the 100 mg/kg nano-TiO2-treated group was significantly lower than that in the control, whereas the numbers of dead fetuses and resorption sites were obviously higher than those in the control (, p<0.05), respectively.
Table 4 Effects of nano-TiO2 on survival and mortality in mouse fetus
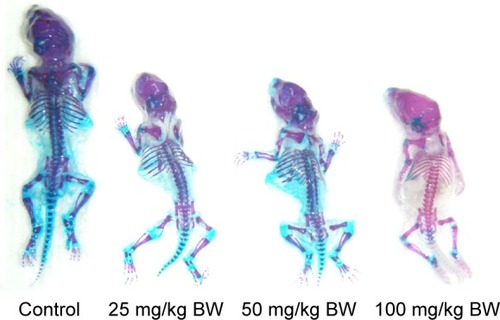
Fetal skeletal development
and show that fetal skeleton development in the 100 mg/kg nano-TiO2-exposed group was significantly slower than that in the control, suggesting the absence of significant cartilage, non-ossification of the sternum and metacarpal bones, or incomplete ossification of the sternum. Following maternal exposure to nano-TiO2, fetal dysplasia was also observed compared with the control; dysplasia included exencephaly, spina bifida, coiled tail, scoliosis, rib absence, and sternum absence.
Table 5 Effects of nano-TiO2 on skeletal ossification in mouse fetuses (n=10)
Discussion
Recently, knowledge on the toxicity of different types of nano-TiO2, including damage to the liver, lung, kidney, spleen, heart, brain, testis, and ovary of mice or rats,Citation11 stomach and thymus of mice,Citation12,Citation13 inflammatory response, membrane injury, and cytotoxicity,Citation11 has increased, resulting in several toxicity studies of nano-TiO2 in different biological systems, such as zebrafish and Caenorhabditis elegans.Citation47,Citation48 However, evidence of developmental toxicity caused by nano-TiO2 in mammals is still limited. It is highly desirable and necessary to have a better understanding of the possible developmental toxicity of nano-TiO2 as humans are often exposed to nano-TiO2 both intentionally and unintentionally.
This study was conducted to examine the maternal and developmental toxic potential of nano-TiO2 orally administered to mice during GD 0–17 of pregnancy. Our findings showed that following maternal exposure, nano-TiO2 crossed the blood–fetal barrier or placental barrier and was deposited in maternal serum, placenta, and fetus, leading to significant decreases in Ca and Zn in the maternal serum, placenta, and fetus. In addition, maternal exposure to 100 mg/kg nano-TiO2 markedly reduced maternal weight gain, placental weight, fetal weight, number of live fetuses, and fetal crown–rump length as well as cauda length, and increased the numbers of both dead fetuses and resorption sites. Skeletal development in fetal mice following maternal exposure to 100 mg/kg nano-TiO2 lagged behind that of the control group, and resulted in absent or reduced ossification, and fetal dysplasia. The main results of the present study are discussed subsequently.
Most of the skeletal system is derived from mesoderm, which is mainly from the ventral and medial mesenchyme of the segment. The critical period of fetal organ development in mice or rats is 7.5–18 days. Therefore, the administration of drugs during this stage can be used to examine their effects on the organs of fetal mice or rats. In the study of embryonic developmental toxicity, evaluation of skeletal deformities is often a major part of the assessment of the effects of external compounds on the body, and bone development is often determined by bone staining. The bones of fetal mice are only partially ossified and cartilage is still present at GD 18. In the present study, we observed that fetal vertebrae, carpal, and metatarsophalangeal bones in the control showed uniform cartilage at GD 18. However, maternal exposure to nano-TiO2 resulted in retardation of skeletal development, for example, cartilage was absent, the ossification centers of some bones were absent, the scale of the ossification center was less than that in the control group, and skeletal deformities (such as scoliosis, vertebral fusion, rib deformity) occurred.
The suppression of embryonic bone development and skeletal abnormalities in fetuses caused by nano-TiO2 may be related to the following factors. 1) The placenta is one of the target organs of nano-TiO2; thus, nano-TiO2 can affect the normal development of embryos by interfering with placental blood flow, substance transport, and endocrine as well as substance metabolism. In this study, we found that maternal exposure to nano-TiO2 not only caused intrauterine developmental retardation of the fetus, but also affected bone ossification, which may have been due to nano-TiO2 interfering with the permeability of Ca2+ in the placenta. Furthermore, we also observed that nano-TiO2 caused deformities including exencephaly, spina bifida, coiled tail, scoliosis, rib absence, and sternum absence. 2) Nano-TiO2 reduced the levels of Ca in pregnant mice and fetuses, which may be an important aspect of nano-TiO2-induced toxicity. In general, the active transport of Ca by osteoblasts is broadly divided into three stages: firstly, Ca channels are open and Ca2+ enters the cells; secondly, Ca2+ binds to the Ca binding protein in the cytoplasm and rapidly spreads from the free surface side of the cell to the basal surface; finally, the Ca pump on the basal plane uses energy from the hydrolysis of ATP to pump Ca2+ from the cells to the extracellular region.Citation49 Exposure to nano-TiO2 may decrease Ca absorption and increase excretion, leading to a negative Ca balance, and a subsequent reduction in Ca levels in the embryo. Ossification of bone, whether osteogenesis or Ca deposition of endochondral ossification, is an important step in bone formation. Our data suggested that maternal nano-TiO2 exposure interfered with Ca metabolism in embryos (Ca reductions in maternal serum, placenta, and fetus), and thus affected ossification in the fetus. Nano-TiO2 may bind to proteins on the cell membrane, thereby changing membrane function and signal transduction resulting in toxicity. The effects of nano-TiO2 on bone Ca included two aspects: on one hand, the inhibition of bone Ca deposition may lead to the formation of a barrier in the ossification center during bone development;Citation49 on the other hand, damage to osteoblasts and chondrocytes, and increased osteoclast activity may result in an increase in bone Ca dissolution.Citation50,Citation51 3) Nano-TiO2 reduced the levels of Zn in pregnant mice, placenta, and fetuses, which may also be an important aspect of nano-TiO2-induced toxicity. It has been shown that Zn is closely related to bone development, metabolism, and function during normal development of the embryo or fetus.Citation52 Osteoblasts secrete organic substances, such as collagen and proteoglycan, which are called osteoids. Osteoblasts are gradually embedded by osteoids and become osteocytes. Serum phosphatase (AKP) is a marker of osteoblasts, and catalyzes the decomposition of phosphate compounds to produce free phosphates that combine with Ca in the intercellular substance to form Ca phosphate, which is involved in the regulation of calcification. AKP is a Zn-dependent enzyme. Zn can stabilize AKP conformation and maintain AKP activity. When Zn is deficient, the conformation and function of AKP are compromised. Epidemiological findings suggest that Zn deficiency delays growth and bone age in animals and humans; a severe lack of Zn in animals can cause skeletal deformities.Citation53 Nano-TiO2 may play a direct role as a promoter of the metallothionein (MT) gene, and induce MT synthesis in maternal liver, kidney, and in placental trophoblast cells. MT rapidly combines with Zn2+, and then decreases the Zn concentration in maternal blood and impairs placental Zn2+ transport, which results in an insufficient supply of Zn to the embryo, leading to a slow ossification center or incomplete calcification of fetal bones. Exposure to nano-TiO2 has been demonstrated to increase MT level in fish and Daphnia.Citation54,Citation55 A previous study showed that exposure to Cd in pregnant rats caused significant reductions in maternal serum and fetal Zn levels.Citation56 In addition, maternal Zn supplementation during pregnancy significantly decreased fetal malformations and growth retardation in mice caused by Cd.Citation57 In the present study, the levels of Zn, Ca, and Ti in the maternal serum of control mice were determined by the contents of Zn, Ca, and Ti in the diet and drinking water, which were strictly controlled according to the standards of mouse rearing from the Animal Center of Soochow University (China) to meet the needs for growth and development of mice. These findings indicate that reductions of Ca and Zn in the maternal serum may be an important cause of nano-TiO2-induced growth retardation in fetal mice.
The placenta is an important link between the mother and the fetus, and has substance transport, barrier, and endocrine functions. In the current study, placental weight in the nano-TiO2-treated group was lower than that in the control group, suggesting that maternal nano-TiO2 exposure inhibited placental growth, which may be associated with decreases in the relative volume of placental villus space, the relative surface area of fetal capillaries, and the absolute volume of the placenta.Citation58 Furthermore, our data suggested that nano-TiO2 content in the maternal blood was positively correlated with placental and fetal nano-TiO2 levels, and negatively correlated with placental and fetal weight. These findings indicate that placenta and embryo may be the second target organs of nano-TiO2 toxicity, and the abnormal development of placenta may be one of the causes of nano-TiO2-induced fetal growth retardation. Taken together, these findings demonstrate that consecutively maternal exposure to nano-TiO2 could suppress embryonic development in mice; however, the underlying molecular mechanism requires further study.
Conclusion
In this experimental study, exposure to nano-TiO2 in pregnant mice caused fetal growth retardation, which was associated with embryonic toxicity and bone toxicity due to nano-TiO2 accumulation in fetal mice. These effects may be due to the direct role or the indirect role of nano-TiO2 interfering with Ca, Zn, and other metabolic processes, or may be the result of a combination of several factors, which require further investigation in the future. Therefore, the use of nano-TiO2 and exposure to nano-TiO2, especially during pregnancy in humans, should be thoroughly investigated.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant nos 31671033, 81473007, 81273036, and 30901218), the National Natural Science Foundation of Jiangsu Province (grant no BK20161306), and the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015A018).
Disclosure
The authors report no conflicts of interest in this work.
References
- GelisCGirardSMavonADelverdierMPaillousNVicendoPAssessment of the skin photoprotective capacities of an organomineral broad-spectrum sunblock on two ex vivo skin modelsPhotodermatol Photoimmunol Photomed20031924225314535895
- SahuKKAlexTCMishraDAgrawalAAn overview on the production of pigment grade titania from titaniarich slagWaste Manag Res200624747916496873
- ShuklaRKSharmaVPandeyAKSinghSSultanaSDhawanAROS mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cellsToxicol In Vitro20112523124121092754
- KaidaTKobayashiKAdachiMSuzukiFOptical characteristics of titanium oxide interference film and the film laminated with oxides and their applications for cosmeticsJ Cosmet Sci20045521922015190897
- ChaudhryQScotterMBlackburnJApplications and implications of nanotechnologies for the food sectorFood Addit Contam Part A Chem Anal Control Expo Risk Assess20082524125818311618
- WeirAWesterhoffPFabriciusLHristovskiKvon GoetzNTitanium dioxide nanoparticles in food and personal care productsEnviron Sci Technol2012462242225022260395
- JengHASwansonJToxicity of metal oxide nanoparticles in mammalian cellsJ Environ Sci Health A Tox Hazard Subst Environ Eng2006412699271117114101
- MikkelsenLSheykhzadeMJensenKAModest effect on plaque progression and vasodilatory function in atherosclerosis-prone mice exposed to nanosized TiO2Part Fibre Toxicol20118324822074227
- ZhaoJBowmanLZhangXTitanium dioxide (TiO2) nanoparticles induce JB6 cell apoptosis through activation of the caspase-8/Bid and mitochondrial pathwaysJ Toxicol Environ Health A2009721141114920077182
- MadlAKPinkertonKEHealth effects of inhaled engineered and incidental nanoparticlesCrit Rev Toxicol20093962965819743943
- HongFSYuXHWuNZhangYQProgress of in vivo studies on the systemic toxicities induced by titanium dioxide nanoparticlesToxicol Res20176115133
- HongFSZhouYJJiLChenTWangLGastric toxicity involving alterations of gastritis-related protein expression in mice following long-term exposure to nano TiO2Food Res Int201795384528395823
- HongFSZhouYMZhouYJWangLImmunotoxic effects of thymus in mice following exposure to nanoparticulate TiO2Environ Toxicol Epub 2017624
- IARC (International Agency for Research on Cancer)Monographs on the evaluation of carcinogenic risks to humans: carbon black, titanium dioxide, and talcLyon, FranceWorld Health Organization, International Agency for Research on Cancer201093
- HayashiANagaokaMYamadaKIchitaniYMiakeYOkadoNMaternal stress induces synaptic loss and developmental disabilities of offspringInt J Dev Neurosci1998162092169785117
- IqbalUDringenbergHCBrienJFReynoldsJNChronic prenatal ethanol exposure alters hippocampal GABAA receptors and impairs spatial learning in the guinea pigBehav Brain Res200415011712515033285
- AndersonLMDiwanBAFearNTRomanECritical windows of exposure for children’s health: cancer in human epidemiological studies and neoplasms in experimental animal modelsEnviron Health Perspect2000108Suppl 357359410852857
- AndersonLMPredictive values of traditional animal bioassay studies for human perinatal carcinogenesis risk determinationToxicol Appl Pharmacol2004199216217415313588
- DelgadoIFPaumgarttenFRCurrent challenges in toxicological research: evaluation of the developmental toxicity of manufactured nanomaterialsVigilância Sanitária em Debate2013141124 Portuguese
- SunJLZhangQWangZPYanBEffects of nanotoxicity on female reproductivity and fetal development in animal modelsInt J Mol Sci20131459319933723629667
- PietroiustiAMassimianiMFenoglioILow doses of pristine and oxidized single-wall carbon nanotubes affect mammalian embryonic developmentACS Nano2011564624463321615177
- HougaardKSJacksonPJensenKAEffects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in micePart Fibre Toxicol201071620546558
- UmezawaMTainakaHKawashimaNShimizuMTakedaKEffect of fetal exposure to titanium dioxide nanoparticle on brain development – brain region informationJ Toxicol Sci20123761247125223208439
- BlumJLXiongJQHoffmanCZelikoffJTCadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growthToxicol Sci2012126247848622240978
- ChanWHShiaoNHCytotoxic effect of CdSe quantum dots on mouse embryonic developmentActa Pharmacol Sin200829225926618215357
- TianXZhuMDuLIntrauterine inflammation increases materno-fetal transfer of gold nanoparticles in a size-dependent manner in murine pregnancySmall20139142432243923761193
- JoESeoGKwonJTExposure to zinc oxide nanoparticles affects reproductive development and biodistribution in offspring ratsJ Toxicol Sci201338452553023824008
- ChuMWuQYangHTransfer of quantum dots from pregnant mice to pups across the placental barrierSmall20106567067820143348
- PhilbrookNAWalkerVKAfroozARMNSalehNBWinnLMInvestigating the effects of functionalized carbon nanotubes on reproduction and development in Drosophila melanogaster and CD-1 miceReprod Toxicol20113244244821963887
- YamashitaKYoshiokaYSafety assessment of nanomaterials in reproductive developmental fieldYakugaku Zasshi20121323331335 Japanese22382838
- HongJSParkMKKimMSEffect of zinc oxide nanoparticles on dams and embryo-fetal development in ratsInt J Nanomedicine20149Suppl 2145157
- RoodbariNHParivarKBadieiBBaroghSZCytotoxic effects of nano-titanium dioxide on forelimb bud development in NMRI mouse embryos in vivoZums J201422911124
- ParivarKHayati RudbariNKhanbabaeeRKhaleghiMThe effect of nano-titanium dioxide on limb bud development of NMRI mouse embryo in vivoCell J201517229630326199908
- ShimizuMTainakaHObaTMizuoKUmezawaMTakedaKMaternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mousePart Fibre Toxicol200962019640265
- TakedaKSuzukiKIIshiharaANanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systemsJ Health Sci200955195102
- TakahashiYShinkaiYMizuoKOshioSTakedaKPrenatal exposure to titanium dioxide nanoparticles increases dopamine levels in the prefrontal cortex and neostriatum of miceJ Toxicol Sci201035574975620930469
- MohammadipourAHosseiniMFazelAThe effects of exposure to titanium dioxide nanoparticles during lactation period on learning and memory of rat offspringToxicol Ind Health201632222122824081627
- MohammadipourAFazelAHaghirHMaternal exposure to titanium dioxide nanoparticles during pregnancy; impaired memory and decreased hippocampal cell proliferation in rat offspringEnviron Toxicol Pharmacol20143761762524577229
- GaoXYinSTangMChenJYangZZhangWEffects of developmental exposure to TiO2 nanoparticles on synaptic plasticity in hippocampal dentate gyrus area: an in vivo study in anesthetized ratsBiol Trace Elem Res201114331616162821331565
- CuiYHChenXYZhuZPrenatal exposure to nanoparticulate titanium dioxide enhances depressive-like behaviors in adult ratsChemosphere2014969910423972732
- HuRPZhengLZhangTMolecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticlesJ Hazard Mater2011191324021570177
- GaoGDZeYGLiBOvarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticlesJ Hazard Mater2012243192723131501
- GaoGDZeYGZhaoXYTitanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male miceJ Hazard Mater2013258–259133143
- MenegolaEBrocciaMLDi RenzoFGiaviniEComparative study of sodium valproate-induced skeletal malformations using single or double staining methodsReprod Toxicol200216681582312401511
- LuoCRenRNHuYHChenXMYeLYHuangJApplication of double staining in the study of skeletal development toxicity in ratsJ Health Toxicol2004184249251 Chinese
- LuoCRenRNHuYHChenXMYeLYHuangJApplication of double staining in the study of skeletal development toxicity in ratsJ Health Toxicol2004184249251 Chinese
- CuiYQYangLYMiaoAJApplication of zebrafish in nanotoxicology: a reviewJ Nanjing Univ (Natural Sciences)2017532316325 Chinese [with English abstract]
- RocheleauSArbourMEliasMSunaharaGIMassonLToxicogenomic effects of nano- and bulk-TiO2 particles in the soil nematode Caenorhabditis elegansNanotoxicology2015950251225211548
- ZhangYLWangJXAdvances in effects of cadium on calcium metabolism and its associated potential mechanismsJ Environ Health2004214269271 Chinese [with English abstract]
- XuSQBaoKGShuBHYaoDCInfluence of cadmium on cartilage and bone formation induced by bone morphogenetic proteinChin J Prev Med1997315292294 Chinese
- WilsonAKCernyEASmithBDWaghABhattacharyyaMHEffects of cadmium on osteoblast formation and activity in vitroToxicol Appl Pharmacol199614014514608887463
- CarlssonLLundholmCECharacterisation of the effects of cadmium on the release of calcium and on the activity of some enzymes from neonatal mouse calvaria in cultureComp Biochem Physiol C Pharmacol Toxicol Endocrinol199611532512569375363
- BadakhshMHKhamsehMEMalekMImpact of maternal zinc status on fetal growth in an Iranian pregnant populationGynecol Endocrinol201127121074107621480766
- ClementeZCastroVLFeitosaLOFish exposure to nano-TiO2 under different experimental conditions: methodological aspects for nanoecotoxicology investigationsSci Total Environ2013463–464647656
- TanCWangWXModification of metal bioaccumulation and toxicity in Daphnia by titanium dioxide nanoparticlesEnviron Pollut2014186364224361562
- MoriTTaniTHanasawaKKodamaMEffects of zinc deficiency and corticosterone elevation on bone marrow in ratsEur Surg Res2001331929811399875
- SorellTLGrazianoJHEffect of oral cadmium exposure during pregnancy on maternal and fetal zinc metabolism in the ratToxicol Appl Pharmacol199010235375452315920
- XuMZWangHWangZZinc supplementation during pregnancy alleviates cadmium-induced developmental toxicity in miceActa Univ Med Anhui2012474425428