?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Development of a novel delivery system has been attempted to deliver viable probiotic cells into the gut for a prolonged period of time while maintaining high numbers of viable cells within the formulation throughout the shelf-life of the product and during the gastrointestinal transit. Core mucoadhesive microspheres of Bacillus coagulans were developed employing several grades of hypromellose, a mucoadhesive polymer, following coacervation and phase separation technique and were subsequently enteric-coated with hypromellose phthalate. Microspheres were evaluated for percent yield; entrapment efficiency; in vitro swelling; surface morphology; particle size, size distribution, and zeta potential; flow property, mucoadhesion property by the ex vivo mucoadhesive strength test and the in vitro wash off test; in vitro release profile and release kinetic; in vivo probiotic activity; and stability. The values for the kinetic constant and regression coefficient of model-dependent approaches and the difference factor (f1), the similarity factor (f2), and the Rescigno index (ξ1 and ξ2) of model independent approaches were determined for comparing in vitro dissolution profiles. Freeze dried B. coagulans cells were successfully formulated as enteric-coated mucoadhesive microspheres with satisfactory physical structure and yield. The viability of B. coagulans was maintained in the simulated gastric conditions and during processing; in simulated intestinal conditions exhibiting mucoadhesion, and controlling and extending the viable cell release following zero-order; and was satisfactorily stable at room temperature. Test results depict statistically significant effects of the hypromellose grade and their concentration on the performance and release profile of formulations.
Introduction
Reported health benefits of probiotics such as suppressing the growth of undesirable microorganisms in the colon and in the small intestine, controlling serum cholesterol level, reducing the risk of colon cancer, stimulating the immune system, improving lactose utilization, and controlling the allergic inflammation associated with food allergy has created a large market of probiotic foods worldwide.Citation1–Citation7 The therapeutic benefit of probiotics is associated with its ability to secrete a bacteriocin, coagulin, which is active against a broad spectrum of enteric microbes.Citation7 To be therapeutically active, probiotics must arrive in the intestine alive and in sufficient numbers which is suggested at 106–107 colony-forming units (cfu).Citation8 B. coagulans, commonly mislabeled as Lactobacillus sporogenes, also suffers with wide variation between the actual content and the labeled claim of viable spores, as in other probiotic strains.Citation7,Citation9 Reduction in cell viability within the dosage form results from the freeze-drying operation of probiotics during the initial manufacturing, the processing conditions during formulation, and noncompliance to storage requirements during transportation and storage.Citation7–Citation12 After their consumption, the hydrolytic enzymes, the acidic conditions of stomach, and the bile salts in the gastrointestinal (GI) tract also adversely affect the viability of probiotics.Citation7,Citation12–Citation15 Various approaches such as the addition of different growth promoters, manipulation of fermentation and storage conditions of the food carriers, careful selection of the culture organisms according to their interrelationships and acid-bile resistance, and employing microencapsulation technology have been investigated to improve the amount of viable cells arriving in the intestine.Citation7,Citation13–Citation15 However, all of these approaches have varying degrees of success, as maintenance of high numbers of viable cells within the product throughout its shelf-life and during GI transit is a challenge. Amongst all the above approaches, microencapsulation technologies have attracted considerable attention but their short residence time at the site of action/absorption limits their performance. Coupling mucoadhesion characteristics to microspheres through developing mucoadhesive microspheres in turn will enhance their residence time and specifically can target drug(s) to the site of action/absorption and thereby enhance performance.Citation16,Citation17 Hypromellose has been used as a polymer in the preparation of mucoadhesive microspheres due to its favorable mucoadhesive properties, and is safe for human oral consumption.Citation16–Citation18 Hypromellose and hypromellose phthalate are compatible with B. coagulansCitation19 and possess aqueous solubility.Citation20
In the context of the above principles, there is a need to develop a dosage form that will deliver B. coagulans into the gut for a prolonged period of time with adequate stability during storage and GI transit. Thus, the present investigation attempted to prepare B. coagulans loaded mucoadhesive microspheres, using several grades of hypromellose and subsequently enteric-coated with hypromellose phthalate, in order to deliver viable B. coagulans into the gut for an extended period of time protecting against the harsh conditions of the GI tract and having an adequate shelf-life.
Materials and methods
Materials
Freeze dried B. coagulans (powder) and hypromellose phthalate (HP-50) were gift samples from Glenmark Pharmaceuticals Limited (Sinnar, Nasik, India). Grades of hypromellose (hydroxypropylmethylcellulose or HPMC) namely Methocel E5 Premium LV, Methocel E15 Premium LV, and Methocel K15M Premium were received from Indoco Remedies Limited (Rabale, Mumbai, India). Glucose Yeast Extract (GYE), agar media, and other analytical grade laboratory chemicals were purchased from HiMedia Laboratories Pvt Limited (Mumbai, India).
Methods
In-house compliance test of B. coagulans
Identification tests (description, microscopic examination, qualitative test for lactic acid production), viable B. coagulans spore count, estimation of the lactic acid-producing capacity, determination of loss on drying, and ensuring the absence of contaminants were done as per the method of analysis provided by the manufacturer.
Preparation of mucoadhesive microspheres
Core B. coagulans loaded mucoadhesive microspheres were prepared with hypromellose, employing coacervation and phase separation technique.Citation16,Citation17 Briefly, hypromellose (2.5 g) was dissolved in 100 mL of cold de-ionized (DI) water (4 ± 2°C). One hundred mg of tween-80 was dissolved in the above solution with stirring. Resultant solution was filtered aseptically using 0.45 μm PVDF filter membrane (Millipore, Bedford, MA). A calculated quantity of B. coagulans was dispersed in the above solution, under stirring. The temperature of the resultant dispersion was raised gradually up to 30 ± 2°C with stirring at 500 ± 25 revolutions per minute (rpm) for 30 minutes. Acetone (25 mL) was added drop-wise with stirring at 300 ± 25 rpm for 10 minutes. Microspheres thus obtained were filtered aseptically with 10 μm nylon filter (NY10; Millipore), washed twice with sterile water for injection (30 ± 2°C) and then kept in a desiccator for 24 hours. The entire process was carried out aseptically on a bench of horizontal laminar flow clean air work station (1500048-24-24; Klenzaids Bioclean Devices (P) Ltd, Mumbai, India). All formulation batches were prepared in triplicate following the above method to verify reproducibility and their composition is summarized in .
Table 1 Formulation formula and percent yield value and entrapment efficiency value of all formulation batches
Coating of microspheres
One hundred mL of hypromellose phthalate solution (10% w/w) was prepared by dissolving HP-50 in phosphate buffer pH-6.8Citation21 with stirring. Two g of polyethylene glycol 200 (PEG-200) and 100 mg of tween-80 was added to it with stirring. The above solution was filtered aseptically using a 0.45 μm PVDF filter membrane. Core microspheres were dispersed in the above solution with stirring at 300 ± 25 rpm followed by drop-wise addition of 20 mL of propan-2-ol. Stirring was continued for 30 minutes. Coated microspheres, thus obtained, were filtered and washed thrice with sterile water for injection (30 ± 2°C), kept in a desiccator for 24 hours, transferred aseptically to a sterile glass vial, sealed hermetically, and stored in a refrigerator for further manipulation. The entire process was carried out aseptically on a bench of horizontal laminar flow clean air work station, in triplicate to observe reproducibility of the process.
Percent yield study
Percent yield (w/w) of microspheres was calculated employing Equationequation-1(1) .
Calibration curve of B. coagulans
Several standard concentrations of dispersions containing B. coagulans were prepared with the sterile simulated gastric fluid TS and the sterile simulated intestinal fluid TS separately. The respective absorbance optical density (OD) was measured at 600 nm with respect to their respective blank, employing UV/Vis spectroscopic method (UV-1700; Shimadzu, Kyoto, Japan), so as to get a calibration curve that relates the OD values to cell concentration.Citation22 When a standard B. coagulans dispersion was analyzed repeatedly (n = 3), the mean error (accuracy) was found to be 0.98% and relative standard deviation (precision) was found to be 1.5%, indicating validity of the method for linearity, accuracy, and precision. The OD test is the best way to enumerate roughly the total number of cells (viable plus nonviable) present in the sample and helps to determine the dilution factor for performing viable spore count but fails to reveal cfu, the unit for expressing the number of viable cells. The simulated gastric fluid TSCitation21 and the simulated intestinal fluid TSCitation21 contains inorganic salts but no carbon source, thus B. coagulans cells will not proliferate in this media but will remain in a state of stasis until they are plated on media containing a carbon source.
Viable B. coagulans spore count
The number of viable spores in the sample was determined by employing the following procedure.Citation19
Dilution and heat-treatment
One gram of sample alternating with 1 mL of sample solution containing B. coagulans was transferred aseptically into a pre-sterilized 10 mL volumetric flask containing 5 mL of sterile saline TS and mixed thoroughly by sonication for 2 minutes. The volume was made up to 10 mL with sterile saline TS. One mL of the above suspension was diluted to 10 mL with sterile saline TS in an autoclaved test tube (25 mm by 150 mm size). This serial dilution method was continued until a suitable dilution was achieved (approximately 100 cell/mL). The final dilution tube was allowed to stand in a water bath at 70°C for 30 minutes and then cooled immediately to about 45°C. Saline TSCitation21 contains inorganic salts but no carbon source; thus B. coagulans will not proliferate in this media but will remain in a state of stasis until they are plated on media containing carbon source.
Plating
GYE agar medium was liquefied and cooled to 45°C in a water bath. One mL of sample from the heat-treated final dilution tube was transferred into each sterile petri dish (six per sample) followed by pouring 15 mL of molten medium and mixing and then incubating in an inverted position at 40°C for 48 hours after solidification.
Counting
Six plates were counted and the average count per plate was calculated. The number of cfu per unit (mL or gram) of sample was calculated by employing Equationequation-2(2) .
Entrapment efficiency study
Two hundred mg of accurately weighed microspheres were transferred aseptically into a sterile glass vial containing 10 mL of sterile simulated intestinal fluid TS, sealed hermetically and kept at 4 ± 2°C for 24 hours. The dispersion was subjected to a viable B. coagulans spore count and the entrapment efficiency value was calculated using Equationequation-3(3) and is reported in percent.
In vitro swelling study
An in vitro swelling test of microspheres was conducted in simulated intestinal fluid TS. The size of dried microspheres and those after incubation in simulated intestinal fluid for 5 hours were measured using a calibrated optical microscope (CX RIII; Labomed, Ambala, India). Percent swelling value was determined from the diameter of microspheres at time t (DT) and initial time t = 0 (D0) using Equationequation-4(4) .Citation23
Morphological examination of microspheres
Coated microspheres were mounted on the aluminum stubs using double-sided adhesive tape. The stubs were then vacuum-coated with a thin layer of gold and examined with a scanning electron microscope (JSM 5610 LV; Jeol, Tokyo, Japan).Citation16
Particle size, size distribution, and zeta potential study
The core and the coated microspheres were dispersed in DI water (pH 6.8) and sonicated for 2 minutes to get a homogenous dispersion (0.5% w/v). The resultant dispersions contained in small volume disposable zeta cells were subjected to particle size study using photon correlation spectroscopy with in-built Zetasizer (Nano ZS; Malvern Instruments, Worcestershire, UK) at 633 nm and 25 ± 0.1°C. Measured electrophoretic mobility (mm/s) is converted to zeta potential by in-built software based on the Helmholtz–Smoluchowski equation.Citation24
Flow property study
The flow property of coated microspheres was determined from the results of study parameters namely: Angle of repose (α), Equationequation-5(5) ; Carr’s index (CI), Equationequation-6
(6) ; and Hausner ratio (HR), Equationequation-7
(7) .Citation21 Angle of repose was determined by the fixed funnel method and is calculated from the height (H) and the radius (R) of the powder hip. Microspheres were placed in a graduated cylinder and the initial volume before tapping (V0) was noted then they were tapped with a tap density apparatus (ETD-1020; Electrolab, Mumbai, India) to a constant volume so as to get tapped volume (VT).
Mucoadhesion test
The mucoadhesion properties of coated microspheres were studied to examine the adhesiveness of the microspheres to the mucosa at the site of absorption/application, following institutional animal ethical committee guidelines and were performed by the following tests:
Ex vivo mucoadhesive strength determination
The coated microsphere suspension was prepared in simulated intestinal fluid TS, and the number of microsphere per mL (No) was determined by optical microscopy. One mL of the above suspension was fed to overnight-fasted Albino rats of either sex (in a group of four) using an oral feeding needle. The rats were sacrificed at an interval of 0, 4, 8, and 12 hours to isolate their stomach and intestinal region. The stomach and intestines were then cut open longitudinally to count the number of microspheres adhering to these regions (NS). Percent adhesive strength test result of all formulation batches was reported in percent adhesive strength and was calculated using Equationequation-8(8) .Citation17
In vitro wash-off test
Freshly excised pieces of intestinal mucosa (2 cm × 2 cm) from sheep were mounted onto a glass slide (8 cm × 3 cm) with cyanoacrylate glue. Coated microspheres (100 numbers) were accurately counted and were spread onto wet rinsed intestinal mucosa tissue followed by hanging it onto one of the groves of a USP tablet disintegration test apparatus (DT 1000; Labindia Instruments Pvt Ltd, Mumbai, India), with continuous oxygen supply. The apparatus was operated giving tissue specimen regular up-and-down movements within the beaker of disintegration test apparatus, containing simulated intestinal fluid TS at 37 ± 1°C.Citation25 The number of microspheres still adhering onto the tissue was counted at hourly intervals up to 12 hours.
In vitro release study
The in vitro release profile of B. coagulans from coated microspheres was studied using a USP basket apparatus (TDT-06T; Electrolab, Mumbai, India) at 37 ± 0.5°C and 100 rpm containing 900 mL of sterile dissolution medium, namely, the simulated gastric fluid TS and the simulated intestinal fluid TS. About 500 mg of accurately weighed coated microspheres was placed in a basket (wrapped with 100 mesh nylon cloth) of the dissolution apparatus. Five mL of dissolution medium was withdrawn at a predetermined time interval up to 18 hours followed by immediate replacement with an equal volume of fresh dissolution medium. After suitable dilution samples were subjected for viable spore count, the result was presented as percent viable B. coagulans cells released.
In vitro release kinetic studies, statistical evaluation, and data fitting
The kinetic model describes drug dissolution from the solid dosage form, where the dissolved amount of the drug as a function of test time was studied. Under appropriate test conditions, a dissolution profile can characterize the product more precisely than a single point dissolution test. A mean value of three determinations at each time point was used to fit an in vitro drug dissolution profile of all formulation batches to different kinetic models so as to find the best fitting kinetic model and to determine their release exponents, while the mean value of twelve determinations was used to estimate the factors of the model-independent approach.Citation26,Citation27 In vitro release kinetic studies, statistical evaluation, data fitting, nonlinear least square curve fitting, simulation, and plotting were performed using Excel software (v 2007; Microsoft Software Inc, Redmond, WA) so as to determine the parameters of each equation.
ANOVA-based procedures
Statistical analysis of in vitro release data and other data were performed using one way ANOVA at a significance level of 5% (P < 0.05) using Excel.
Model-independent methods (pair-wise procedures)
A comparison of the dissolution profile between products helps to assure similarity in product performance and shows bioequivalence. The model-independent mathematical approach is employed to compare the dissolution profile using three factors: f1, Equationequation-9(9) ; f2, Equationequation-10
(10) ; and Rescigno index (ξi), Equationequation-11
(11) . According to the nature of measurement, f1 was described as the difference factor and f2 as the similarity factor and are determined by employing cumulative data. The factor f1 is proportional to the average difference between the two profiles and indicates the percent error between two curves over all time points, whereas factor f2 is inversely proportional to the average squared difference between two profiles and is a logarithmic transformation of the sum-squared error of difference between the test Tt and reference products Rt. Rescigno index (ξ1 and ξ2), a dimensional index, refer to area differences for noncumulative data and refer to the difference between the dissolved amount of the test and reference product in a given time interval.
Note that Rt is the cumulative percentage dissolved from the reference product; Tt is from the test product at each of the selected time points, n, of the test and the product; dT (t) is the test product dissolved amount; dR (t) is the reference product dissolved amount at each time point; and i is any positive integer number.
Model-dependent methods
Model-dependent mathematical approaches including zero order, first order, Hixson–Crowell and Weibull model, as listed in ,
Table 2 Mathematical models used to describe dissolution curves
In vivo probiotic activity evaluation
An in vivo probiotic activity evaluation of coated microspheres was performed using the mouse model Enterococci stool colonization method described by Donskey et al following institutional animal ethical committee guidelines. One mL of coated microspheres dispersion (102 cfu/mL), in simulated intestinal fluid TS, was orally fed to mice (in a group of six) using an oral feeding needle. The stools were collected periodically at six hour intervals up to 48 hours and subjected for enterococci colonization density study.Citation9
Accelerated stability study of microspheres
Following ICH guidelines, formulation batches of microspheres were stored at several conditions of temperature and humidity (30 ± 2°C/65 ± 5% RH and 40 ± 2°C/75 ± 5% RH) in a stability analysis chamber (Darwin Chambers Company, St Louis, MO) and in a refrigerator (2–8°C) for a period of 12 weeks.Citation28 The samples were subjected for viable B. coagulans cell content, color, and texture analysis at 2-week intervals. These results were compared with those of the initial results (results of analysis of samples prior to stability charging) and control samples kept at 2–8°C. The absence of any statistically significant decrease in viable B. coagulans cell content indicates product stability with the excipient and at the storage condition.
Results and discussion
The coacervation and phase separation technique described here appears to be a suitable method for the preparation of enteric-coated hypromellose microspheres loaded with B. coagulans. It is a two-step process, simple and less time consuming, and eliminates the exposure of B. coagulans to high temperatures, organic solvents, and mechanical stress, thereby maintaining their viability during processing. Temperatures above 20°C and nonaqueous solvents adversely affect and decrease the viability of B. coagulans, thus the developmental processes of enteric-coated microsphere was carried out below 20°C in aqueous medium. Hypromellose is soluble in cold water and its solubility in water decreases with increase in temperature and is insoluble in organic solvents such as chloroform, dichloromethane, ether, and acetone.Citation20 Hypromellose phthalate is soluble in aqueous alkali and is insoluble in water and propan-2-ol.Citation20 Hypromellose as a mucoadhesive polymer and hypromellose phthalate as a coating polymer were selected due to their abovementioned properties. Tween-80 was incorporated in the formulation as a dispersing agent for homogenously dispersing B. coagulans cells and PEG-200 was incorporated in the coating solution formulation as a plasticizer to impart plasticity to the coat and to prevent it from splitting and cracking.
The percent yield value of the formulation batches lie within the range 54.6%–68.1% w/w while entrapment efficiency value lies within the range 67.68%–85.36% w/w, and both were found to vary with differences in the grade of hypromellose and the B. coagulans-to-hypromellose ratio (). The highest percent yield value was observed with the formulation containing Methocel E5 Premium LV. The effect of the grade of hypromellose on percent yield value is the following order: Methocel E5 Premium LV > Methocel E15 Premium LV > Methocel K15M Premium. A similar trend was also found in the case of the entrapment efficiency value and the highest value was also observed with formulations containing Methocel E5 Premium LV. It was also observed that the percent yield and entrapment efficiency values increase with a decrease in B. coagulans-to-hypromellose ratio ().
The percent swelling value varies with differences in the grade of HPMC, and the maximum percent swelling value was observed with Methocel E5 Premium LV. The effect of the grade of hypromellose on the percent swelling value follows the order of Methocel E5 Premium LV > Methocel E15 Premium LV > Methocel K15M Premium while the B. coagulans-to-HPMC ratio does not have a statistically significant effect on the percent swelling value. The percent swelling value of all formulation batches lies between 0.85 and 1.36 ().
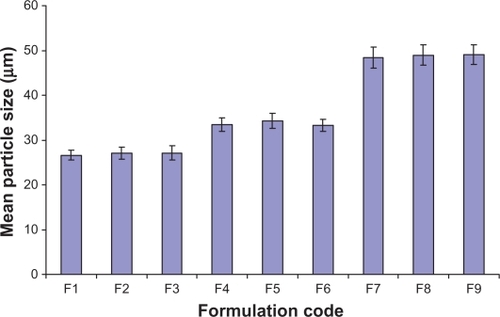
Table 3 Percent swelling, mean particle size, zeta potential, and percent adhesive strength values of microspheres of all formulation batches
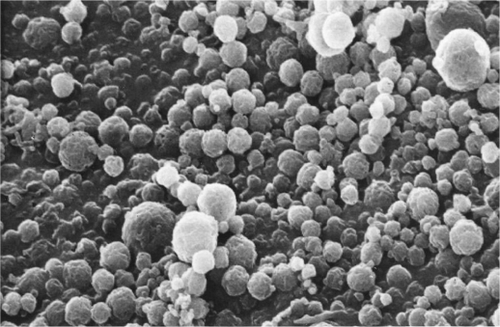
Prepared B. coagulans loaded mucoadhesive microspheres were spherical in shape with a smooth surface and had no holes/ruptures on the surface. A scanning electron microscopy photograph of formulation-F1 which enumerates microsphere particle size and morphology is shown in .
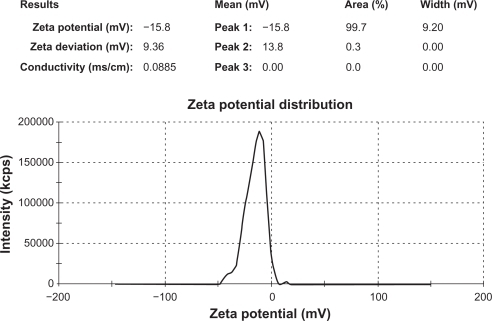
The mean particle size value of all formulation batches lies between 26.61 μm and 49.01 μm (). Variation in the mean particle size was observed with differences in the grade of hypromellose. The highest mean particle size value was observed with formulations containing Methocel K15M Premium. The effect of the grade of hypromellose on the mean particle size value follows the order of Methocel K15M Premium > Methocel E15 Premium LV > Methocel E5 Premium LV ( and ). Variation in B. coagulans-to-hypromellose ratio does not influence the mean particle size value of formulation batches. The zeta potential value () of core microspheres was negative as expected due to the methoxy group of hypromellose. Core microspheres prepared with the Methocel E15 Premium LV and the Methocel E5 Premium LV possessed nearly the same zeta potential value as both have the same ratio of methoxy and hydroxypropoxy groups, and are both higher than that prepared with Methocel K15M Premium. This may be due to an increase in the methoxy content of hypromellose polymer. A nearly constant negative zeta potential value (−7.66 ± 0.87) was observed with coated microspheres of all formulation batches, which is lower than that of uncoated microspheres, confirms the presence of hypromellose phthalate on the surface of microspheres. The zeta potential report of uncoated microspheres from formulation-F1 is presented in . The flow properties of formulation batches lie between the passable and very poor range.
The percent adhesive strength value of all formulation batches, as a measure of ex vivo mucoadhesive strength test, lies between 72.3% and 83.1%, and an increase in B. coagulans-to-hypromellose ratio does not affect it (). The highest percent adhesive strength value was observed with Methocel E5 Premium LV and the effect of the grade of hypromellose on percent adhesive strength value follows the order of Methocel E5 Premium LV > Methocel E15 Premium LV > Methocel K15M Premium. The in vitro wash-off test results (),
Table 4 Results of in vitro wash-off test of all formulation batches
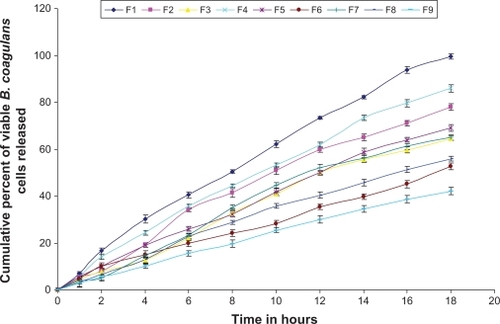
In simulated gastric fluid TS, the amount of B. coagulans released from the coated microsphere system is negligible, while in simulated intestinal fluid TS, B. coagulans release is almost regulated and extended within the investigational period of 18 hours (). This indicates that enteric-coating of microspheres has been competently protecting the viability of B. coagulans in gastric pH, preventing the release of B. coagulans in gastric pH, and releasing B. coagulans in the intestinal pH.
Figure 4 Comparative dissolution profile (model dependent, Zero-order kinetic model) of all formulation batches.
The model independent (pair wise approach) release exponents (f1, f2, ξ1 and ξ2) values are listed in .
Table 5 Mean value of dissimilarity factor (f1), similarity factor (f2), and two indices of rescigno (ξ1 and ξ2)
The values for the kinetic constant and release exponent of model-dependent approaches (zero-order, Weibull) are listed in .
Table 6 Linearization of B. coagulans dissolution profile using model-dependent approach, ie, the Zero-order and the Weibull
A plot showing the viable B. coagulans release profile following the zero-order kinetic model of all formulation batches is shown in , and reveals that an increase in the B. coagulans-to-polymer ratio significantly decreases the rate of viable B. coagulans release from microspheres while variation in the grade of hypromellose influences the rate of viable B. coagulans release from microspheres following the order of Methocel E5 Premium LV > Methocel E15 Premium LV > Methocel K15M Premium.
In vivo probiotic activity evaluation showed that oral administration of coated microspheres of B. coagulans from all formulation batches resulted in statistically significant reductions in the density of enterococci colonization in the stool of Albino mice from 24 hours to 36 hours. Satisfactory in vivo probiotic activity in the mouse model showed that the prepared B. coagulans loaded microspheres have potential for follow-up studies to verify its potentiality for human use.
The stability results reveal that prepared B. coagulans loaded microspheres exhibit adequate stability at the storage condition of 30 ± 2°C/65 ± 5% RH, as no statistically significant decreases in viable B. coagulans cell content were observed, and also signifies that the B. coagulans cells are compatible with the excipients used in the development of microspheres. At the storage condition of 40 ± 2°C/75 ± 5% RH, statistically significant decrease in viable B. coagulans cell content was observed, indicating product instability at this storage condition.
Formulation F1 (containing B. coagulans-to-Methocel E5 Premium LV with ratio of 1:1) was found to be superior to the other prototype formulations with respect to product performance, as it exhibited the highest entrapment efficiency, highest mucoadhesion efficiency, the ability to protect the viability of B. coagulans cells during storage and GI transit, and releases viable B. coagulans cells in the gut for an extended period of time, as shown via zero-order kinetics.
Conclusion
Experimental results suggest that enteric-coated hypromellose microspheres loaded with B. coagulans could be prepared by the conventional coacervation and phase separation method, and have the potential to deliver viable B. coagulans cells into the gut for an extended period of time, while maintaining the viability of B. coagulans during storage and GI transit, and could be viewed as an alternative to conventional dosage forms. However, extensive in vivo studies are required to establish the use of mucoadhesive microspheres as an alternative to conventional dosage forms of B. coagulans.
Acknowledgements
The author wishes to thank Glenmark Pharmaceuticals Limited, Sinnar, Nasik, India, for the sample of freeze dried B. coagulans and hypromellose phthalate (HP-50). Words are insufficient to express my gratitude to Indoco Remedies Limited, Rabale, Mumbai, India, for samples of used grades of methocel. The author will remain indebted to Jadavpur University, Kolkata, India, for support to complete this work.
Disclosure
No conflicts of interest were declared in relation to this paper.
References
- MitsuokaTRole of intestinal flora in health with special reference to dietary control of intestinal floraHgaBHLeeYKMicrobiology applications in food biotechnologyLondon, UKElsevier Science Publishers Ltd1992135148
- OrrhageKNordCEBifidobacteria and lactobacilli in human healthDrugs Exp Clin Res20002639511110941602
- NakajimaHSuzukiYKaizuHHirotaTCholesterol lowering activity of ropy fermented milkJ Food Sci199257613271329
- SinghJRivensonATomitaMShimamuraSIshibashiNReddyBSBifidobacterium longum, a lactic acid-producing intestinal bacterium inhibits colon cancer and modulates the intermediate biomarkers of colon carcinogenesisCarcinogenesis19971848338419111222
- KimHSGillilandSELactobacillus acidophilus as a dietary adjunct for milk to aid lactose digestion in humansJ Dairy Sci19836659599666409948
- KirjavainenPVApostolouESalminenSJIsolauriENew aspects of probiotics-a novel approach in the management of food allergyAllergy199954990991510505453
- SandersMEMorelliLTompkinsTASporeformers as human probiotics: Bacillus, Sporolactobacillus, and BrevibacillusCompr Rev Food Sci Food Saf200323101110
- KrasaekooptWBhandariBDeethHEvaluation of encapsulation techniques of probiotics for yoghurtInt Dairy J2003131313
- DonskeyCJHoyenCKDasSMFarmerSDeryMBonomoRAEffect of oral Bacillus coagulans administration on the density of vancomycin-resistant enterococci in the stool of colonized miceLett Appl Microbiol2001331848811442822
- ShahNPProbiotic bacteria: selective enumeration and survival in dairy foodsJ Dairy Sci200083489490710791807
- GilliandSESpeckMLInstability of Lactobacillus acidophilus in yogurtJ Dairy Sci197760913941398
- LankaputhraWEVShahNPSurvival of Lactobacillus acidophilus and Bifidobacterium spp in the presence of acid and bile saltsCultured Dairy Products J199530327
- O’RiordanKAndrewsDBuckleKConwayPEvaluation of microencapsulation of a Bifidobacterium strain with starch as an approach to prolonging viability during storageJ Appl Microbiol20019161059106611851814
- SultanaKGodwardGReynoldsNArumugaswamyRPeirisPKailasapathyKEncapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurtInt J Food Microbiol2000621–2475511139021
- ChandramouliVKailasapathyKPeirisPJonesMAn improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditionsJ Microbiol Methods2004561273514706748
- ChowdaryKPRRaoYSMucoadhesive microspheres for controlled drug deliveryBiol Pharm Bul2004271117171724
- BelgamwarVShahVSuranaSJFormulation and evaluation of oral mucoadhesive multiparticulate system containing metoprolol tartarate: an in vitro-ex vivo characterizationCurr Drug Deliv20096111312119418963
- LiCLMartiniLGFordJLRobertsMThe use of hypromellose in oral drug deliveryJ Pharm Pharmacol200557553354615901342
- BoraPSPuriVBansalAKPhysicochemical properties and excipient compatibility studies of probiotic Bacillus coagulans SporesSci Pharm2009773625637
- RoweRCSheskeyPJOwenSCHandbook of pharmaceutical excipients5th ed.London, UKPharmaceutical PressWashington, DCAmerican Pharmacists Association2006346358
- United States Pharmacopoeial Convention (US)United States Pharmacopoeia-National Formulary (USP-NF) 2008Rockville, MDUS Pharmacopoeial Convention, Inc2007639641814820
- ShakooriARAnwarSKhurshedNRiaz-ul-HaqBiocidal activity of Bacillus species for Anopheles larvaeFolia Biol (Krakow)1999473–414314810754794
- El-GibalyIDevelopment and in vitro evaluation of novel floating chitosan microcapsules for oral use: comparison with non-floating chitosan microspheresInt J Pharm20022491–272112433430
- VyasTKBabbarAKSharmaRKSinghSMisraAIntranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targetingJ Pharm Sci200695357058016419051
- LehrCMBowstraJATukkerJJJunginerHEIntestinal transit of bioadhesive microspheres in an in-situ loop in the ratJ Control Release19901315162
- CostaPSousa LoboJMModeling and comparison of dissolution profilesEur J Pharm Sci200113212313311297896
- SoniTChoataiNAssesment of dissolution profile of marketed aceclofenac formulationsJ Young Pharma2010212126
- DandagiPMMastiholimathVSGadadAPIligerSRMucoadhesive microspheres of propranolol hydrochloride for nasal deliveryIndian J Pharm Sci2007693402407