?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The p53 tumor-suppressor gene encodes a nuclear phosphoprotein with cancer- inhibiting properties. The most probable cancerous mutations occur as point mutations in exons 5 up to 8 of p53, as a base pair substitution that encompasses CUA and GAT sequences. As DNA drug design represents a direct genetic treatment of cancer, in the research reported computational drug design was carried out to explore, at the Hartree–Fock level, effects of solvents on the thermochemical properties and nuclear magnetic resonance (NMR) shielding tensors of some atoms of CUA involved in the hydrogen-bonding network. The observed NMR shielding variations of the solutes caused by solvent change seemed significant and were attributed to solvent polarity, and solute–solvent and solvent–solute hydrogen-bonding interactions. The results provide a reliable insight into the nature of mutation processes. However, to improve our knowledge of the hydration pattern more rigorous computations of the hydrated complexes are needed.
Introduction
The p53 tumor-suppressor gene encodes a nuclear phosphoprotein with cancer- inhibiting properties. However, the development of human cancer often involves inactivation of this suppressor function through various mechanisms including gene deletions and point mutation. The most probable cancerous mutations occur as point mutations in exons 5 up to 8 of p53, as a base pair substitution that encompasses CUA and GAT sequences. Including uracil and adenine, the positions where the mutations occur are called the ‘hot spots’ of mutations.Citation1,Citation2 The hydrogen-bonded complexes generated by solute are the main reason for these changes. Hydrogen bonds play a key role in maintaining the structure and specificity of biological systems.Citation3–Citation6 Further studies have focused on the acidity and basicity of uracil. The proton affinities and the deprotonation enthalpies of nucleobases have been also studied, in particular their relationship with the interaction with one water molecule.Citation7–Citation10 The important point of the study reported here is that experimental investigation of nucleic acid base pairing is difficult. However, gas phase association energies have been reported for some systems and in nonpolar solvents.Citation12,Citation13 Due to the limited experimental NMR data, the extent to which a simple dielectric medium model affects the dominating solute–solvent interactions of CUA sequence in different solvents remains unknown.Citation14,Citation15 This lack of experimental NMR data motivated us to calculate NMR shielding tensors of nitrogen, oxygen, and phosphorous atoms involved in the hydrogen-bonding network of a CUA model and to investigate the solvent-induced effect on these parameters. Due to the importance of hydrogen-bonding interactions in biological systems, the main theoretical attention has been focused on NMR parameters of nitrogen, oxygen, and phosphorous nuclei involved in the hydrogen-bonding network of CUA.
In order to identify the most probable nucleobases for mutation among CUA, all energy values as well as relative energies (ΔE) of the studied systems were calculated in vacuum at the level of RHF/6–31G theory and a logical trend was obtained in different solvent media.
Computational details
In the present work, we optimized the CUA codon () with 3 basis sets Sto-3g, 3–21g, 6–31g in the gas phase with the Gaussian 03 packageCitation19 by the Hartree–Fock (HF) method. The calculations including the intermolecular interactions give semiquantitative information on the effects of hydrogen bonding on the principal values of chemical shift tensors. We studied the influence of acetone, dimethyl sulfoxide (DMSO), ethanol, methanol, and water on chemical shielding tensors. There are different methods of salvation. One family of models for systems in solution is referred to as the self-consistent reaction field (SCRF) method. The simplest SCRF model is the Onsager reaction field model. For the simulation of a polar environment this model was used as implemented in Gaussian 03. In general, the following quantities are often used to describe NMR shielding tensors, namely, the isotropic, anisotropic shielding, and the asymmetry parameters:
The isotropic value σiso of the shielding tensor which can be defined as:Citation16,Citation18
(1)
The anisotropy parameter (Δσ) defined as:
(2)
and
The asymmetry parameter (η) which is given by:Citation18
(3)
The polarized continuum model is the most frequently used method employed to study solvent effects. However, the capability of the method for describing the effect of the formation of hydrogen bonds between the solvent and the solute is always controversial.Citation19
Results
The treatment of large biological systems in aqueous solution using ab initio methods is extremely expensive. However, analysis of NMR parameters is essential for understanding the role they play in biological processes. The calculated NMR shielding tensors of nitrogen, oxygen, and phosphorous atoms of CUA are listed in .
Table 1 Nuclear magnetic resonance parameters of nitrogen, oxygen, and phosphorus atoms involved in hydrogen-bonding network of CUA codon in different solvent media at the level of RHF/6–31G theory
The theoretical values of σiso, Δσ, and η of oxygen, nitrogen, and phosphorous atoms of CUA in different solvents are shown in . On the basis of the obtained results, it can be understood that NMR shielding values of the CUA model often yielded maximum dielectric constant values of 78.39, 32.65, and 46.8. So, it can be concluded that hydrogen bonding is the most important reason for this behavior that causes deshielding. For nitrogen atoms in the CUA structure, the highest isotropic shielding values have been obtained in water and ethanol as protic solvents whereas the lowest values have been obtained in DMSO as a protic solvent. However, for both N25 and N6 atoms, the differences in these values are insignificant. More interestingly, in the case of N25 atoms involved in uracil, the differences between maximum and minimum values of asymmetry parameter (η) seem insignificant and had a trivial effect on this parameter. For P38 the maximum values of σiso were obtained in protic solvents such as water and ethanol while the minimum values were observed in DMSO. Conversely, for P18 involved in uracil the opposite trend was observed. For O17 atom of uracil, the obtained negative values of σiso may indicate that in protic solvents including water and methanol the charge density around nuclei tended to be deshielded.
According to the table of σiso versus dielectric constants of different solvents, it can be seen that in most of the ethanol nuclei considered (ɛ = 24.55) the expected trend of variation will change. Also, in the gas phase, it can be seen that the lowest value of σiso for O7 and P18 corresponds to uracil. In the case of CUA sequences, the most negative value was observed for σiso for O27. Moreover, the graph of δiso of all the nitrogen atoms versus dielectric constant revealed that the deshielded points were observed at ɛ = 46.8 and the more shielded regions were observed at ɛ = 78.39 and ɛ = 32.63.
Discussion
To the best of our knowledge, there have been numerous reports about the analysis of thermochemical parameters of isolated uracil and its hydrated model.23–25 However, there are no experimental data on the relative energies or enthalpies of these systems.26
The current study focuses on the variations in thermochemical parameters due to effects of temperature in different solvents. Let us focus first on the uracil part of the CUA model, as a hot spot in mutation. Certainly, from the thermochemical parameters in solvent media, at different temperatures, we can gain further information and about the stability of uracil structure as a mutation hot spot, and then obtain useful results about solvent and temperature effects on the point mutation of CUA. All the relative thermochemical parameters were calculated. According to the thermochemical parameters reported in ,
Table 2 The Hartree–Fock calculations of thermochemical parameters of CUA in different solvent media at 3 different temperatures
Solvent effects on the relative structural stabilities of hot spots
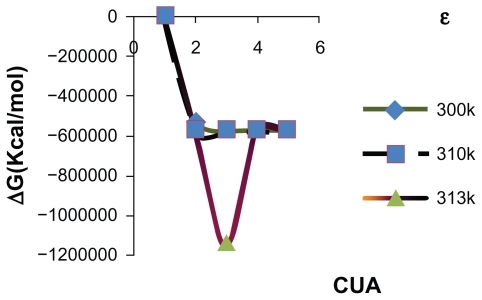
According to the graph of relative energy values of CUA versus dielectric constant, a dramatic decrease was observed, and the relative energy value of CUA reached its lowest point at ɛ = 24.55 (). Because polar solvents are molecules with a dipole moment that forms a hydrogen bond, the stability of the CUA system was logically found in ethanol. Meanwhile, along with the increasing trend of the dielectric constant the increase of energy values has been observed after the optimal point.
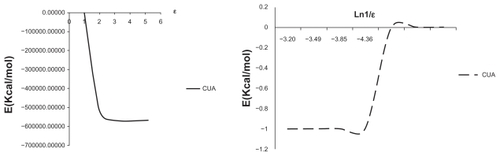
Figure 2 Relative energies (Erelatives) of CUA sequence versus ɛ and Ln (1/ɛ) in different solvent media.
Indeed, one of the key roles of a solvent is to avoid the initial rise in energy and a solvent can also stabilize biological systems.
On the other hand, shows a linear relationship of energy values of CUA versus ln (1/ɛ), which revealed the contribution of electrostatic interaction with the solvent-induced effect. However, based on the graph of energy values of CUA versus ln (1/ɛ) a linear relationship has been found which revealed the contribution of electrostatic interaction of the solvent-induced effect rather than the hydrophobic contribution of solvent effect.
Hydrophobic interaction is associated with the energy required to move apart solvent molecules to make space for the solute, which is greater in water and smaller in nonhydrogen-bonding systems. The thermochemical functions of CUA at three different temperatures and with five solvents are shown in . The energy graphs of CUA and also the graph of Gibbs hydration energies versus dielectric constants are shown in and , respectively.
Conclusion
The results described in this article cover extensive developments in reproducing and predicting a wide variety of theoretical physicochemical and structural parameters of a modeled CUA sequence involved in the p53 tumor-suppressor gene. These findings open the way to determine local geometries and also reveal more confidence in using ab initio methods to probe target-drug interactions as a useful application of quantum chemical technology to determine structure–stability correlations of specified sequences.
Based on the energy calculation of CUA it was observed that the relative energies (ΔE) of CUA in solution were smaller than in the gas phase, which is due to interactions in solution that were larger than in the gas phase. Moreover, the lowest ΔE value was found at the lowest dielectric constant and the maximum value was in water with a high dielectric constant and high polarity. Consequently, it can be concluded that the electrostatic and hydrophobic effects as well as dipole effects are important factors in solvation.
Disclosure
The authors disclose no conflicts of interest.
References
- DongMNioYYamasawaKTogaTYueLHaradaTp53 alteration is not an independent prognostic indicator, but affects the efficacy of adjuvant chemotherapy in human pancreatic cancerJ Surg Oncol20038211120
- SherrCJPrinciples of tumor suppressionCell200411623524614744434
- MessiasACSattlerMStructural basis of single-stranded RNA recognitionAcc Chem Res20043727928715147168
- BrameldKDasguptaSGoddardWADistance dependent hydrogen bond potentials for nucleic acid base pairs from ab initio quantum mechanical calculations (LMP2/cc-pVTZ)J Phys Chem B199710148514859
- ShihCTRocheSRomerRAPoint-mutation effects on charge-transport properties of the tumor-suppressor gene p53Phys Rev Lett200810001810518232825
- KurinovichMALeeJKThe acidity of uracil from the gas phase to solution: the coalescence of the N1 and N3 sites and implications for biological glycosylationJ Am Chem Soc200012262586262
- HocquetAGhomiMThe peculiar role of cytosine in nucleoside conformational behaviour: Hydrogen bond donor capacity of nucleic basesPhys Chem Chem Phys200025351
- PodolyanYGorbLLeszczynskiJProtonation of nucleic acid bases. A comprehensive post-Hartree−Fock study of the energetics and proton affinitiesJ Phys Chem A200010473467352
- MillerTMAronoldSTViggianoAAStevens MillerAEAcidity of a nucleobase: uracilJ Phys Chem A200410834393446
- ChandraAKNguyenMTHuyskensTZTheoretical study of the interaction between thymine and water. protonation and deprotonation enthalpies and comparison with uracilJ Phys Chem A199810260106016
- BartikKThe role of water in the structure and function of biological macromoleculesCurr Opin Struct Biol20001018219610753816
- YansonIKTeplitskyABSukhodubLFExperimental studies of molecular interactions between nitrogen bases of nucleic acidsBiopolymers19791811491170435611
- CornellWDCieplakPBaylyCIA second generation force field for the simulation of proteins, nucleic acids, and organic moleculesJ Am Chem Soc199511751795197
- KupkaTKolaskiMPasternaGRundKTowards more reliable prediction of formaldehyde Multinuclear NMR parameters and harmonic vibrations in the gas phase and solutionJ Mol Struct19994676378
- AuffingerPHashemYNucleic acid solvation: from outside to insightCurr Opin Struct Biol20071732533317574833
- LeppertJHeiseBRamachandranR15N chemical shift tensor magnitude and orientation in the molecular frame of uracil determined via MAS NMRJ Magn Reson200014530731410910699
- PeculMSadlejJ15N Chemical Shift Tensor Magnitude and Orientation in the Molecular Frame of Uracil Determined via MAS NMRChemical Physics1998234111119
- DiezNMSenentMLGarciaBAb initio study of solvent effects on the acetohydroxamic acid deprotonation processesChem Phys2006324350358
- GaigeotMPSprikMAb initio molecular dynamics computation of the infrared spectrum of aqueous uracilJ Phys Chem B200310710344
- NguyenMTZhangRBNamPCCeulemansASinglet-triplet energy gaps of gas phase RNA and DNA bases: a quantum chemical studyJ Phys Chem A200410865546561
- ZhangRBCeulemansANguyenMTA theoretical study of uracil and its tautomers in their lowest-lying triplet stateMol Phys2005103983994
- ZhangRHuyskensdTZCeulemeansANguyenMTInteraction of triplet uracil and thymine with waterChem Phys20053163544