?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Paeonol (Pae; 2′-hydroxy-4′-methoxyacetophenone) has attracted intense attention as a potential therapeutic agent against various cancers. However, the use of Pae is limited owing to its hydrophobicity. Recently, biodegradable polymeric nanoparticles with amphiphilic copolymers have been used as drug carriers; these have better bioavailability and are promising tumor-targeted drug delivery systems. In the current study, we prepared Pae-loaded nanoparticles (Pae-NPs) with amphiphilic block copolymers using nanoprecipitation. The physiochemical characteristics and antitumor effects of nanoparticles were evaluated in different cancer cells. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays showed substantial inhibition of cell growth by Pae-NPs. Moreover, lower doses of Pae-NPs inhibited cell growth more efficiently than the equivalent doses of free Pae. Inhibition was characterized by significant elevation of intracellular reactive oxygen species and subsequent inhibition of Akt and regulation of apoptotic proteins, which could be partly reversed by pretreatment with the antioxidant N-acetylcysteine. In vivo results also demonstrated that Pae-NPs could exert much stronger antitumor effects than free Pae. Therefore, Pae-NPs represent a promising delivery system to overcome the low solubility of Pae and enable its use in treating cancer.
Keywords:
Background
Lung cancer is the leading cause of cancer-related deaths worldwide. Non-small-cell lung cancer (NSCLC) accounts for about 85% of cases of lung cancer, while small-cell lung cancer is another major type. Chemotherapy is one of the main treatments used in advanced NSCLC.Citation1 However, the effectiveness of chemotherapeutic agents is often hampered by drug resistance and side effects.Citation2 There is a necessity to discover novel agents to improve treatment efficacy in patients with advanced NSCLC.
Recently, drug-loaded nanoparticles using amphiphilic copolymers as drug carriers have shown better bioavailability against tumors than free drugs and have potential as tumor-targeted drug delivery systems.Citation3 Amphiphilic block copolymer-based polymeric micelles can automatically assemble into nanosized core-shell spherical particles, with the hydrophobic part (eg, poly(caprolactone) [PCL]) as the inner core and the hydrophilic part (eg, poly(ethylene glycol) [PEG]) as the outer shell. Hence, the inner core is amenable to incorporating hydrophobic drugs, which enables a controlled release of the loaded drug due to the strong affinity between the hydrophobic core and the encapsulated drug. Furthermore, the hydrophilic outer shell prevents the nanoparticles from being scavenged effectively by the reticuloendothelial systems, which facilitates their internalization by cells.Citation4
Paeonol (Pae; 2′-hydroxy-4′-methoxyacetophenone) is a micromolecular phenolic component isolated from the root bark of moutan cortex (Paeonia suffruticosa). There is a growing body of evidence indicating that Pae has antitumor capacity in vitro and in vivo.Citation5–Citation13 Pae has been reported to induce apoptosis in various cancer cells, including gastric cancer, esophageal cancer, ovarian cancer, and hepatocellular carcinoma.Citation5,Citation6,Citation8,Citation12 One study reported that Pae had a radiosensitizing effect on lung adenocarcinoma cell both in vitro and in vivo.Citation9 This radiosensitizing effect is closely related to apoptotic induction, which is caused by the downregulation of the PI3K/Akt signaling pathway.Citation9 However, the unsatisfactory bioavailability of Pae, which is due to its poor solubility, greatly limits its applications.Citation14 So far, few studies have explored novel formulations of Pae to enhance its bioavailability. Pae-loaded liposomes were successfully constructed by Wu et al, but there was no further antitumor evaluation.Citation15
Previous studies have shown that, when used as a sustained drug delivery system, drug-loaded nanoparticles can effectively accumulate in tumor cells by endocytosis, and at the tumor site through the enhanced permeability and retention effect.Citation3,Citation16 In this study, methoxy PEG–PCL (mPEG–PCL) nanoparticles were adopted as drug carriers to prepare Pae-loaded nanoparticles (Pae-NPs). First, the size, zeta potential, loading efficiency, and in vitro release pattern of Pae-NPs were characterized by physiochemical methods, and the cytotoxic discrepancy between free Pae and Pae-NPs was measured by 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assays. Next, we measured changes in apoptosis and oxidative stress in A549 NSCLC cancer cells in response to treatment with free Pae and Pae-NPs, to elucidate the differential antitumor effects of Pae and Pae-NPs. Finally, we examined the in vivo antitumor effects of Pae-NPs in a NSCLC xenograft cancer model using A549 cells. We hypothesize that Pae-NPs activate pro-apoptotic, and suppress anti-apoptotic, signal molecules simultaneously in cancer cells, through inducing intracellular reactive oxygen species (ROS) generation and inhibiting the phosphorylation of Akt.
Materials and methods
Materials
Pae was purchased from Nanjing Haoxuan Biotechnological Co Ltd (Nanjing, China). Human NSCLC cell lines A549 and NCI-H1975 were obtained from the Shanghai Institute of Cell Biology (Shanghai, China). Cell culture material (RPMI 1640 media and fetal bovine serum) was from Thermo Fisher Scientific (Waltham, MA, USA). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C in a 5% CO2 humidified atmosphere. MTT and N-acetylcysteine (NAC) were purchased from Sigma-Aldrich (St Louis, MO, USA). Antibodies against phosphate-Akt, Akt, Bcl-XL, Bax, Bcl-2, caspase-3, cleaved caspase-3, and β-actin were obtained from Cell Signaling Technology, Inc (Danvers, MA, USA). All other chemicals were of analytical grade.
Methods
Formulation of nanoparticles
Pae-NPs were constructed by a nanoprecipitation method as described previously,Citation16,Citation17 with minor modifications.Citation16,Citation17 Forty milligrams of mPEG10k–PCL30k block copolymers and 8 mg Pae were dissolved in 3 mL acetone, which was then added dropwise into 30 mL double-distilled water with slight-to-moderate stirring at room temperature. The solution was then transferred into a dialysis bag (molecular weight [MW] 12 kD) and rinsed in double-distilled water for 2 hours, changing the medium every 10 minutes to remove all acetone. The solution was then filtered through a 0.22 μm membrane to remove free drugs and copolymer aggregates. Coumarin-6 (a green hydrophobic fluorescent marker) and Pae co-loaded nanoparticles were prepared by simultaneous encapsulation of Pae and coumarin-6. Blank nanoparticles were prepared without addition of drugs. Solutions of nanoparticles were then freeze-dried with F-68 as a cryoprotectant before use.
Characterization of nanoparticles and stability test
Dynamic light scattering (DLS) was applied to detect the mean diameter and distribution of Pae-NPs (Brookhaven BI-9000AT instrument; Brookhaven Instruments Corporation, NY, USA), while laser Doppler anemometry was adopted to measure the zeta potential of the particles (ZetaPlus Zeta Potential Analyzer; Brookhaven Instruments Corporation). The solution of Pae-NPs was kept at room temperature. The size of the nanoparticle was measured by DLS every 2 days for 11 days.
Drug loading content (DLC) and encapsulation efficiency (EE)
High-performance liquid chromatography (HPLC) was used to analyze the loading efficiency of Pae-NPs (Shimadzu LC-10AD; Shimadzu, Japan). The mobile phase consisted of methanol (spectral grade; EMD Millipore, Billerica, MA, USA) and double-distilled water (60/40, v/v) pumped at a flow rate of 0.8 mL/min, with a determination wavelength of 274 nm.Citation18
EquationEquations 1(1) and Equation2
(2) were applied to calculate the DLC and EE, respectively.
In vitro release of Pae-NPs
In vitro release was detected by dialysis as reported previously.Citation16,Citation17 Briefly, 10 mg Pae-NPs powder was dissolved in 1 mL of 0.1 M phosphate-buffered saline (PBS; pH 7.4) and then transferred into a dialysis bag, followed by immersion in 20 mL PBS at room temperature. At each time point, 1 mL liquid was taken from the medium outside the dialysis bag and the Pae concentration was measured using HPLC as described above.
Nanoparticle uptake by cancer cells
To assess the uptake efficiency of Pae-NPs, we applied coumarin-6 as a tracer of nanoparticles incubated with cancer cells.Citation16,Citation17 A549 or NCI-H1975 cells (5×105) were seeded in 6-well plates with RPMI 1640 supplemented with 10% fetal bovine serum and allowed to adhere at 37°C with 5% CO2 for 24 hours. Next, the cells were incubated with coumarin-6 and Pae co-loaded nanoparticles for 2 hours with or without co-treatment with 10 mM NaN3, an endocytosis inhibitor. The cells were washed three times with PBS and then observed under a fluorescent microscope (LSM 410; Carl Zeiss Meditec AG, Jena, Germany). For the assessment of the uptake efficiency of Pae, free Pae was incubated with cancer cells at the same concentration and subjected to HPLC detection.
To quantify the uptake efficiency of Pae and Pae-NPs, we used HPLC to measure the intracellular drug concentration. Cancer cells were incubated with Pae-NPs for 2 hours and then washed out with PBS three times. The suspension was centrifuged at 1,500× g. The pellet was reconstituted in the mobile phase, sonicated for 30 seconds, and subjected to HPLC detection.Citation18
In vitro cytotoxicity studies
The in vitro antitumor effect of Pae-NPs against A549 and NCI-H1975 cells was evaluated by MTT assay, as reported in our previous study.Citation16 Briefly, both cells were seeded in 96-well plates with a density of around 5,000 cells/well and allowed to adhere for 24 hours prior to the assay. Cells were exposed to a series of equivalent doses of free Pae or Pae-NPs (20, 40, 80, 160, 320, and 640 μg/mL) at 37°C for 48 hours. The cells were then washed with PBS three times, and 50 μL MTT indicator dye (5 mg/mL in PBS, pH 7.4) was added to each well and incubated for 2 hours at 37°C in the dark. The cultured medium was replaced by 200 μL acidified isopropanol (0.33 mL HCl in 100 mL isopropanol) to dissolve the formazan crystals. The absorption was detected at a wavelength of 550 nm by a microkinetics reader.
Detection of intracellular ROS levels
Intracellular ROS levels were measured with dihydroethidium (DHE) as described previously.Citation16 Briefly, A549 cells at a density of 2×105 were co-incubated with Pae or Pae-NPs at an equivalent dose of 50 μg/mL for 48 hours, with or without the co-treatment of NAC at a concentration of 1 mM. Next, 10 μM DHE was incubated with cells for 30 minutes and then washed by PBS. The red fluorescence of DHE was immediately observed by a fluorescent microscope and simultaneously measured by a fluorescent spectrophotometer with an emission wavelength at 610 nm and an excitation wavelength of 535 nm.
Apoptosis analysis
For apoptosis analysis, A549 cells were treated with 50 μg/mL free Pae or Pae-NPs for 48 hours in the presence or absence of 1 mM NAC pretreated for 2 hours. Quantification of apoptotic cells was performed with annexin-V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) Apoptosis Detection Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Cells were then washed and transferred into 1× annexin-V binding buffer at a concentration of 1×106 cells/mL. An aliquot of 100 μL of suspension (1×105 cells) was stained with 5 μL annexin-V-FITC and 5 μL PI, and then incubated for 15 minutes at room temperature in the dark. Cell analyses were then performed using a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot analysis
For protein extraction, A549 cells were treated with 50 μg/mL free Pae or Pae-NPs for 48 hours in the presence or absence of 1 mM NAC pretreated for 2 hours. Confluent cells were denatured in lysis buffer (containing 20 mM Tris-HCl, 200 mM NaCl, 0.2% NP-40, 0.5% Triton X-100, and protease inhibitors) and boiled at 95°C for 3 minutes. Equal amounts of protein extract were separated on 12% polyacrylamide gels using standard sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories Inc, Hercules, CA, USA). Following blocking in Tris-buffered saline with 0.1% Triton X-100 and 5% milk, the membranes were incubated at 4°C overnight with different primary antibodies against phosphate-Akt (dilution, 1:1,000; no 4060), Akt (dilution, 1:1,000; no 4685), Bcl-XL (dilution, 1:1,000; no 2762), Bax (dilution, 1:1,000; no 5023), Bcl-2 (dilution, 1:1,000; no 2870), caspase-3 (dilution, 1:1,000; no 9665), cleaved caspase-3 (dilution, 1:1,000; no 9664), and β-actin (dilution, 1:1,000; no 4970), respectively. Following washing, the membranes were incubated with secondary antibody (dilution, 1:3,000; no 7074) conjugated to horseradish peroxidase at room temperature for 2 hours. Signal detection was carried out on an imager (Syngene, Frederick, MD, USA) using an enhanced chemiluminescence system (Thermo Fisher Scientific).
In vivo evaluation of Pae-NPs in a xenograft model
Five-week-old BALB/C-nu/nu nude mice were obtained from the Shanghai Laboratory Animal Center of China. The Animal Care Committee of Nanjing University of Chinese Medicine approved this study. Housing and all of the animal experiments were conducted according to the protocol approved by the Animal Care Committee of Nanjing University of Chinese Medicine. A549 cells (1–1.5×106) were harvested, washed, and resuspended in 200 μL serum-free medium, and were subcutaneously implanted into the right flanks of nude mice. Tumor volumes (mm3) were defined using the following formula: V = (length × widthCitation2)/2.Citation19 When the tumors reached a mean volume of 100 mm3, the mice were randomly divided into four groups (n=8). Each group of mice was then treated with vehicle (PBS), empty NPs (E-NPs), free Pae, or Pae-NPs, respectively, administered through the tail vein in a volume of 200 μL as shown in . Free Pae solution was administered at doses of 40 mg/kg. Pae-NPs were administered as a saline solution at equivalent doses of 40 mg/kg. The day that mice were treated with drugs was designated day 1. Tumor volumes were then monitored every other day with calipers during the treatment. To evaluate possible toxic effects of the therapy, the body weight of each mouse was also measured during the period of study. At the end of the experiment, survival curves were calculated by Kaplan–Meier analysis.
Table 1 Parameters of the in vivo study
Statistical analysis
Values are presented as mean ± SD from triplicate experiments. The results were analyzed by analysis of variance or Student’s t-test with SPSS 11.5 (SPSS Inc, Chicago, IL, USA). A difference of p<0.05 was considered statistically significant.
Results and discussion
Characterization of nanoparticles
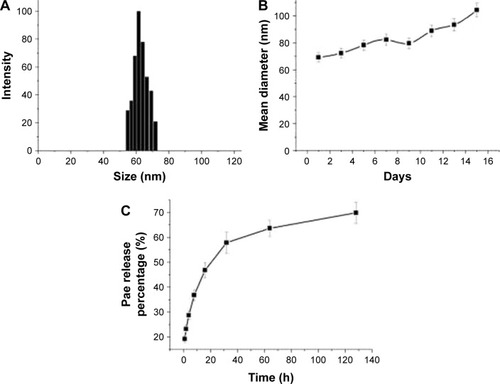
The diameters of Pae-NPs are around 60 nm, with a negative zeta potential slightly below 0 mV (; ). In a previous study, a PEG coating was shown to greatly neutralize the negative zeta potential of nanoparticles.Citation20,Citation21 The satisfactory loading efficiency was attributed to the affinity between lipophilic Pae and the inner PCL core.Citation22,Citation23 By varying the feeding ratio of the copolymer and Pae, the highest DLC for Pae in mPEG10k–PCL30k nanoparticles was 16.3%±2.4%, and EE was >94.3%±4.4% (). In addition, shows that the size of Pae-NPs slightly increased over time at room temperature, which indicates that Pae-NPs had good stability in vitro. shows the sustained release pattern of Pae-NPs. After the initial burst of about 30% release in the first few hours, the release speed gradually decreased, with nearly 70% of Pae being released from the nanoparticles. This indicates that Pae can be released from mPEG–PCL nanoparticles in a controlled manner, meaning that Pae-NPs could be used as a potential controlled release system for cancer therapy.
Table 2 Mean particle size and drug loading efficiency of Pae-NPs
In vitro cytotoxicity of Pae-NPs against cancer cells
shows the in vitro cytotoxicity of Pae-NPs. E-NPs were almost nontoxic to A549 cells, even at a concentration of 5 mg/mL (). Both Pae and Pae-NPs dose-dependently inhibited the proliferation of A549 cells when incubated with A549 cells for 48 hours; however, Pae-NPs induced more cell death than did the equivalent dose of Pae. Most importantly, lower concentrations of Pae-NPs (20, 40, and 80 μg/mL) significantly inhibited cell growth compared with the equivalent dose of Pae. Specifically, 20 μg/mL Pae-NPs resulted in nearly 30% cell death, compared with <15% for treatment with the same dose of Pae (p<0.05). It is also notable that more significant cell growth inhibition was observed in cells exposed to 40 or 80 μg/mL Pae-NPs compared with the equivalent doses of Pae (p<0.01). The same trend was observed in the cytotoxicity curve for NCI-H1975 cells (). Pae-NPs showed a dose-dependent cytotoxicity, which was stronger than that observed for the equivalent dose of free Pae.
Figure 2 Dose-dependent cytotoxicity of Pae and Pae-NPs against (A) A549 and (B) NCI-H1975 cells.
Notes: Data are presented as mean ± SD (n=3). *p<0.05, **p<0.01, compared to the control group.
Abbreviations: Pae, paeonol; Pae-NPs, Pae-loaded nanoparticles; E-NPs, empty nanoparticles; SD, standard deviation.
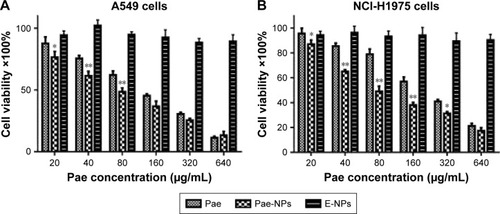
It has been demonstrated in earlier studies that drug-loaded nanoparticles exhibit stronger cytotoxicity than the free drug owing to endocytosis-based cellular uptake,Citation24 which is supported by the current findings. As a significant difference is observed in cells exposed to lower doses of Pae and Pae-NPs, a dose of 50 μg/mL was applied in further experiments to study the discrepancy between Pae-NPs and free Pae by detection of apoptosis and relative apoptotic protein expression.
Uptake of nanoparticles by cancer cells
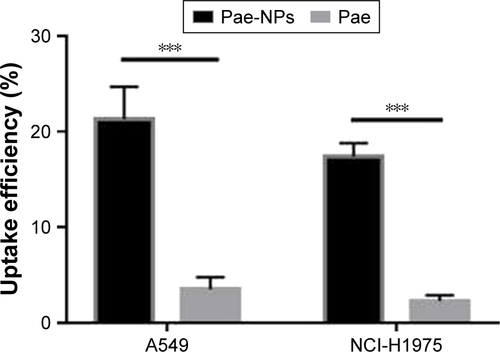
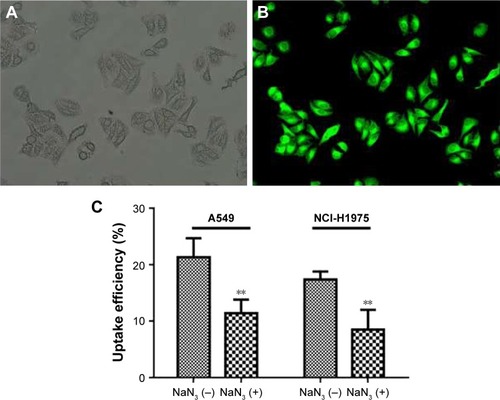
The cellular uptake of coumarin-6-loaded Pae-NPs by A549 cells was measured by fluorescence microscopy. The fluorescence, which indicates the location of the nanopar-ticles, was observed mainly in the cytoplasm rather than the nucleus (). A 2-hour incubation allowed the A549 cells to take up nanoparticles efficiently, with >20% of Pae located intracellularly. The same fluorescence was observed in NCI-H1975 cells when incubated with coumarin-6-loaded Pae-NPs. Quantification studies showed that the uptake efficiency of Pae-NPs by NCI-H1975 cells was <20% (). Moreover, the uptake efficiency could be significantly decreased by NaN3, an endocytosis inhibitor, which demonstrated that the uptake process was significantly dependent on endocytosis (). In addition, the uptake efficiency of Pae-NPs by cancer cells was much higher than that of free Pae (Figure S1), which demonstrates the efficient penetration of nanoparticles through endocytosis. Previous studies have also reported that the uptake of nanoparticles by cells occurs mainly through endocytosis. However, if it exceeds the saturable capacity of cellular endocytosis, the uptake efficiency will no longer increase as the concentration elevates.Citation25 Here, A549 cells were used because of the relatively higher uptake efficiency and stronger cytotoxicity of Pae-NPs.
Figure 3 Cellular uptake of coumarin-6-loaded fluorescent Pae-NPs by cancer cells.
Notes: (A, B) Representative microscopic images of A549 cells incubated with coumarin-6-loaded Pae-NPs for 2 hours; (A) bright fields with higher magnification; (B) fluorescent fields with higher magnification. (C) Cellular uptake efficiency of Pae-NPs by A549 and NCI-H1975 cells. Data are presented as mean ± SD (n=3). **p<0.01, compared to the control group.
Abbreviations: Pae-NPs, Pae-loaded nanoparticles; SD, standard deviation.
Apoptotic induction of Pae-NPs
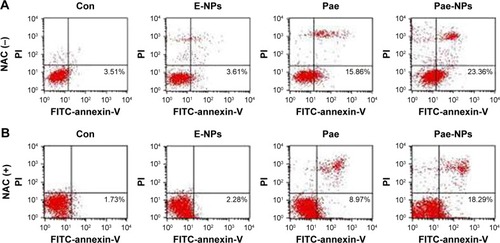
Several studies have reported that Pae could promote apoptosis in several tumor cells.Citation5,Citation6,Citation8,Citation12 To examine the apoptotic effect of E-NPs, free Pae, and Pae-NPs, cells were treated with a dose of 50 μg/mL of these agents for 48 hours. Apoptosis was detected using flow cytometry assays with an annexin-V-FITC/PI kit. Both the free Pae and Pae-NPs promoted apoptosis in A549 cells compared with E-NPs (). It is notable that the proportion of early apoptotic cells induced by Pae-NPs was significantly higher than that induced by free Pae (23.36% vs 15.86%). These results indicate that Pae-NPs elicited significantly more apoptosis at almost equivalent dose and incubation time.
Detection of ROS level induced by Pae-NPs
It is known that the balance between ROS and antioxidant capacity is a major determinant of cell destiny, as elevation of ROS can induce apoptosis.Citation26 In addition, many studies have shown that some chemotherapeutic agents can induce apoptosis in certain types of cancer cells through overproduction of intracellular ROS.Citation13,Citation27–Citation29 Therefore, we investigated whether Pae treatment could increase ROS levels in A549 cells. We found that both free Pae and Pae-NPs increased ROS generation, and Pae-NPs induced more accumulation of ROS. There was no significant difference in intracellular ROS levels between cells treated with control and with E-NPs (). However, cells treated with free Pae or Pae-NPs showed increased intracellular ROS levels of 1.80- or 2.29-fold of control values, respectively (p=0.01, Pae vs control; p=0.01, Pae-NPs vs control) (). These data show the increase in intracellular ROS levels with Pae treatment while Pae-NPs induced more intracellular ROS.
Figure 5 Detection of intracellular ROS.
Notes: (A) DHE fluorescence images of A549 cells treated with different agents (control; E-NPs; Pae [50 μg/mL]; Pae-NPs [at an equivalent dose of 50 μg/mL]). (B) Intracellular DHE fluorescence intensity. Data are represented as mean ± SD. *p<0.05, compared to the control group; #p<0.05, compared to the Pae group or Pae-NPs group.
Abbreviations: Pae, paeonol; Pae-NPs, Pae-loaded nanoparticles; E-NPs, empty nanoparticles; SD, standard deviation; DHE, dihydroethidium; ROS, reactive oxygen species; NAC, N-acetylcysteine; Con, control.
![Figure 5 Detection of intracellular ROS.Notes: (A) DHE fluorescence images of A549 cells treated with different agents (control; E-NPs; Pae [50 μg/mL]; Pae-NPs [at an equivalent dose of 50 μg/mL]). (B) Intracellular DHE fluorescence intensity. Data are represented as mean ± SD. *p<0.05, compared to the control group; #p<0.05, compared to the Pae group or Pae-NPs group.Abbreviations: Pae, paeonol; Pae-NPs, Pae-loaded nanoparticles; E-NPs, empty nanoparticles; SD, standard deviation; DHE, dihydroethidium; ROS, reactive oxygen species; NAC, N-acetylcysteine; Con, control.](/cms/asset/f70a07fb-ed27-461a-9b8b-e0b0b656f67a/dijn_a_12193775_f0005_c.jpg)
Next, to further investigate whether ROS plays an important part in Pae-induced apoptosis, NAC was used to block accumulation of ROS in A549 cells. ROS accumulation was blocked more obviously by NAC in Pae-treated cells (up to 27.2%) in contrast to Pae-NP-treated cells (up to 13.5%) (). Simultaneously, with NAC pretreatment, the apoptosis induced by free Pae was attenuated more significantly than that induced by Pae-NPs (18.29% vs 8.97%), which is consistent with ROS attenuation being blocked by NAC (). These data suggest that the pro-apoptotic effect of free Pae and Pae-NPs is partly dependent on the generation of ROS.
Modulation of AKT/apoptotic pathways by Pae-NPs
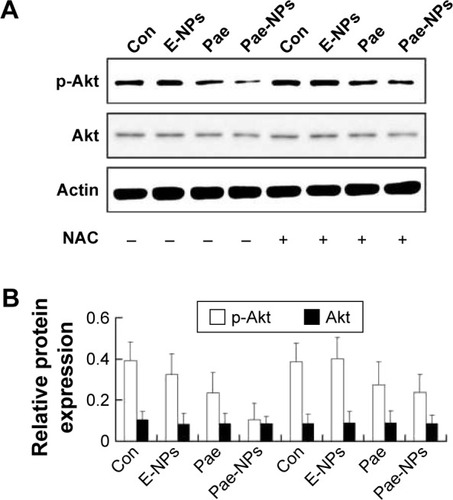
The regulation of Akt in apoptosis through its downstream pathways remains to be implicated in cancer.Citation30–Citation32 It is known that suppression of Akt activation is an important mechanism in ROS-induced apoptosis in tumor cells.Citation26 To determine the role of Akt in Pae-NP-induced lung cancer cell apoptosis, Western blot analysis was used to evaluate Akt-related proteins after Pae-NPs treatment. A549 cells were treated with a dose of 50 μg/mL of either free Pae or Pae-NPs. The results showed that phosphorylated Akt levels decreased more significantly after Pae-NPs treatment than after free Pae treatment ().
Figure 6 Effects of free Pae or Pae-NPs on the expression of Akt pathway proteins in A549 cells.
Notes: (A) Blots of p-Akt and Akt from cells treated with different agents. (B) Semi-quantification of protein expression. Data are represented as mean ± SD.
Abbreviations: Pae, paeonol; Pae-NPs, Pae-loaded nanoparticles; E-NPs, empty nanoparticles; SD, standard deviation; NAC, N-acetylcysteine; Con, control.
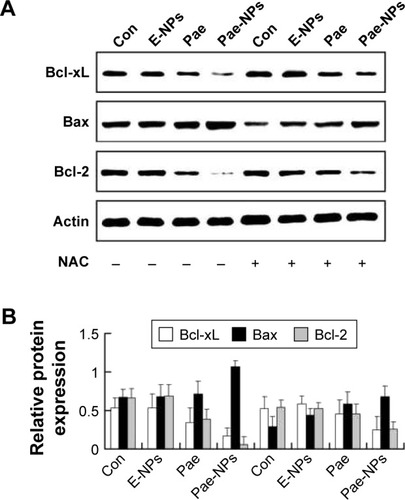
Our data further indicated that protein expression of Bcl-2 and Bcl-XL was significantly decreased, whereas the expression of Bax proteins was increased, in A549 cells treated with free Pae or Pae-NPs (). In accordance with the apoptosis results, the expression of the anti-apoptotic proteins Bcl-2 and Bcl-XL was decreased more significantly in Pae-NP-treated A549 cells than in free Pae-treated cells. Similarly, the expression of the pro-apoptotic protein Bax was increased more significantly in Pae-NP-treated A549 cells. These results indicate that Pae-NPs exert a greater pro-apoptotic effect than free Pae.
Figure 7 Effect of free Pae or Pae-NPs on the expression of apoptotic proteins in A549 cells.
Notes: (A) Blots of Bcl-XL, Bax, and Bcl-2 from cells treated with different agents. (B) Semi-quantification of protein expression. Data are represented as mean ± SD.
Abbreviations: Pae, paeonol; Pae-NPs, Pae-loaded nanoparticles; E-NPs, empty nanoparticles; SD, standard deviation; NAC, N-acetylcysteine; Con, control.
During the initiation and progression of apoptosis, caspase-3 has a key role, and its activation is a characteristic of apoptosis.Citation33,Citation34 The expression of caspase-3 was examined to determine whether Pae led to caspase-dependent apoptosis. The cleavage of caspase-3 was markedly elevated in A549 cells treated with Pae-NPs compared with free Pae ().
Figure 8 Effect of free Pae or Pae-NPs on the expression of caspase-3 and cleaved caspase-3 proteins in A549 cells.
Notes: (A) Blots of caspase-3 and pro-caspase-3 from cells treated with different agents. (B) Semi-quantification of protein expression. Data are represented as mean ± SD.
Abbreviations: Pae, paeonol; Pae-NPs, Pae-loaded nanoparticles; E-NPs, empty nanoparticles; SD, standard deviation; NAC, N-acetylcysteine; Con, control.
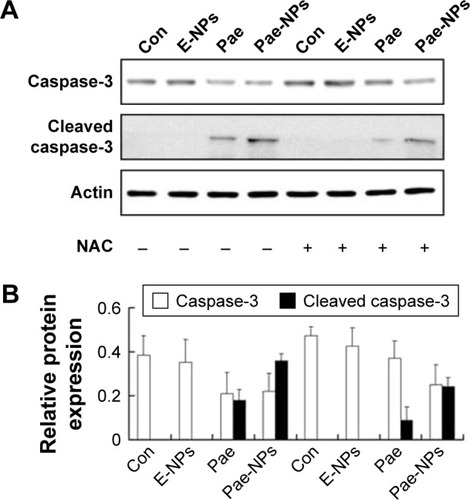
Following removal of the ROS by NAC, we next studied the expression levels of phosphorylated Akt, Bcl-2, Bcl-XL, and Bax, and cleavage of caspase-3 in A549 cells treated with free Pae or Pae-NPs. We found that the increased levels of phosphorylated Akt and caspase-3 cleavage by Pae or Pae-NPs were rescued with NAC pretreatment, especially in the case of free Pae treatment ( and ). NAC pretreatment could partially prevent free Pae or Pae-NPs from inducing the expression of Bax and reversing the decrease of Bcl-2 and Bcl-XL (). Most importantly, NAC inhibited the expression of Bax and recovered the expression of Bcl-2 and Bcl-XL induced by free Pae much more significantly. Therefore, these results show that Pae-induced apoptosis results from the initial generation of ROS and the following inhibition of the Akt pathway, which appears to be the downstream event of ROS elevation. Pae-NPs statistically surpassed free Pae in apoptotic induction through regulating the ROS-dependent Akt pathway and the downstream caspase cascade.
Although it has been shown that Pae-induced apoptosis is characterized by the activation of caspase-3 and inhibition of surviving,Citation6 decrease in the Bcl-2/Bax ratio,Citation5,Citation8,Citation12 and stimulation of interleukin (IL)-2 and tumor necrosis factor (TNF)-α production,Citation12 the exact mechanism remains unclear. Previous studies have demonstrated that the mechanism of Pae’s antitumor effect is via induction of apoptosis, stimulation of IL-2, and TNF-α production.Citation5,Citation12,Citation35
In the current study, we showed that Pae-induced apoptosis is closely related to the induction of ROS and the suppression of Akt activation in human lung cancer cells. Furthermore, drug-loaded nanoparticles exhibit clear advantages over free drug in inhibiting cell growth by the enhancement of cellular uptake and the subsequent generation of ROS, thereby strongly inducing cell apoptosis through regulation of the Akt pathway. Recent studies have shown that Pae has a synergistic effect with cisplatin in esophageal and hepatoma cancer cell lines, which indicates the potential of Pae as a chemosensitizer in conventional chemotherapy.Citation36,Citation37 A similar study is ongoing in the authors’ laboratory, focusing on the synergistic effect and the underlying mechanisms of Pae and paclitaxel in several cancer cell lines.
In vivo evaluation of the antitumor effect of Pae-NPs
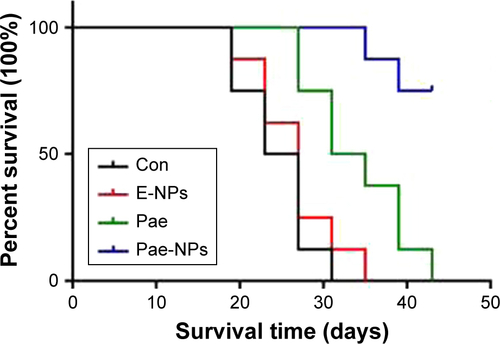
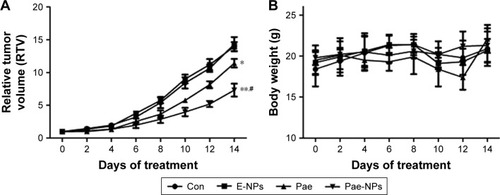
The aforementioned promising antitumor effects of Pae-NPs encouraged us to study the in vivo antitumor effects of Pae-NPs in mice bearing A549 cell xenografts. Both free Pae and Pae-NPs showed superior antitumor effects compared with control and E-NPs. Furthermore, Pae-NPs exhibited the strongest antitumor efficacy (). As shown in , the mice in the control group and the treatment group showed no statistically significant differences in body weight throughout the evaluation. A literature search found no previous study of the antitumor effect of Pae-NPs, which makes the current study the first report of a nano-delivery system for Pae in cancer therapy. Moreover, the Kaplan–Meier analysis in Figure S2 demonstrates the superior therapeutic effect of Pae-NPs with the best survival among all the groups, which is in accordance with the tumor growth results in .
Figure 9 In vivo antitumor efficacy of Pae-NPs.
Notes: The mice were intravenously administered with PBS, E-NPs, free Pae, or Pae-NPs every 2 days for three consecutive injections. (A) Growth inhibition study in the A549 xenograft model. (B) Body weight changes for the tumor bearing mice after various formulations were given to mice on the indicated days. Data are represented as mean ± SD (n=6). *p<0.05, **p<0.01, compared to the control group; #p<0.05, compared to the Pae group.
Abbreviations: Pae, paeonol; Pae-NPs, Pae-loaded nanoparticles; E-NPs, empty nanoparticles; SD, standard deviation; PBS, phosphate-buffered saline; Con, control.
Used in traditional Chinese medicine, Pae has a variety of pharmacological effects with lower toxicity compared with commonly used chemotherapeutics. It has previously been reported that Pae is a good chemo/radiosensitizer, which points to a novel strategy to improve the efficacy of chemotherapy or radiotherapy.Citation9,Citation36–Citation38 Pae, in combination with cisplatin, had a significantly synergistic growth inhibitory effect on esophageal cancer cell lines.Citation37 Meanwhile, Pae has protective effects on cisplatin-induced acute renal failure in mice.Citation39 In addition, Pae also showed the potential property of the reversal of paclitaxel resistance, facilitating the sensitivity of breast cancer chemotherapy.Citation38 Moreover, delivery of Pae using nanoparticles will enhance its antitumor effects and improve its pharmacokinetic and pharmacodynamic profile.
Conclusion
In this study, we prepared Pae-loaded mPEG–PCL nanoparticles with satisfactory DLC and EE. In vitro cytotoxicity studies indicated that, at lower doses, Pae-NPs achieved more cell apoptosis than the equivalent dose of Pae, as well as greater induction of intracellular ROS and consequent downregulation of p-Akt. The superior effect of Pae-NPs compared with free Pae was partly reversed by the antioxidant NAC. In vivo evaluation demonstrated a superior antitumor effect of Pae-NPs in a xenograft cancer model.
Author contributions
CC and FJ were involved in the fabrication and characterization of the nanoparticles. CC, HZ, and FJ were involved in the in vitro and in vivo evaluations. SR, HZ, and LQ were involved in the design and supervision of the whole study. HZ was involved in the analysis of protein expression. All authors contributed toward data analysis, drafting and revising the paper, read and approved the final manuscript, and agree to be accountable for all aspects of the work.
Acknowledgments
The study was partly supported by the National Natural Science Foundation of China (grant no 81572937; 81603647).
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- GalluzziLVitaleIMichelsJSystems biology of cisplatin resistance: past, present and futureCell Death Dis20145e125724874729
- LiXLiRQianXSuperior antitumor efficiency of cisplatin-loaded nanoparticles by intratumoral delivery with decreased tumor metabolism rateEur J Pharm Biopharm200870372673418634874
- HuYXieJTongYWWangCHEffect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cellsJ Control Release2007118171717241684
- SunGPWanXXuSPWangHLiuSHWangZGAntiproliferation and apoptosis induction of paeonol in human esophageal cancer cell linesDis Esophagus200821872372918522637
- YinJWuNZengFChengCKangKYangHPaeonol induces apoptosis in human ovarian cancer cellsActa Histochem2013115883583923768958
- ChenBNingMYangGEffect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma ratsMolecules20121744672468322522397
- LiNFanLLSunGPPaeonol inhibits tumor growth in gastric cancer in vitro and in vivoWorld J Gastroenterol201016354483449020845518
- LeiYLiHXJinWSThe radiosensitizing effect of paeonol on lung adenocarcinoma by augmentation of radiation-induced apoptosis and inhibition of the PI3K/Akt pathwayInt J Radiat Biol201389121079108623875954
- KimSALeeHJAhnKSPaeonol exerts anti-angiogenic and anti-metastatic activities through downmodulation of Akt activation and inactivation of matrix metalloproteinasesBiol Pharm Bull20093271142114719571375
- ChunhuZSuiyuHMeiqunCGuilinXYunhuiLAntiproliferative and apoptotic effects of paeonol on human hepatocellular carcinoma cellsAnticancer Drugs200819440140918454050
- SunGPWangHXuSPAnti-tumor effects of paeonol in a HepA-hepatoma bearing mouse model via induction of tumor cell apoptosis and stimulation of IL-2 and TNF-alpha productionEur J Pharmacol20085842–324625218329639
- TanSYeJQianCJiCLiuCWangJPaeonol inhibits the proliferation of human colorectal carcinoma cells and synergic with chemotherapeutic agentsSaudi Med J200728464264317457498
- TsaoJYTsaiHHWuCPRelease of paeonol-beta-CD complex from thermo-sensitive poly(N-isopropylacrylamide) hydrogelsInt J Pharm20104021–212312820933068
- WuRGDaiJDWuFGZhangXHLiWHWangYRCompetitive molecular interaction among paeonol-loaded liposomes: differential scanning calorimetry and synchrotron X-ray diffraction studiesInt J Pharm20124381–2919722981687
- LiXZhenDLuXEnhanced cytotoxicity and activation of ROS-dependent c-Jun NH2-terminal kinase and caspase-3 by low doses of tetrandrine-loaded nanoparticles in Lovo cells – a possible Trojan strategy against cancerEur J Pharm Biopharm201075333434020438840
- LiuJXuHZhangYNovel tumor-targeting, self-assembling peptide nanofiber as a carrier for effective curcumin deliveryInt J Nanomedicine2014919720724399876
- ZhaoJYanWSimultaneous determinations of paeonol and palmatine hydrochloride in Shangshi aerosols by HPLC methodJ Pharm Biomed Anal200538357157515925262
- ChenYShiLZhangLThe molecular mechanism governing the oncogenic potential of SOX2 in breast cancerJ Biol Chem200828326179691797818456656
- ZhangLYangMWangQ10-Hydroxycamptothecin loaded nanoparticles: preparation and antitumor activity in miceJ Control Release2007119215316217400320
- ZhangLHuYJiangXYangCLuWYangYHCamptothecin derivative- loaded poly(caprolactone-co-lactide)-b-PEG-b-poly(caprolactone-co-lactide) nanoparticles and their biodistribution in miceJ Control Release200496113514815063036
- LiYDongLJiaAChangXXueHPreparation and characterization of solid lipid nanoparticles loaded traditional Chinese medicineInt J Biol Macromol2006383–529629916600362
- WangMChenWZhangHSynthesis and characterization of PLLA–PLCA–PEG multiblock copolymers and their applications in modifying PLLA porous scaffoldsEur Polym J2007431146834694
- van VlerkenLEDuanZSeidenMVAmijiMMModulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancerCancer Res200767104843485017510414
- DavdaJLabhasetwarVCharacterization of nanoparticle uptake by endothelial cellsInt J Pharm20022331–2515911897410
- IvanovaDBakalovaRLazarovaDGadjevaVZhelevZThe impact of reactive oxygen species on anticancer therapeutic strategiesAdv Clin Exp Med201322689990824431321
- ZhouYShuFLiangXAmpelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathwayPloS One201492e8902124551210
- ParkKWKunduJChaeIGCarnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cellsInt J Oncol20144441309131524481553
- KimTHWooJSKimYKKimKHSilibinin induces cell death through ROS-dependent down-regulation of Notch-1/ERK/Akt signaling in human breast cancer cellsJ Pharmacol Exp Ther2014349226827824472723
- LiuCGongKMaoXLiWTetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinomaInt J Cancer201112961519153121128229
- CassinelliGZucoVGattiLTargeting the Akt kinase to modulate survival, invasiveness and drug resistance of cancer cellsCurr Med Chem201320151923194523410153
- MartelliAMTabelliniGBressaninDThe emerging multiple roles of nuclear AktBiochim Biophys Acta20121823122168217822960641
- MeierPVousdenKHLucifer’s labyrinth – ten years of path finding in cell deathMol Cell200728574675418082600
- ChiantoreMVVannucchiSManginoGSenescence and cell death pathways and their role in cancer therapeutic outcomeCurr Med Chem200916328730019149578
- OuYLiQWangJLiKZhouSAntitumor and apoptosis induction effects of paeonol on mice bearing EMT6 breast carcinomaBiomol Ther (Seoul)201422434134625143814
- XuSPSunGPShenYXPengWRWangHWeiWSynergistic effect of combining paeonol and cisplatin on apoptotic induction of human hepatoma cell linesActa Pharmacol Sin200728686987817506946
- WanXASunGPWangHXuSPWangZGLiuSHSynergistic effect of paeonol and cisplatin on oesophageal cancer cell linesDig Liver Dis200840753153918339596
- CaiJChenSZhangWPaeonol reverses paclitaxel resistance in human breast cancer cells by regulating the expression of transgelin 2Phytomedicine201421798499124680370
- LeeHLeeGKimHBaeHPaeonol, a major compound of moutan cortex, attenuates cisplatin-induced nephrotoxicity in miceEvid Based Complement Alternat Med2013201331098924171038