Abstract
Pathogens usually invade hair follicles when skin infection occurs. The accumulated bacteria in follicles are difficult to eradicate. The present study aimed to assess the cutaneous and follicular delivery of chloramphenicol (Cm)-loaded liposomes and the antibacterial activity of these liposomes against methicillin-resistant Staphylococcus aureus (MRSA). Skin permeation was conducted by in vitro Franz diffusion cell. The anti-MRSA potential was checked using minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), a well diffusion test, and intracellular MRSA killing. The classic, dimyristoylphosphatidylcholine (DMPC), and deoxycholic acid (DA) liposomes had a vesicle size of 98, 132, and 239 nm, respectively. The incorporation of DMPC or DA into the liposomes increased the bilayer fluidity. The malleable vesicles containing DMPC and DA showed increased follicular Cm uptake over the control solution by 1.5- and 2-fold, respectively. The MIC and MBC of DA liposomes loaded with Cm were 62.5 and 62.5–125 μg/mL, comparable to free Cm. An inhibition zone about 2-fold higher was achieved by DA liposomes as compared to the free control at a Cm dose of 0.5 mg/mL. DA liposomes also augmented antibacterial activity on keratinocyte-infected MRSA. The deformable liposomes had good biocompatibility against keratinocytes and neutrophils (viability >80%). In vivo administration demonstrated that DA liposomes caused negligible toxicity on the skin, based on physiological examination and histology. These data suggest the potential application of malleable liposomes for follicular targeting and the treatment of MRSA-infected dermatologic conditions.
Introduction
Bacterial infections are becoming increasingly serious health problems. Among these, Staphylococcus aureus has emerged as a leading infectious bacterium strain, especially for superficial and invasive skin infection.Citation1 The substantial challenge in S. aureus infection management is antibiotic resistance, as demonstrated by the methicillin-resistant S. aureus (MRSA). Resistance to methicillin is found in >60% of S. aureus, the most prevalent bacteria in cutaneous infection.Citation2 Topically applied antibacterial chemotherapy is fundamental in the treatment of skin infection. The use of nanoparticles as the carriers of antibacterial agents represents a novel strategy to overcome drug-resistant strains in skin. The unique features of nanoparticles, such as controllable size, high surface-area-to-mass ratio, and high reactivity to pathologic microorganisms, contribute to their advantages for antimicrobial therapy.Citation3
Approximately 25% of cutaneous bacteria accumulate within the hair follicles.Citation4 S. aureus violation in the follicles causes folliculitis, furuncle, and hidradenitis suppurativa.Citation5,Citation6 An ideal antimicrobial treatment would be efficiently delivered to the infection area and targeted to the bacteria. The nanoparticles provide a means to specifically transfer the drugs into cutaneous appendages.Citation7 Liposomes, functioning as nanocarriers with a phospholipid bilayer membrane, have been demonstrated to be useful for follicular targeting.Citation8,Citation9 Liposomes possess some beneficial effects such as good biocompatibility, sustained drug release, and improved bioavailability. The delivery and therapeutic potential of the drugs can be controlled by modulating liposomal compositions and properties. The incorporation of ethanol or edge activators into liposomes can increase deform-ability, producing malleable vesicles. The soft and flexible structure of malleable liposomes can enhance cutaneous permeation through their ability to squeeze through inter-cellular spaces.Citation10 Treating S. aureus invasion by follicular delivery of malleable liposomes is conceivable, although studies related to this possibility are currently lacking. The aim of the present research was to prepare and assess malleable liposomes for improved follicular transport and anti-MRSA activity.
Chloramphenicol (Cm) was employed as the model drug. Previous studyCitation11 has indicated an antagonist effect on antimicrobial activity of combined Cm and silver nanoparticles, suggesting the importance of selecting feasible nanosystems for Cm. To enhance cutaneous permeation and follicular targeting of Cm, we added dimyristoylphosphatidylcholine (DMPC) or deoxycholic acid (DA) to liposomes, in order to produce flexible vesicles. The ability of DMPC and DA to elasticize liposomes has been previously shown.Citation12,Citation13 The physicochemical properties of the liposomes were evaluated with regard to Cm skin penetration and anti-MRSA effect. S. aureus is difficult to kill using conventional antibiotics because of its intracellular persistence in host cells.Citation14 The antimicrobial efficacy of the liposomes was also investigated using MRSA-infected keratinocytes.
Materials and methods
Preparation of liposomes
Soybean phosphatidylcholine (SPC, Phospholipon® [American Lecithin Company, Oxford, CT, USA] 80H, 2.5%) and cholesterol (0.7%) were dissolved in 5 mL of a chloroform/ethanol (2:1) solution. DMPC (1%) or DA (0.15%) was added to the solution if necessary. The solvent was evaporated in a rotary evaporator at 50°C, and solvent traces were removed under vacuum overnight. The phospholipid film was hydrated with double-distilled water containing Cm using a probe-type sonicator at 35 W for 30 minutes. The Cm concentration in the final product was 4 mg/mL. The liposomal system was centrifuged at 48,000× g and 4°C for 40 minutes to withdraw the unencapsulated drug in the supernatant. The liposomes were reconstituted by adding water to the pellets to achieve a Cm concentration of 1 mg/mL.
Vesicle diameter and zeta potential
The average diameter (z-average) and zeta potential of the prepared liposomes were measured using a laser scattering method (Nano ZS90; Malvern Instruments, Malvern, UK). The determination was performed at the liposomal concentration after a 100-fold dilution with double-distilled water. The measurement was repeated three times per sample for three batches. Nano ZS90 software version 7.11 (Malvern Instruments) was used.
Molecular environment
The molecular environment of all vesicles was detected by fluorescence spectrophotometry (F2500; Hitachi Ltd, Tokyo, Japan) based on the solvatochromism of Nile red. The liposomes were incorporated with Nile red (1 ppm). The emission spectra of the dye-loaded liposomes were scanned from 550 to 700 nm. The excitation wavelength was set to 546 nm. A representative image from three independent measurements was shown.
Differential scanning calorimetry (DSC)
The transition point of SPC in the liposomal suspension was measured using a Q2000 calorimeter (TA Instruments, New Castle, DE, USA). All liposomes were lyophilized before the analysis. The samples were weighed and sealed in the calorimeter. The heating curve was recorded from 20°C to 90°C at a scan rate of 10°C/min under nitrogen. The software used to calculate the enthalpy was Advantage® (TA Instruments). A representative image from three independent measurements was shown.
Animals
Eight-week-old female nude mice (ICR-Foxn1nu) purchased from National Laboratory Animal Center (Taipei, Taiwan) were used in the in vitro skin permeation and in vivo cutaneous irritation experiments. The protocol was approved by the Institutional Animal Care and Use Committee of Chang Gung University. All animals were housed and handled according to the institutional guidelines.
In vitro skin permeation
In vitro Cm absorption was conducted by Franz diffusion cell. The dorsal skin of a female nude mouse (8 weeks old) was mounted between the donor and receptor with the stratum corneum (SC) facing up toward the donor. The donor and receptor were filled with liposomal suspension (0.5 mL) and pH 7.4 buffer (5.5 mL), respectively. The Cm dose in the donor was 1 mg/mL. The other procedures and the drug amount extracted from the skin were the same as in our previous study.Citation15 Differential stripping and cyanoacrylate casting were employed to detect Cm uptake in the hair follicles as previously described.Citation16 The Cm content was quantified by high performance liquid chromatography (HPLC). The HPLC system was the Hitachi 2-series. A 25-cm-long C18 column (Merck Millipore, Billerica, MA, USA) was used as the stationary phase. The mobile phase used methanol and pH 2 double-distilled water adjusted by phosphoric acid (55:45). The ultraviolet wavelength detected for Cm was 280 nm.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
MIC and MBC were determined to evaluate the antibacterial effect of classic, DMPC, and DA liposomes against MRSA (ATCC 33591). A 2-fold broth-dilution method was used to detect MIC. The overnight culture of MRSA was diluted in tryptic soy broth (TSB) medium to attain an optical density at 600 nm (OD600) of 0.01 (about 2×106 colony forming units [CFU]/mL). The MRSA population was exposed to several dilutions of liposomes containing Cm ranging from 3.9 to 1,000 μg/mL and incubated at 37°C for 20 hours. An enzyme-linked immunosorbent assay reader was used to detect MIC at 595 nm. MIC was recorded as the highest dilution revealing no bacterial growth. For the MBC assay, the bacteria were diluted in phosphate-buffered saline (PBS) and positioned on plates. The liposomes with different dilutions were incubated with MRSA for 20 hours at 37°C. CFU were counted. The highest dilution, which resulted in 99.9% reduction of cell numbers, was recognized as MBC.
Disk diffusion assay
This assay was performed, based on a previous study,Citation17 by plating MRSA (OD600 =0.8) on the agar medium. MRSA grown during the mid-logarithmic phase were added to 30 mL of molten 7.5% TSB agar at 47°C. This suspension was gently mixed using agitation for 30 seconds; then, a 5-mL portion of this mixture was added onto the dish. The agar was allowed to cool until solidified. The 6-mm-diameter disks were put on the agar surface, and liposomes containing Cm at 0.5, 1, and 2 mg/mL with a volume of 10 μL were pipetted into the disk. The plates were incubated at 37°C for 12 hours. After this procedure, the diameter of the inhibition zone was measured. The inhibition zone size around each disk represented the mean of three axial measurements for each disk.
Detection of MRSA death rate by flow cytometry
The viability of MRSA after liposomal treatment was examined using a Live/Dead BacLight® kit (Molecular Probes, Eugene, OR, USA). MRSA with OD600 =0.1 was treated with free drug or Cm-loaded liposomes (Cm concentration =200 μg/mL) for 8 hours. The bacteria pellet was then obtained by centrifugation at 12,000 rpm for 3 minutes. The pellet was resuspended in culture medium to obtain an OD600 of 0.2. The aim of this process was to increase the bacterial population for facile detection by flow cytometry. After incubation at 37°C for 8 hours, the samples were stained with the kit and incubated for 15 minutes. The samples were analyzed by a flow cytometer (BD Biosciences, San Jose, CA, USA).
Intracellular MRSA killing
Cultured human keratinocytes (HaCaT) were used as the host cells to assess the activity of liposomes toward intracellular MRSA. The HaCaT cell line was provided free by Dr Chi-Feng Hung (Fu Jen Catholic University, New Taipei City, Taiwan). This experiment was approved by the Institutional Review Board at Chang Gung University. The method was modified according to a previous study.Citation18 Briefly, keratinocytes were infected with MRSA at a multiplicity of infection (MOI) =50 for 20 minutes. MOI is the ratio of the MRSA population to the infection target cells. After being washed with PBS, the cells were incubated in 1 mL fresh medium supplemented with DA liposomes containing Cm at 31.25, 62.5, or 125 μg/mL. After a 16-hour period, the species were rinsed with PBS. Triton X-100 (1%) was added to the cell medium to obtain the lysate.Citation19 The 1% Triton X-100 was examined using a disk diffusion test to treat MRSA for 1 hour. No inhibition zone was detected after Triton X-100 treatment, indicating that this lysis solution only damaged the mammalian cells but not the bacteria. The resultant solution was cultured on the agar dish for 20 hours and the CFU were counted. Fluorescence microscopy was employed to visualize HaCaT cells and viable MRSA. DAPI (4′-6-diamidino-2-phenylindole) and anti-S. aureus antibody (Abcam, Cambridge, UK) were used to stain the keratinocyte nucleus and MRSA, respectively. The secondary antibody used in this experiment was Alexa Fluor® 488 goat anti-mouse IgG. The staining of DAPI and antibody to the mammalian cells and MRSA was performed according to a previous protocol.Citation20
Cytotoxicity of liposomes against keratinocytes and neutrophils
The possible cytotoxicity of liposomes on keratinocytes was determined by using the MTT method as described previously.Citation21 The cytotoxicity of neutrophils was measured by detecting lactate dehydrogenase (LDH) as demonstrated in a previous study.Citation22 The SPC concentrations in the liposomal systems for testing cytotoxicity were 0.25, 0.50, and 1.25 μg/mL.
In vivo cutaneous irritation test
Control solution (25% ethanol in water) or DA liposomes with a volume of 0.6 mL were applied daily to the nude mouse back for 7 consecutive days with possible irritation being monitored. The application area was 1.5×1.5 cm2. After 7-day administration, the treated skin region was examined for transepidermal water loss (TEWL), skin surface pH, and erythema index (a*). TEWL and pH were measured by Cutometer® MPA580 (Courage and Khazaka, Cologne, Germany). Erythema was analyzed using a CD100 spectrocolorimeter (Yokogawa, Tokyo, Japan). Hematoxylin and eosin (H&E) were utilized to stain skin slices for histological observation under optical microscopy.
Statistical analysis
The data are presented as mean and standard deviation. The difference in the data of different experimental groups was evaluated using the Kruskal–Wallis test. The post hoc test used for checking individual differences was Dunn’s test. Significance was indicated as * for p<0.05, ** for p<0.01, and *** for p<0.001.
Results
Physicochemical properties of liposomes
Two types of soft liposomes containing DMPC and DA were prepared. Physicochemical data allowed comparison of rigid and soft liposomes as shown in . Classic liposomes were found to have a hydrodynamic size of 98 nm. Intercalation of DMPC and DA resulted in the increase of diameter to 132 and 239 nm, respectively. DA incorporation significantly increased the polydispersity index from 0.22 to 0.42. The zeta potential of the vesicles was highly negative and was not influenced by DMPC or DA loading. The negative charge corroborated the anionic nature of SPC. The encapsulation percentage for Cm was 15.0%±3.6%, 14.1%±2.0%, and 12.5%±2.3% for classic, DMPC, and DA liposomes, respectively. This loading capacity can be regarded as low since Cm is categorized as a hydrophilic drug. Free Cm from vesicles could be removed after ultracentrifugation; therefore, the completely encapsulated form was utilized throughout the experiment.
Table 1 The characterization of liposomes by vesicle size, PDI, zeta potential, chloramphenicol encapsulation efficiency, and antibacterial activity
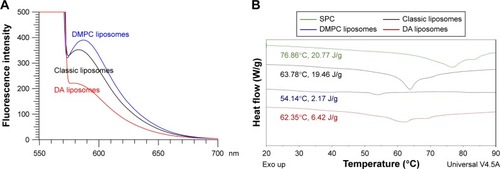
demonstrates the emission spectra of Nile red in liposomes. DMPC vesicles exhibited the highest fluorescence intensity, which implied that this nanosystem had the strongest lipophilicity. DA liposomes revealed weaker emission as compared to classic and DMPC liposomes, indicating a more hydrophilic nature of this deformable formulation. The crystallinity of the lyophilized liposomes was characterized using DSC (). For the bulk material of SPC, the melting peak was observed at 76.86°C. A DSC thermogram of classic liposomes showed an endotherm at 63.78°C, attributed to SPC melting in the vesicles. The shifted endotherm of SPC (54.14°C) and minimization of enthalpy (from 19.46 to 2.17 J/g) by DMPC intercalation were attributed to the reduced crystallinity. The peak of SPC in DA liposomes also showed a shift to the lower temperature and enthalpy (62.35°C and 6.42 J/g).
Figure 1 Comparison of physicochemical properties of various liposomes.
Notes: (A) Fluorescence emission profiles of Nile red in liposomes. (B) DSC profiles of the melting process of bulk SPC and liposomes after freeze drying.
Abbreviations: DSC, differential scanning calorimetry; SPC, soybean phosphatidylcholine; DA, deoxycholic acid; DMPC, dimyristoylphosphatidylcholine.
In vitro skin permeation
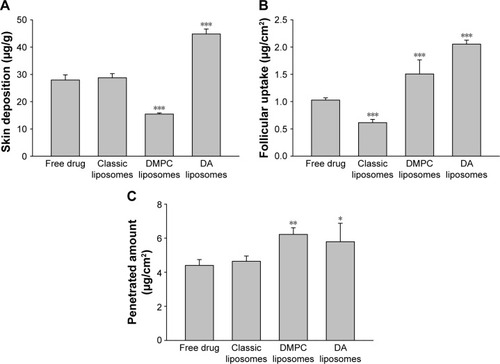
The prepared liposomes were first investigated to establish skin delivery of Cm (). Permeation experiments conducted with nude mouse skin demonstrated a Cm skin deposition of 28 μg/g for aqueous solution and 29 μg/g for classic liposomes, with no significant difference between the groups (). Application with DMPC liposomes reduced skin deposition by 2-fold. The highest Cm deposition was found in DA liposomes (45 μg/g). As depicted in , classic liposomes delivered less Cm to the hair follicles as compared to free Cm. Both malleable liposomes increased follicular targeting. There was a 2-fold increase in the Cm follicular level after DA liposome treatment compared to the aqueous control. The cumulative amount of Cm that penetrated across the skin was 4.4 and 4.6 μg/cm2 for the control group and classic liposomes, respectively (). The amount of penetrated Cm was higher in the case of flexible liposomes (6.2 μg/cm2 for DMPC liposomes and 5.8 μg/cm2 for DA liposomes).
Figure 2 In vitro skin permeation of Cm from control solution and various liposomes after 24-hour application.
Notes: (A) Skin deposition, (B) follicular uptake, and (C) penetrated amount in receptor. Each value represents the mean and SD (n=4). *p<0.05, **p<0.01, and ***p<0.001.
Abbreviations: DA, deoxycholic acid; DMPC, dimyristoylphosphatidylcholine; Cm, chloramphenicol; SD, standard deviation.
Anti-MRSA activity
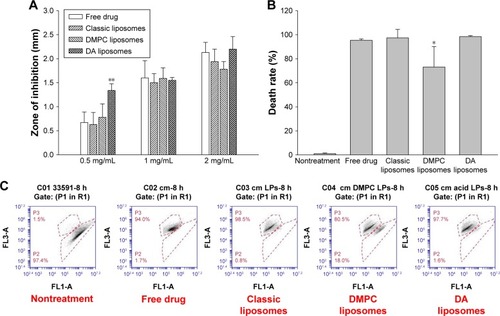
The liposomes were evaluated for antibacterial activity against MRSA. summarizes the MIC and MBC values for free control and liposomes. Vehicles without Cm showed no anti-MRSA effect. The MIC and MBC for free Cm were 62.5 and 62.5–125 μg/mL, respectively. Classic and DMPC liposomes exhibited a slightly higher MIC and MBC than the free control. Cm loading in DA liposomes maintained the same levels of MIC and MBC as free Cm. compares the antibacterial activity of free Cm and Cm containing liposomes judged by the inhibition zone. The inhibition diameter for free Cm at 0.5, 1, and 2 mg/mL was 0.67, 1.60, and 2.13 mm, respectively. The classic and DMPC vesicles retained the antibiotic activity of free Cm against MRSA. Higher anti-MRSA activity was noted in DA liposomes with 0.5 mg/mL Cm compared to the other vehicles. An inhibition diameter was obtained for DA liposomes about 2-fold wider than for the other liposomes. The liposomes without Cm were also examined using the disk diffusion test. We found no inhibition zone for the three nanovesicles tested. This indicated no action of the anti-MRSA effect by the liposomes in the absence of the drug. We further examined the bacterial death rate by using flow cytometry. As shown in , free Cm treatment induced 95% killing of MRSA. Classic and DA liposomes showed a comparable MRSA death rate compared to the control solution. The death rate of DMPC liposomes against MRSA was 73%, showing less potency than with the other formulations. illustrates the representative flow cytometry diagram of the live/dead strain.
Figure 3 Anti-MRSA activity of Cm in control solution and various liposomes.
Notes: (A) Zone of inhibition measured from disk diffusion assay, (B) death rate measured by flow cytometry, and (C) representative flow cytometry diagram of live/dead strain. Each value represents the mean and SD (n=3). *p<0.05 and **p<0.01.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; DA, deoxycholic acid; DMPC, dimyristoylphosphatidylcholine; Cm, chloramphenicol; SD, standard deviation.
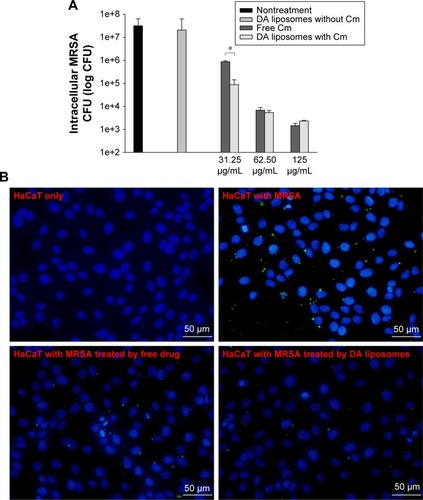
MRSA is an extracellular and intracellular pathogen. Since DA nanocarriers showed the strongest anti-MRSA activity among the three liposomal formulations tested, the capability of DA liposomes to serve as a force against intracellular MRSA in infected keratinocytes was evaluated. Both free Cm and Cm-entrapped liposomes did not exert a cytotoxic effect on HaCaT cells at the concentrations tested. As shown in , MRSA-infected HaCaT treated by DA liposomes containing Cm exhibited a lower microbial burden than did the free control at a lower Cm concentration (31.25 μg/mL). DA liposomes reduced the number of MRSA by 2–3 log units in comparison to nontreatment. Dose-dependent intracellular killing of MRSA was observed. There was no significant difference between the intracellular MRSA survival of free and liposomal Cm at higher concentrations. shows fluorescence microscopic images of MRSA incubated with keratinocytes. Green fluorescence from MRSA was visualized inside and outside HaCaT cells after incubation for 16 hours. The extracellular MRSA could be derived from the intracellular replication and the spread from bacteria. The treatment of infected HaCaT with free and liposomal Cm at 31.25 μg/mL produced a dramatic decrease of MRSA, verifying the data obtained in .
Figure 4 Intracellular MRSA killing in keratinocytes by Cm in control solution and DA liposomes.
Notes: (A) Intracellular MRSA burden and (B) keratinocytes (stained by DAPI) and MRSA (stained by anti-S. aureus antibody) viewed by fluorescence microscopy. Each value represents the mean and SD (n=3). *p<0.05.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; DA, deoxycholic acid; Cm, chloramphenicol; SD, standard deviation; DAPI, 4′-6-diamidino-2-phenylindole; CFU, colony forming units.
Cytotoxicity and cutaneous irritation examination
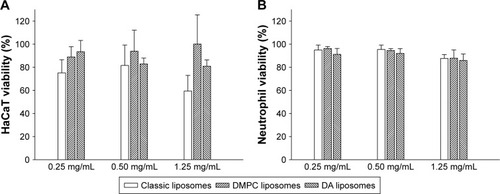
The impact of liposomes on cell viability was evaluated in two cell types, keratinocytes and neutrophils. The empty liposomes without Cm were examined in this experiment. The vesicle concentration is presented in units of SPC concentration as shown in . SPC concentrations of 0.25, 0.50, and 1.25 mg/mL used in this experiment corresponded with the Cm concentrations of 40, 80, and 200 μg/mL in liposomes, though there was no Cm in the nanovesicles in this experiment. DMPC liposomes did not have a toxic effect on HaCaT as demonstrated in the MTT assay (). DA liposomes without Cm showed mild cytotoxicity to HaCaT at the highest SPC concentration (1.25 mg/mL), which led to 19% less viability compared to the control. A higher magnification of cytotoxicity was observed for classic liposomes over the others, showing 59% viability at the highest concentration. Neutrophils are the predominant leukocytes in the body. The LDH assay indicates that all liposomes could maintain neutrophil viability >85% at concentrations of 0.25–1.25 μg/mL (). This suggests good biocompatibility of liposomes to neutrophils. The malleable liposomes showed negligible cytotoxicity at the highest SPC concentration of 1.25 mg/mL. The Cm dose in this SPC concentration in liposomes reached 200 μg/mL, which surpassed the MIC level (62.5 μg/mL) against MRSA. This result demonstrated that malleable liposomes could eradicate MRSA without affecting the viability of mammalian cells.
Figure 5 Cell viability by treatment of control solution and various liposomes without Cm.
Notes: (A) Keratinocytes and (B) neutrophils. Each value represents the mean and SD (n=4).
Abbreviations: DA, deoxycholic acid; DMPC, dimyristoylphosphatidylcholine; Cm, chloramphenicol; HaCaT, keratinocytes; SD, standard deviation.
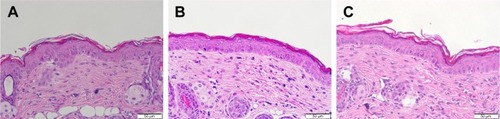
The change in skin physiology such as TEWL, skin surface pH, and erythema was measured by a 7-day consecutive administration of free drug and DA liposomes. TEWL is a reflection of skin barrier function. As shown in , ΔTEWL was comparable between the free control and DA liposomes. The pH values were slightly shifted to an alkaline condition. Erythema (a*) detection indicated no skin rash in the nude mouse treated by the aqueous control and malleable liposomes. Possible skin damage was checked by H&E staining as illustrated in . The histology indicated no observable disruption in the intact skin that received no treatment (). The biopsied skin specimens after treatment of aqueous solution and DA liposomes showed comparative morphology versus the non-treated skin (). The inflammation and immune cell infiltration could be categorized as negligible.
Table 2 Skin physiological parameters after treatment of free chloramphenicol and DA liposomes
Discussion
Bacteria are becoming resistant even to second-line therapy as shown in the resistance of MRSA to most β-lactam antibiotics. Increasing attention has been paid to nanomedicine as an approach to efficiently treat drug-resistant microbes. In this study, we developed malleable liposomes, as they were capable of specifically delivering Cm to hair follicles for treating cutaneous pathogens. The experimental results indicated that DA liposomes showed superior anti-MRSA activity compared to the control solution and other liposomes without being harmful to mammalian cells and skin. It was also observed that DA liposomes allowed Cm to reach a therapeutic intracellular level when MRSA had infected the keratinocytes.
Cholesterol embedded in liposomal bilayers increases the lateral packing density, hence lowering the volume of the lipophilic phaseCitation10 and resulting in the small size of classic liposomes of <100 nm. Incorporation of DMPC and DA impaired the packing density and produced larger vesicles. The deformity of DMPC and DA liposomes was confirmed by DSC. The liposomal systems provided a net negative charge, assuring physical stability due to electrostatic repulsion.Citation23
Cm is a broad-spectrum bacteriostatic antibiotic with poor skin permeation.Citation24 Cm skin absorption and follicular uptake were improved with the flexible liposomes, especially DA containing nanocarriers. A vesicle size of <300 nm is suitable to deliver the drugs into deeper skin strata.Citation25 Our liposomes fitted this criterion. The smaller vesicles may ensure close contact with the SC and can enhance drug permeation. This was not the case in our study since the size of the malleable liposomes was much greater than that of the classic liposomes. The vesicle–skin interaction can be strongly affected by liposomal stiffness. Both SPC and cholesterol offer rigidity of liposomal bilayers.Citation25,Citation26 DA loading into the liposomes led to crystal order imperfection and increased flexibility of the liposomal membrane. The malleable liposomes are thought to permeate facilely into the intercellular lipid bilayers, thereby enhancing drug absorption into the skin.Citation27 It is also proposed that the soft liposomes interact strongly with SC lipids,Citation25,Citation28 creating a cutaneous reservoir for drug delivery. Follicular accumulation is generally more efficient with nanoparticles of <300 nm.Citation29 Our liposomes demonstrated an ideal size for follicular delivery. The easy transport of malleable vesicles into the appendages can be anticipated due to facile squeezing through the follicular space.Citation7
Although DMPC liposomes showed a deformable characteristic, the skin permeation behavior of this nanocarrier was quite different from that of DA liposomes. DMPC liposomes revealed lower skin deposition but higher follicle accumulation and a greater amount of penetrated Cm in the receptor as compared with classic liposomes. The capability of DMPC-loaded liposomes for follicular targeting has been previously reported.Citation8 Classic liposomes are of little value for topical Cm delivery as they are difficult to accumulate in the follicles compared to free Cm in alcohol/water solution. Alcohol can extract sebum in sebaceous units, making the transfollicular route predominant for drug absorption.Citation9 Due to the low resistance of the follicular epithelium, the nanocarriers diffuse quickly into the deeper strata via the follicles,Citation30 resulting in the greater Cm amount in the receptor by topical application of flexible liposomes. Easy Cm penetration into the receptor by DMPC liposomes may contribute to the limited Cm deposition remaining in the skin, although this effect was not detected with respect to DA liposomes. The follicular delivery of DMPC vesicles was less than that of DA liposomes, though the DSC profiles indicated that the DMPC system was more fluid than the DA system. The significant reduction of the melting peak and enthalpy by DMPC liposomes was attributed not only to the crystalline deficiency but also to the reduction of vesicle size. The smaller size of DMPC compared to DA liposomes led to further enthalpy decrease. It has been reported that liposomes made from DMPC are easily elongated and fluctuated.Citation13 The DMPC vesicles may be quickly disrupted in the skin because of the instability, which causes loss of benefits from the malleable feature.
It was found that DMPC incorporation raised the liposomal lipophilicity. This may be due to the long alkyl chains of DMPC increasing lipophilicity. Contrary to this result, DA had decreased liposomal lipophilicity due to the high hydrophile–lipophile balance of DA.Citation16 The follicular ducts mainly contain lipophilic material such as sebum. Lipophilic nanoparticles may exhibit a potent affinity to sebum. Our results showed no correlation between vesicle lipophilicity and follicular transport, suggesting that sebum partitioning was not the major factor predominating follicular uptake of vesicles. Elastic liposomes can keep the vesicles intact after penetrating into the lipid bilayers and follicles.Citation10,Citation31 For antibacterial activity, the drug should be present in a sufficient concentration at the infection site for a period of time. Cutaneous bacteria were found within the entire epidermis and appendages.Citation4,Citation32 The epidermis and follicles can be an efficient reservoir for drug delivery. Topically applied malleable liposomes provided a possibility of sustained and targeted drug permeation in the sites of action, achieving efficient treatment and minimizing side effects.
The experimental results clearly indicated that the presence of DA in the liposomes enhanced the anti-MRSA action of Cm. The elastic vesicles have a high surface-to-volume ratio and a deformable characteristic, translating into increased interaction and fusion to bacteria and thus greater antimicrobial reaction.Citation33,Citation34 It has been reported that the antibacterial property of nanoparticles increases following size reduction.Citation35 This was not the case in this work since DA liposomes exhibited a larger diameter than the others. Surface active nanocarriers can interact with bacteria such as S. aureus toward adhering and penetrating bacteria.Citation3 A low concentration of DA is found to facilely intercalate with membrane lipids of bacteria.Citation36 This DA exposure to sublethal levels allows the bacteria to adapt and protect themselves against subsequent exposure to lethal levels of DA.Citation37 This adaptation does not cause notable damage to the bacterial membrane/wall. Our results proved that DA liposomes without Cm did not display a germicidal effect. When DA liposomes directly fused with the membrane, the entrapped drug was released into the bacteria, resulting in increased contact time and a high local antimicrobial concentration. A prolonged period of antimicrobial drug treatment is always necessary to kill drug-resistant bacteria. The sustained drug release from nanoparticles provided a sufficient quantity and duration required for inhibiting bacterial growth.Citation38
The inhibition zone treated by DA liposomes was larger than that treated by free Cm although MIC and MBC were comparable in the two groups. This may be due to the DA liposomes being able to interact with MRSA and retain an adequate drug concentration near the strain, whereas free Cm might diffuse rapidly in the agar medium. DA liposomes showed a superior anti-MRSA effect at a lower Cm concentration (0.5 mg/mL) but not at a higher concentration (1 and 2 mg/mL). The hydrophilic drugs had restricted penetration across the bacterial membrane. The hydrophilic Cm might have difficulty penetrating into the cells at a low level. As the Cm level increased, the free Cm could efficiently diffuse into the cells by the concentration gradient between the extrabacterial and intrabacterial environments. On the other hand, the capacity of DA liposomes to adhere to bacterial membrane might be able to reach saturation at a high concentration.
Intracellular infection remains difficult to treat because of the antibiotic transport obstruction into the cytoplasm and low activity inside the host cells.Citation39 Treatment of intracellular pathogens and the subsequent spread outside the cells is an essential issue. It has been reported that the bacteria within macrophages are protected from the lethal action of Cm.Citation40 Liposomes may ensure drug passage into the cells via phospholipid bilayer-membrane fusion.Citation14 For instance, liposomal vancomycin has a marked ability to kill intracellular MRSA.Citation41 Our results indicated that Cm delivered by DA liposomes exerted significant bactericidal activity on the MRSA present within the keratinocytes. The increased cellular uptake and subsequent sustained Cm release inside the host cells effectively ameliorated the antibacterial effect. The elastic property of the DA vesicles may further promote liposomal fusion and uptake to the cells.Citation42 The entry of the DA liposomes into the mammalian cells to kill pathogens remained unclear and could not be concluded from this study. Further work is needed to clarify the mechanisms. Antimicrobial agents topically applied to treat epidermal or follicular bacteria must be able to transport into the epidermis or follicles so that they are active against intracellular and extracellular pathogens. Improved therapy of Cm entrapped in DA liposomes is expected through penetration into the skin in an intact form, followed by the facile interaction with MRSA and host cells for manifesting antibacterial efficacy.
We have shown that DA liposomes had minimal toxicity toward keratinocytes and neutrophils. Due to the intention of developing topically applied liposomes, the cytotoxicity to skin cells was inspected. Neutrophils were also examined because of the neutrophil-rich infiltration in the MRSA-infected wounds.Citation43 Neutrophils can attack MRSA to achieve infection reduction. The neutrophil viability decrease may result in the insufficient defense against MRSA. Our results demonstrated that DA liposomes did not affect this self-protection capability. According to the results of an in vivo skin irritation test, a comparable TEWL and erythema were found for the aqueous control and DA liposomes. Histology also suggested a minimal change after topical administration. No sign of skin irritation was caused by DA liposomes, though this formulation displayed a high cutaneous Cm accumulation. The materials of the elastic liposomes are mainly composed of SPC and DA, which are generally recognized as having a safe status, enabling harmless application to the skin. It is thought that topical application of the malleable vesicles can be effective and safe for treatment of MRSA infection.
Conclusion
It was concluded that the cutaneous delivery and anti-MRSA activity of liposomal formulations depended on the compositions. The experimental data proved that malleable liposomes facilely entered the skin reservoir and hair follicles. DA liposomes enhanced the antimicrobial properties of Cm, probably due to the increased surface area, the interaction between DA and the bacterial membrane, and sustained drug release. DA liposomes showed both extracellular and intracellular anti-MRSA activity. The results suggest that DA liposomes may be useful for the treatment of infected follicular diseases such as folliculitis, furuncle, and hidradenitis suppurativa. For good clinical implementation, further preclinical studies are required to survey the anti-MRSA effect of malleable liposomes on in vivo skin infection.
Acknowledgments
The authors are grateful for financial support from Chang Gung Memorial Hospital (CMRPD1F0231-3 and CMRPD1D0432-3).
Disclosure
The authors report no conflicts of interest in this work.
References
- MartinezLRHanGChakoMAntimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infectionJ Invest Dermatol20091292463246919387479
- HeunisTDJDicksLMTNanofibers offer alternative ways to the treatment of skin infectionsJ Biomed Biotechnol2010201051068220798871
- TaylorEWebsterTJReducing infections through nanotechnology and nanoparticlesInt J Nanomedicine201161463147321796248
- Lange-AsschenfeldtBMarenbachDLangCDistribution of bacteria in the epidermal layers and hair follicles of the human skinSkin Pharmacol Physiol20112430531121757977
- LaureanoACSchwartzRACohenPJFacial bacterial infections: folliculitisClin Dermatol20143271171425441463
- PrensEDeckersIPathophysiology of hidradenitis suppurativa: an updateJ Am Acad Dermatol201573S8S1126470623
- FangCLAljuffaliIALiYCFangJYDelivery and targeting of nanoparticles into hair folliclesTher Deliv20145991100625375342
- JungSOtbergNThiedeGInnovative liposomes as a transfollicular drug delivery system: penetration into porcine hair folliclesJ Invest Dermatol20061261728173216645589
- TabbakhianMTavakoliNJaafariMRDaneshamouzSEnhancement of follicular delivery of finasteride by liposomes and niosomes 1. In vitro permeation and in vivo deposition studies using hamster flank and ear modelsInt J Pharm200632311016837150
- KasetvatinCRujivipatSTiyaboonchaiWCombination of elastic liposomes and low frequency ultrasound for skin permeation enhancement of hyaluronic acidColloids Surf B Biointerfaces201513545846426280820
- JainJAroraSRajwadeJMOmrayPKhandelwalSPaknikarKMSilver nanoparticles in therapeutics: development of an antimicrobial gel formulation for topical useMol Pharm200961388140119473014
- FangJYHwangTLHuangYLFangCLEnhancement of the transdermal delivery of catechins by liposomes incorporating anionic surfactants and ethanolInt J Pharm200631013113816413711
- KatoNIshijimaAInabaTNomuraFTakedaSTakiguchiKEffects of lipid composition and solution conditions on the mechanical properties of membrane vesiclesMembranes20155224725611306
- XieSTaoYPanYBiodegradable nanoparticles for intracellular delivery of antimicrobial agentsJ Control Release201418710111724878179
- LeeWRShenSCSunCKFractional thermolysis by bipolar radiofrequency facilitates cutaneous delivery of peptide and siRNA with minor loss of barrier functionPharm Res2015321704171325392230
- LeeWRShenSCAljuffaliIALiYCFangJYErbium-yttrium-aluminum-garnet laser irradiation ameliorates skin permeation and the follicular delivery of antialopecia drugsJ Pharm Sci20141033542355225187109
- HoopmanTCLiuWJoslinSNPybusCBrautigamCAHansenEJIdentification of gene products involved in the oxidative stress response of Moraxella catarrhalisInfect Immun20117974575521098105
- MuHTangJLiuQSunCWangTDuanJPotent antibacterial nanoparticles against biofilm and intracellular bacteriaSci Rep201661887726728712
- RichardsonARLibbySJFangFCA nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunityScience20083191672167618356528
- JohnsonMBCrissAKFluorescence microscopy methods for determining the viability of bacteria in association with mammalian cellsJ Vis Exp201379e50729
- PanTLWangPWAljuffaliIAHuangCTLeeCWFangJYThe impact of urban particulate pollution on skin barrier function and the subsequent drug absorptionJ Dermatol Sci201578516025680853
- HwangTLSungCTAljuffaliIAChangYTFangJYCationic surfactants in the form of nanoparticles and micelles elicit different human neutrophil responses: a toxicological studyColloids Surf B Biointerfaces201411433434124246197
- SandriGBonferoniMCD’AutiliaFWound dressings based on silver sulfadiazine solid lipid nanoparticles for tissue repairingEur J Pharm Biopharm201384849023207329
- KalitaSDeviBKandimallaRChloramphenicol encapsulated in poly-ε-caprolactone-pluronic composite: nanoparticles for treatment of MRSA-infected burn woundsInt J Nanomedicine2015102971298425931822
- VermaPPathakKNanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulationNanomedicine2012848949621839053
- CorveraEMouritsenOGSingerMAZuckermannMJThe permeability and the effect of acyl-chain length for phospholipid bilayers containing cholesterol: theory and experimentBiochim Biophys Acta199211072612701504071
- ElmoslemanyRMAbdallahOYEl-KhordaguiLKKhalafallahNMPropylene glycol liposomes as a topical delivery system for miconazole nitrate: comparison with conventional liposomesAAPS PharmSciTech20121372373122566173
- ManconiMMuraSSinicoCFaddaAMVilaAOMolinaFDevelopment and characterization of liposomes containing glycols as carriers for diclofenacColloids Surf A20093425358
- MorgenMLuGWDuDTargeted delivery of a poorly water-soluble compound to hair follicles using polymeric nanoparticle suspensionsInt J Pharm201141631432221722722
- AljuffaliIAPanTLSungCTChangSHFangJYAnti-PDGF receptor β antibody-conjugated squarticles loaded with minoxidil for alopecia treatment by targeting hair follicles and dermal papilla cellsNanomedicine2015111321133025933696
- SubongkotTWonglertnirantNSongprakhonPRojanarataTOpanasopitPNgawhirunpatTVisualization of ultradeformable liposomes penetration pathways and their skin interaction by confocal laser scanning microscopyInt J Pharm2013442151161
- GriceEASegreJAThe skin microbiomeNat Rev Microbiol2011924425321407241
- HadinotoKCheowWSNano-antibiotics in chronic lung infection therapy against Pseudomonas aeruginosaColloids Surf B Biointerfaces201411677278524656614
- AljuffaliIAHuangCHFangJYNanomedical strategies for targeting skin microbiomesCurr Drug Metab20151625527126264194
- PanacekAKvitekLPrucekRSilver colloid nanoparticles: synthesis, characterization, and their antibacterial activityJ Phys Chem B2006110162481625316913750
- BegleyMGahanCGMHillCThe interaction between bacteria and bileFEMS Microbiol Rev20052962565116102595
- O’DriscollBGahanCGMHillCAdaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulenceAppl Environ Microbiol199662169316988633868
- KalhapureRSSonawaneSJSikwalDRSolid lipid nanoparticles of clotrimazole silver complex: an efficient nano antibacterial against Staphylococcus aureus and MRSAColloids Surf B Biointerfaces201513665165826492156
- ZazoHColinoCILanaoJMCurrent applications of nanoparticles in infectious diseasesJ Control Release20162248610226772877
- KiruthikaVMayaSSureshMKKumarVAJayakumarRBiswasRComparative efficacy of chloramphenicol loaded chondroitin sulfate and dextran sulfate nanoparticles to treat intracellular Salmonella infectionsColloids Surf B Biointerfaces2015127334025645750
- ZhangLPornpattananangkulDHuCMJHuangCMDevelopment of nanoparticles for antimicrobial drug deliveryCurr Med Chem20101758559420015030
- WenCJSungCTAljuffaliIAHuangYJFangJYNanocomposite liposomes containing quantum dots and anticancer drugs for bioimaging and therapeutic delivery: a comparison of cationic, PEGylated, and deformable liposomesNanotechnology20132432510123867977
- KinoshitaMMiyazakiHOnoSEnhancement of neutrophil function by interleukin-18 therapy protects burn-injured mice from methicillin-resistant Staphylococcus aureusInfect Immun2011792670268021536791