?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Brucellosis is a group of closely associated zoonotic bacterial illnesses caused by members of the genus Brucella. B. melitensis Omp31 is a promising candidate for a subunit vaccine against brucellosis. This study surveyed the immunogenicity of Omp31 alone and with incomplete Freund’s adjuvant (Omp31-IFA) and N-trimethyl chitosan (TMC/Omp31) nanoparticles (NPs), as well as the effect of Omp31 immunization route on immunological responses and protection. After expression and purification, the recombinant Omp31 (rOmp31) was loaded onto TMC NPs by ionic gelation. The particle size, loading efficiency and in vitro release of the NPs were examined. Omp31-IFA was administered intraperitoneally, while TMC/Omp31 NPs were administered orally and intraperitoneally. According to the antibody subclasses and cytokine profile, intraperitoneal immunization by Omp31-IFA and TMC/Omp31 NPs induced T helper 1 (Th1) and Th1–Th2 immune responses, respectively. On the other hand, oral immunization with TMC/Omp31 NPs elicited a mixed Th1–Th17 immune response. Data obtained from the cell proliferation assay showed that vaccination with Omp31 stimulated a vigorous antigen-specific cell proliferative response, which could be further increased after oral immunization with TMC/Omp31 NPs. Vaccinated groups of mice when challenged with B. melitensis 16M were found to be significantly protected in the orally administered group in comparison with the intraperitoneally immunized mice. Results of this study indicated that the reason for high protection after oral vaccination can be via elicited Th17 response.
Introduction
Brucellosis is a group of closely associated zoonotic bacterial illnesses caused by members of the genus Brucella, a group of facultative intracellular Gram-negative, nonmotile and nonspore-forming bacteria.Citation1 There are different Brucella species that infect a wide range of mammals. The disease is mostly acquired by ingestion, inhalation, or direct contact such as conjunctiva or skin lesions contaminated with animal products.Citation2,Citation3
Owing to Brucella intracellular lifestyle, a few antibiotics are useful against these pathogens upon entering their intracellular niche. Antibiotics are applied to treat brucellosis in human beings including rifampicin, tetracycline, trimethoprim–sulfamethoxazole, aminoglycosides, quinolones, and chloramphenicol. Since there is a high possibility for relapse in single-agent therapy, the antibiotics are mostly administered in combination.Citation4,Citation5 Hence, there is a huge demand for efficient vaccines or treatments against human brucellosis. At present, there is no accessible safe vaccine against brucellosis in human beings, and all commercially accessible animal vaccines are based on live attenuated strains of Brucella such as B. melitensis Rev.1 and Brucella abortus S19 and RB51.Citation6 Despite their effective role in controlling brucellosis in animals, these vaccines have some drawbacks such as being infectious in human beings, interfering with diagnosis, causing abortion when administered to pregnant domestic animals, and allowing the regional transmission of vaccine strain.Citation6,Citation7
Owing to disadvantages of live attenuated vaccines, replacing these vaccines by subunit ones would be a great improvement for safety reasons, which would make them suitable for vaccination.Citation8 Several cell surface and intracellular Brucella spp. components were designed and examined as subunit vaccine against brucellosis.Citation9–Citation13 Among these antigens, 31 kDa outer membrane protein (Omp31) conferred protection against B. melitensis and Brucella ovis in BALB/c mice.Citation14 The main subunit vaccine’s drawback is poor immunogenicity. To improve their immunogenicity, these types of vaccines can be formulated into particulate vaccine delivery systems.Citation15,Citation16
Owing to their simplicity and convenience, mucosal immunizations (particularly oral immunization) are a subject of great interest. Overall, advantages of oral immunization include infection control at pathogen entry site, induction of mucosal and systemic immune responses, and no requirement for needle.Citation17,Citation18 However, oral immunization is difficult due to extremely low bioavailability. Development of oral vaccine formulations requires overcoming various obstacles such as the low permeability of large molecules, lack of drug lipophilicity, and fast enzymatic degradation or inactivation in the gastrointestinal tract.Citation19,Citation20 To overcome these problems, different types of polymeric nanoparticles (NPs) have been investigated as delivery systems to the intestine, which can protect their cargo from adverse situations that could affect vaccine bioactivity.Citation21 Particles composed of bioadhesive materials such as N-trimethyl chitosan (TMC), known by its stable positive charges regardless of pH, can increase the vaccine residence time in the intestine and enhance their permeation and immunogenicity. Furthermore, TMC NPs can stimulate maturation of dendritic cells (DCs); hence, they have intrinsic adjuvant properties. These properties make TMC NPs a promising delivery system for immunization.Citation22–Citation24
The aim of this study was to examine the immunogenicity property of Omp31 alone and with TMC NPs and Freund’s adjuvant and to investigate the administration route influence on the type of immune response.
Materials and methods
Animals and ethics statement
Female BALB/c mice (4–6 weeks old; obtained from Pasteur Institute of Iran) were acclimated and randomly divided into six experimental groups. Mice were kept in conventional animal facilities and received water and food ad libitum. All experiments were approved by the ethical committee of Razi Vaccine and Serum Research Institute (No 515.92 GD, 26.1.2010) and performed following the guidelines of ethical committee of Razi Vaccine and Serum Research Institute.
Bacterial strains and plasmid
B. melitensis 16M and B. melitensis Rev.1 were obtained from Razi Vaccine and Serum Research Institute (Karaj, Iran) and were grown on Brucella broth (Difco Laboratories, Detroit, MI, USA) under aerobic conditions at 37°C in a humidified chamber. Escherichia coli DH5α strain (Thermo Fisher Scientific, Waltham, MA, USA) was applied for gene cloning. E. coli BL21 (DE3) and pET28a vector (Novagen, Madison, WI, USA) were used to express recombinant Omp31 (rOmp31).
Antigen production
The selected open reading frame (ORF) was amplified by polymerase chain reaction (PCR) from a synthetic gene (GenBank Accession Number: JQ965699) with forward primer (5′-ACTAGAATTCGCCACCATG GTTGTGGTCAGC-3′) and reverse primer (5′-GGTACTCGAGATTAGTGATGGTG ATGGTGATG-3′). The underlined regions of the primer sequences represent the restriction sites for EcoRI and XhoI, respectively. The amplified DNA fragment was cloned into the pET28a vector. The recombinant protein was expressed in E. coli. The recombinant protein expression was validated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The rOmp31 was then purified using affinity chromatography on Ni2+-conjugated chelating sepharose. Protein expressed as inclusion bodies was solubilized with 8 M urea and refolded by serial dialysis against 4, 2, 1 and 0 M of urea in phosphate buffered saline (PBS). The purified protein was finally solubilized in PBS-1% glycine (0.01 M, pH 8.0). The rOmp31 purity was confirmed by Western blotting. Briefly, protein was separated on a 12% SDS-PAGE gel and transferred into polyvinylidene fluoride membrane. The recombinant protein was determined by probing the membrane with mouse anti-His antibodies (1:5,000; Sigma-Aldrich Co., St Louis, MO, USA). The concentration of purified protein was determined by the Bradford method.
Preparation of TMC formulations
TMC was provided by Dr Abbas Sahebghadam Lotfi (Department of Clinical Biochemistry, Faculty of Medicine, Tarbiat Modares University, Tehran, Iran). TMC NPs were prepared by ionic complexation with pentasodium tripolyphosphate (TPP) (Merck, Darmstadt, Germany) as a cross-linking agent. Omp31 and TMC were dissolved in a 5 mM HEPES (Sigma-Aldrich Co.) buffer (pH 7.4) to a final concentration of 0.1 and 1 mg/mL, respectively. TPP was added under continuous stirring to an Omp31:TPP:TMC weight ratio of 1:3.6:10 until the solution became slightly opalescent. The NPs were harvested by centrifugation (30 minutes, 14,000× g) on a glycerol bed to avoid aggregation. The supernatant was removed, and the NPs were resuspended in PBS.
Characterization of antigen-loaded NPs
The size of the NPs was determined by using a NanoSizer ZS (Malvern Instruments, Malvern, UK) in PBS at 25°C. NPs’ morphology was assessed using field emission scanning electron microscopy (FESEM, 7500F; JEOL, Tokyo, Japan). The samples were coated with gold prior to examination by FESEM.
The amount of encapsulated Omp31 in the NPs was determined by measuring the amount of protein remaining in the supernatant by the Bradford method after centrifugation (30 minutes, 14,000× g). The loading efficiency (LE) was computed by the following equation:
Protein integrity
Omp31-loaded TMC NPs were destabilized by adding 10% (w/v) NaCl to the NPs suspension, resulting in a solution with a protein concentration of 0.41 mg/mL. The protein was electrophoresed at 115 V under reducing conditions in a 12% SDS-PAGE gel.
In vitro release study
Omp31-loaded TMC NPs were separated by centrifugation at 12,000× g and 4°C for 20 minutes. The supernatant was decanted, and the NPs were resuspended in 10 mL of 0.1 M PBS (pH 7.4) and then kept at 37°C under magnetic stirring (150 rpm). At various time intervals, 0.5 mL of the suspension was removed and centrifuged (16,000× g, 20 minutes). The Omp31 concentration in the supernatant was determined by the Bradford method. The same amount of fresh PBS was added to the release medium to reach the primary volume. A sample consisting of only nonloaded TMC NPs was resuspended in PBS to be used as a negative control.
Vaccination
Mice were vaccinated by the oral and intraperitoneal (ip) routes. Six groups of mice either receiving vaccine or as negative control groups are shown in . The positive control group was administered intraperitoneally on the 15th day with 1×105 CFU of B. melitensis Rev.1. TMC NPs and PBS were used as negative control groups.
Table 1 The groups of immunized mice
Antibody responses
After harvesting of the whole blood, the blood was allowed to clot by leaving it undisturbed at room temperature. This usually took ~30 minutes. The clot was removed by centrifuging at 1,800× g for 15 minutes at 4°C. The resulting supernatant was designated serum. After centrifugation, it was important to immediately transfer the serum into a clean polypropylene tube using a Pasteur pipette. The samples were maintained on ice while handling. Antibody titer and isotypes of IgG, namely, IgG1 and IgG2a, in vaccinated mice sera were determined by the enzyme-linked immunosorbent assay (ELISA) as described previously.Citation25 The threshold value for titer determination was taken as the absorbance plus three times the standard deviation obtained at 1:250 dilution of preimmune sera. Isotypes of IgG were analyzed using anti-mouse IgG1–HRP- and anti-mouse IgG2a–HRP-conjugated antibodies (Santa Cruz Biotechnology Inc., Dallas, TX, USA). Dilution of anti-mouse IgG–HRP, IgG1–HRP and IgG2a–HRP used was 1:8,000 (50 ng/mL).
Anti-Omp31 IgA was determined in fecal extracts by indirect ELISA via a goat anti-mouse IgA-specific HRP conjugate (Santa Cruz Biotechnology Inc.). Fecal extracts were prepared by suspending five fecal pellets in 0.5 mL of extraction buffer (100 mg/mL soybean trypsin inhibitor [Sigma-Aldrich Co.], 10 mg/mL bovine serum albumin [Sigma-Aldrich Co.] and 30 mM disodium EDTA in PBS, pH =7.6). After homogenization and centrifugation at 4°C, the supernatants of the fecal extracts (dilution 1:2) were applied for IgA analysis in feces. Dilution of anti-mouse IgA–HRP used was 1:400 (1 µg/mL). All antibody assays were performed in triplicate.
Cytokine responses
Four weeks after the final immunization, five mice from each group were sacrificed and their spleens were aseptically removed. Spleens from mice were removed and teased apart between two ground glass slides. Cells were washed three times in Hanks’ balanced salt solution (BioWhittaker, Walkersville, MD, USA) and resuspended in Roswell Park Memorial Institute medium containing 10% fetal bovine serum, 5×10−5 M 2-mercaptoethanol, 1% sodium pyruvate, 1% nonessential amino acids, 2 mM l-glutamine, and 10 µg of gentamicin per milliliter. Splenocytes (4×106/well) were seeded in 96-well cell culture plates in the complete medium. Cells were stimulated with 10 µg/mL purified rOmp31 and incubated at 37°C in 5% CO2. Supernatants were harvested from cultures after 48 hours of incubation. Cytokine responses were analyzed by mouse ELISA kits according to the manufacturer’s instructions: IFN-γ, interleukin (IL)-12, IL-4, and IL-17 (R&D Systems, Inc., Minneapolis, MN, USA). All assays were performed in triplicate.
Protection experiments
Vaccinated mice were challenged by ip injection with 2×107 CFUs of B. melitensis 16 M 1 month after the last immunization. One month after being challenged, mice were sacrificed by cervical dislocation and their spleens were removed aseptically. Each spleen was homogenized in a stomacher bag, serially diluted and plated on Brucella agar, and CFUs were counted after 48–72 hours of incubation at 37°C. The results are shown as the mean of the standard deviation of the common logarithm of CFU ± per group. Units of protection were obtained by calculating the differences between the common logarithm of CFU obtained from control mice and the common logarithm of CFU obtained from the corresponding experimental mice groups.
Lymphocyte proliferation assay
Mice were sacrificed 1 month after the last immunization. Splenocytes from vaccinated mice were adjusted to 2×105 cells/well in complete DMEM, and then stimulated with rOmp31 (0.1 µg/mL). The plates were then incubated at 37°C, 5% CO2 and 95% humidity for 72 hours. Lymphocyte proliferation was assessed by MTT assay. In all, 1 mL of 5 µg/mL MTT in incomplete media was prepared and 10 µL was added to each well and incubated in dark at 37°C, 5% CO2 and 95% humidity for 30 minutes. After removal of supernatant from each well, the formazan crystals were solubilized using 75 µL of 5% dimethyl sulfoxide (DMSO). Finally, color density absorbance was measured at 540 nm.
Statistical analysis
Comparative analyses of lymphocyte proliferation assay, antibody responses, and cytokine responses were performed by applying the Student’s t-test with values of p calculated accordingly. The CFU data were normalized by log transformation and evaluated by analysis of variance, followed by Dunnett’s post hoc test.
Results
Antigen production
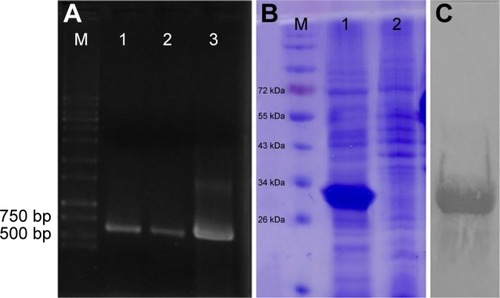
Omp31 gene amplification produced a 708 bp DNA fragment (). The PCR product was ligated in pET28a vector at EcoRI and XhoI restriction sites in frame with 6× His tag at N and C terminals. The Omp31 gene was expressed in E. coli BL21 (DE3). Recombinant protein expression was analyzed by SDS-PAGE and then confirmed by Western blot using anti-His antibody (). Protein concentration was estimated by the Bradford method and then used for vaccination and other experiments.
Figure 1 PCR product, SDS-PAGE and Western blotting of Omp31.
Notes: A PCR product of Omp31 gene (lanes 1–3) followed by agarose gel electrophoresis (A). Expression analysis of recombinant Escherichia coli. After induction with IPTG, the rOmp31 protein produced by recombinant cells was analyzed by SDS-PAGE (B). Lanes 1 and 2 show the induced and uninduced cell lysates of rOmp31 expressing E. coli cells, respectively. Western blot analysis of purified Omp31 with anti-His tag monoclonal antibody (C). 96 dpi (720*451).
Abbreviations: IPTG, isopropyl β-D-1-thiogalactopyranoside; PCR, polymerase chain reaction; Omp31, 31 kDa outer membrane protein; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Characterization of antigen-loaded NPs
Dynamic light scattering (DLS) showed that most TMC/Omp31 NPs had a mean size distribution of 300–400 nm (data not shown). Owing to sample dehydration, SEM images displayed the size of particles to be smaller than measured with DLS (200–300 nm). Furthermore, TMC/Omp31 NPs had a spherical appearance and a smooth surface (). The LE of Omp31 was 70.8%±6.3%.
Protein integrity
The protein integrity of Omp31 after encapsulation was examined by SDS-PAGE analysis. The Omp31 integrity within NPs was found to be intact after protein loading. SDS-PAGE gel analysis results validated the structural integrity of the recombinant protein in the NPs, which shows that the Omp31 integrity has been maintained during the entrapment procedure (data not shown).
In vitro release study
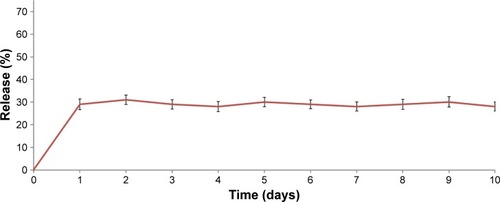
TMC NPs showed ~29% release within the first day, followed by no release over the next 9 days ().
Antibody responses
Ip immunization with Omp31-IFA, TMC/Omp31, and oral immunization induced specific IgG production. Then, Omp31-specific IgG isotypes (IgG1 and IgG2a) were determined in sera from the immunized animals. The obtained results indicated that the main subtype produced after ip immunization with Omp31-IFA and oral immunization with TMC/Omp31 NPs was IgG2a, whereas both IgG1 and IgG2a titers were induced after ip immunization with TMC/Omp31 NPs ().
Figure 4 Anti-Omp31 antibody levels: the sera were analyzed in triplicates for Omp31-specific IgG antibodies by ELISA with comparison to the control group.
Notes: Sera obtained from mice belonging to different experimental groups were collected at regular intervals up to day 45 post-primary immunization, dilution 1:250 (A). Antibody level of intraperitoneally and orally immunized mice. Antibody isotyping (B and C): the isotype profiles of Omp31-specific antibodies in serum of orally and intraperitoneally immunized mice were analyzed by ELISA using HRP-conjugated anti-mouse IgG1 and IgG2a (dilution 1:8,000) antibodies. Omp31-specific mucosal IgA antibody levels in fecal samples from immunized mice, dilution 1:2 (D). Ratio of IgG2a/IgG1 (E), p#0.01. Immunization groups are based on .
Abbreviations: Omp31, 31 kDa outer membrane protein; ELISA, enzyme-linked immunosorbent assay; IFA, incomplete Freund’s adjuvant; PBS, phosphate buffered saline; NP, nanoparticle; ip, intraperitoneal; TMC, N-trimethyl chitosan; HRP, horseradish peroxidase.
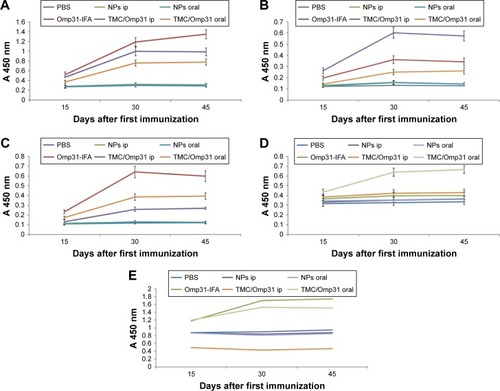
Secretary IgA is a major antibody in mucosal immunity. Ip immunization did not induce any detectable IgA levels in the fecal extracts, whereas oral immunization with TMC/Omp31 NPs showed increased IgA levels (). Additionally, ratio of IgG2a/IgG1 is shown in .
Cytokine responses
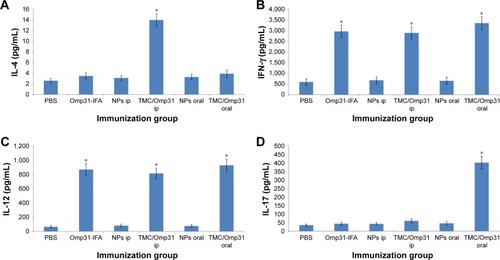
According to the cytokine profile, splenocyte culture supernatants from all vaccinated mice contained high levels of IFN-γ and IL-12 compared to the negative control groups. IL-4 production was significantly higher in mice immunized with TMC/Omp31 in ip route but not in the other groups (). Additionally, a significantly high IL-17 production was observed in orally vaccinated mice ().
Figure 5 IL-4 (A), IFN-γ (B), IL-12 (C), and IL-17 (D) levels in cell supernatants were determined by ELISA.
Notes: Spleen cells (4×106 mL−1 in duplicate wells) were stimulated with rOmp31 for 48 hours (p≤0.01). Immunization groups based on . *Significant difference between groups.
Abbreviations: IL, interleukin; ELISA, enzyme-linked immunosorbent assay; Omp31, 31 kDa outer membrane protein; IFA, incomplete Freund’s adjuvant; NP, nanoparticle; PBS, phosphate buffered saline; ip, intraperitoneal; TMC, N-trimethyl chitosan.
Protection experiments
The Omp31 ability to protect against virulent B. melitensis 16M challenges was determined in BALB/c mice vaccinated with Omp31. The infection level was computed by determining the CFU numbers in spleens 4 weeks after challenge. As expected, the Rev.1 vaccine displayed high protection with 1.91 log units of protection. However, ip vaccination with TMC/Omp31 NPs and Omp31-IFA produced 1.17 and 1.32 log protection units against B. melitensis, respectively (p≤0.01; ). In comparison with the control group, mice that were immunized with Omp31 orally showed a higher level of protection when challenged with B. melitensis, the log units of protection obtained being 1.77.
Table 2 Protection against Brucella melitensis 16M in BALB/c mice immunized with Omp31 compared with the vaccine strain Rev.1
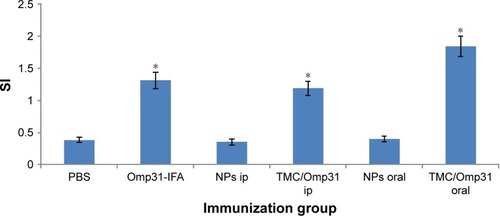
Lymphocyte proliferation assay
The results of MTT proliferation assay were displayed as stimulation index (SI). The SI corresponds to the count per minute of induced spleen cells divided by the count per minute of unstimulated spleen cells. As shown in , the SI values for intraperitoneally vaccinated mice with Omp31-IFA and TMC/Omp31 were determined to be 1.31 and 1.19, respectively, whereas in orally vaccinated mice, it was found to be 1.84 when stimulated with rOmp31 (p≤0.01; ). Thus, this high SI value suggests that cell stimulatory activity of rOmp31 may be one of the reasons behind a robust immune response.
Figure 6 Lymphocyte proliferation assay of splenocytes from mice vaccinated with rOmp31.
Notes: Mice immunized with PBS and NPs were used as negative controls. Splenocytes from vaccinated mice (2×105 cells/well) were stimulated with rOmp31 (0.1 µg/well) for 72 hours, and the proliferative response was determined by in vitro MTT assay. The SI corresponds to the count per minute of stimulated spleen cells divided by the count per minute of unstimulated spleen cells. The data are mean SI ± SD of five individual mice from each group with three repeats (p≤0.01). Immunization groups are based on . *Significant difference between groups.
Abbreviations: PBS, phosphate buffered saline; Omp31, 31 kDa outer membrane protein; NP, nanoparticle; SI, stimulation index; IFA, incomplete Freund’s adjuvant; ip, intraperitoneal; TMC, N-trimethyl chitosan.
Discussion
Polymeric NPs in which the antigen is encapsulated were applied for oral vaccination.Citation26–Citation28 Previous studies have shown that chitosan (CS) and TMC NPs are efficient vaccine delivery systems for systemic vaccination.Citation29–Citation32 In another study, Slutter et al compared CS and TMC NPs’ function in oral immunization. They had indicated that application of TMC NPs is a promising strategy for oral immunization.Citation24 Additionally, TMC NPs, but not CS NPs, displayed intrinsic adjuvant effect on DCs. These NPs have successfully been applied for immunization via different administration routes.Citation33–Citation35 In this study, we assessed the TMC NPs’ ability as a delivery system via the oral and ip routes.
The Omp31 release profile from the TMC NPs showed a primary burst release (). After the primary surge, equilibrium was reached, indicating no further release during the next 9 days. The data obtained from release study are in accordance with data obtained by Amidi et alCitation32 and Bal et alCitation34 who attributed the burst release to a protein that was weakly bound to the NP surface. This means that most of the Omp31 is encapsulated in the TMC NPs.
The subunit vaccine success is related to its composition and immunization route. Currently, licensed human or animal vaccines are generally administered by the parenteral route, but then again, oral vaccines have many advantages compared to systemic injections.Citation36 As the Brucella bacterium often enters the body via contaminated food and/or water, mucosal immunity can act as a first barrier against the infection before it reaches the bloodstream.Citation37 Hence, one of the goals of this study was the elicitation of anti-Brucella IgA in mucosal sites. There is not defined role for IgA in protection against Brucella infection; however, IgA can reflect upon the induction of common mucosal immune response. Our data indicated that when TMC/Omp31 NPs were orally administered, the specific anti-Omp31 IgA was detected in feces of the immunized mice (). Ip vaccination with both Omp31-IFA and TMC/Omp31 NPs showed high IgG titer, whereas oral vaccination with TMC/Omp31 showed a lower antibody titer. Our results are in line with the observations by Chen et al indicating that subcutaneous immunization with another antigen (urease/TMC NPs) into mice generated high levels of IgG titers but low levels of IgA titers. In contrast, orally administered urease/TMC NPs elicited high titers of both IgA and IgG antibodies.Citation26 However, our results are in contrast with those of the study of Boontha et al that had suggested that oral immunization with TMC/ovalbumin elicits low IgA titers.Citation38 Since the antigen type affected the immune response type, this might be the reason behind the difference.
With respect to the IgG subclass detected via pattern of cytokines produced by CD4+ helper T cells, Omp31-specific IgG1 and the IgG2a antibodies titers were analyzed by ELISA. Significant levels of IgG1 and IgG2a were detected from the mice sera that were intraperitoneally immunized with TMC/Omp31, whereas a high amount of IgG2a was determined in the mice sera that were intraperitoneally and orally immunized with Omp31-IFA and TMC/Omp31, respectively. The IgG2a isotype plays a key role in anti-Brucella immunity, since the binding of its Fc portion to Fc receptors on the surface of phagocytes activates a wide range of antimicrobial responses.Citation39 In a study conducted by Mohanan et al, immunogenicity of protein ovalbumine (OVA)-containing dimethyldioctadecylammonium (DDA) liposomes, PLGA microspheres or TMC NPs or adjuvanted OVA-containing DDA/TDB liposomes, or TMC: LPS NPs or PLGA: CpG microspheres was assessed by various immunization routes. They indicated that IgG2a titer is sensitive to vaccination route, whereas IgG1 titer is somewhat insensitive to vaccination route of the particulate delivery systems.Citation33 In contrast, our results showed that both IgG1 and IgG2a were sensitive to immunization route. Since the composition of a subunit vaccine affected the type of immune responses, this might be the reason behind this difference.
Owing to its intracellular survival, efficient immune responses against Brucella include cell-mediated immunity. Additionally, T helper 1 (Th1) and cytotoxic T lymphocyte (CTL) responses are crucial components involved in anti-Brucella protection. Proinflammatory cytokines such as TNF-α, IFN-γ and IL-12 produced at the beginning of infection have shown to play a key role in the battle against this illness. Principally, IFN-γ, which activates the bactericidal function of macrophages, is generally considered crucial in anti-Brucella immunity.Citation40,Citation41 Our results showed that the significant IFN-γ and IL-12 production was achieved in Omp31-IFA in the ip route and TMC/Omp31 NPs in orally and intraperitoneally immunized mice (). Furthermore, the significant IL-17 titer was determined in orally immunized mice. By contrast, IL-4 production was significantly higher only in the group immunized with TMC/Omp31 in the ip route but not in the other groups. It has been reported that Omp31 DNA vaccination stimulates partial protection against B. ovis and B. melitensis infections. This protection was related to the induction of Omp31-specific CD8+ T cells that eradicate Brucella-infected cells via the perforin pathway, a low humoral response, and an absence of Th1 response.Citation42 On the other hand, plasmids encode the Omp31 priming followed by rOmp31 boosting resulting in moderately improved degree of protection against a challenge with B. ovis or B. melitensis.Citation43 Cassataro et al showed that ip immunization with rOmp31-IFA elicits a strong IgG response. After rOmp31 in vitro stimulation, spleen cells from ip rOmp31-vaccinated mice produced significant amounts of the IFN-γ and IL-2 cytokines, but not IL-10 or IL-4, which suggests the elicitation of a Th1 response. The immunization induced partial protection against B. ovis and B. melitensis infections.Citation14 Hence, our data were in accordance with the report of Cassataro et al that had suggested that ip immunization with Omp31-IFA induces Th1 immune response. We showed that oral administration of TMC/Omp31 NPs induces a significant cellular-mixed Th1–Th17 immune response that is in accordance with the observations by Pasquevich et alCitation36 and Abkar et alCitation44 that had shown that oral administration of another antigen (plant-expressed Omp19) and TMC/Omp19 elicits a significant cellular-mixed Th1–Th17 immune response. In a study conducted by Abkar et al, oral vaccination with TMC/Omp19 induced immune responses and a high level of protection against systemic infection. Similar to our report, the researchers did not examine the protection against oral challenge.Citation44
The results obtained in this study showed the importance of administration route in Omp31 protective efficiency. Compared with the Rev.1 vaccine strain, oral immunization of TMC/Omp31 conferred equivalent protection in mice against B. melitensis 16M challenge at 4 weeks post-challenge. The obtained protection after oral immunization was higher than that obtained by ip immunization of Omp31. It has been indicated that an IL-4-dependent Th2 response does not develop or play a negative role during the Brucella pathogenesis, whereas pathogen-specific Th17 cells may boost or work synergistically with Th1 cells for high protection against Brucella infection.Citation36,Citation45 Hence, high levels of Th17 immune responses in oral administration can be one of the reasons behind the high level of protection obtained against B. melitensis challenges compared to the protection level attained in ip immunization.
Indeed, the cell proliferative response observed in Omp31-vaccinated mice represents the activation of cellular immune responses, which is considered to be important for controlling Brucella infections. Data obtained from the cell proliferation assay showed that the vaccination with Omp31 stimulates vigorous production of antigen-specific cytokines secreting cells in spleen, which could be further increased after oral vaccination with TMC/Omp31 NPs.
Conclusion
Our data suggest that administration route plays a main role in determining the immune response type. Furthermore, the protection units obtained show that Omp31 when administered orally confers more protection, which can be due to the elicited Th17 response. TMC/Omp31 may bind and activate a specific cell population present at the gut that positively regulated the differentiation of IL-17-producing T helper cells. It is an ongoing project, and further investigations focusing on increasing efficacy of Omp31 and other Brucella antigen-based vaccines using various adjuvants or evaluation of protection against oral challenges are underway in our laboratory.
Acknowledgments
The authors would like to thank Dr Bram Slutter and Dr Nima Khoramabadi for their scientific advice. The authors also wish to thank the Research Consultation Center (RCC) at Shiraz University of Medical Sciences for their invaluable assistance in editing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- CloeckaertAVergerJMGrayonMClassification of Brucella spp. isolated from marine mammals by DNA polymorphism at the omp2 locusMicrobes Infect20013972973811489421
- PappasGPapadimitriouPAkritidisNChristouLTsianosEVThe new global map of human brucellosisLancet Infect Dis200662919916439329
- Luna-MartinezJEMejia-TeranCBrucellosis in Mexico: current status and trendsVet Microbiol2002901–4193012414130
- ArizaJGudiolFPallaresRTreatment of human brucellosis with doxycycline plus rifampin or doxycycline plus streptomycin. A randomized, double-blind studyAnn Intern Med1992117125301596044
- MontejoJMAlberolaIGlez-ZaratePOpen, randomized therapeutic trial of six antimicrobial regimens in the treatment of human brucellosisClin Infect Dis19931656716768507759
- SchurigGGSriranganathanNCorbelMJBrucellosis vaccines: past, present and futureVet Microbiol2002901–447949612414166
- AshfordDAdi PietraJLingappaJAdverse events in humans associated with accidental exposure to the livestock brucellosis vaccine RB51Vaccine200422253435343915308369
- PerkinsSDSmitherSJAtkinsHSTowards a Brucella vaccine for humansFEMS Microbiol Rev201034337939420180858
- AbkarMAmaniJSahebghadam LotfiANikbakht BrujeniGAlamianSKamaliMSubcutaneous immunization with a novel immunogenic candidate (urease) confers protection against Brucella abortus and Brucella melitensis infectionsAPMIS2015123866767525939375
- FuSXuJLiXImmunization of mice with recombinant protein CobB or AsnC confers protection against Brucella abortus infectionPLoS One201272e2955222383953
- GoelDBhatnagarRIntradermal immunization with outer membrane protein 25 protects Balb/c mice from virulent B. abortus 544Mol Immunol201251215916822464098
- AbkarMLotfiAAmaniJGhorashiSBrujeniGKamaliMDesign of a chimeric DNA vaccine against Brucella sppMinerva Biotecnol2014264223233
- LalsiamtharaJLeeJHBrucella lipopolysaccharide reinforced Salmonella delivering Brucella immunogens protects mice against virulent challengeVet Microbiol2017205849128622869
- CassataroJEsteinSMPasquevichKAVaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infectionInfect Immun200573128079808816299302
- ReedSGBertholetSColerRNFriedeMNew horizons in adjuvants for vaccine developmentTrends Immunol2009301233219059004
- LimaKMdos SantosSARodriguesJMJrSilvaCLVaccine adjuvant: it makes the differenceVaccine200422192374237915193397
- GaucherGSatturwarPJonesMCFurtosALerouxJCPolymeric micelles for oral drug deliveryEur J Pharm Biopharm201076214715820600891
- YamanakaYJLeongKWEngineering strategies to enhance nanoparticle-mediated oral deliveryJ Biomater Sci Polym Ed200819121549157019017470
- KostrzakACervantes GonzalezMGuetardDOral administration of low doses of plant-based HBsAg induced antigen-specific IgAs and IgGs in mice, without increasing levels of regulatory T cellsVaccine200927354798480719539581
- BaumannUMucosal vaccination against bacterial respiratory infectionsExpert Rev Vaccines2008781257127618844598
- MahapatroASinghDKBiodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccinesJ Nanobiotechnology201195522123084
- Jabbal-GillIWattsPSmithAChitosan-based delivery systems for mucosal vaccinesExpert Opin Drug Deliv2012991051106722708875
- GargNKMangalSKhambeteHTyagiRKMucosal delivery of vaccines: role of mucoadhesive/biodegradable polymersRecent Pat Drug Deliv Formul20104211412820380624
- SlutterBPlapiedLFievezVMechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccinationJ Control Release2009138211312119445980
- SinghaHMallickAIJanaCEscheriosomes entrapped DNA vaccine co-expressing Cu-Zn superoxide dismutase and IL-18 confers protection against Brucella abortusMicrobes Infect20081010–111089109618602490
- ChenFZhangZRYuanFQinXWangMHuangYIn vitro and in vivo study of N-trimethyl chitosan nanoparticles for oral protein deliveryInt J Pharm20083491–222623317825506
- ChenWPatelGBYanHZhangJRecent advances in the development of novel mucosal adjuvants and antigen delivery systemsHum Vaccin2010691156120861683
- Fasihi-RamandiMGhobadi-GhadikolaeeHAhmadi-RenaniSTaheriRAAhmadiKVibrio cholerae lipopolysaccharide loaded chitosan nanoparticle could save life by induction of specific immunoglobulin isotypeArtif Cells Nanomed Biotechnol20172816
- Garcia-FuentesMAlonsoMJChitosan-based drug nanocarriers: where do we stand?J Control Release2012161249650422480607
- SandriGBonferoniMCRossiSNanoparticles based on N-trimethylchitosan: evaluation of absorption properties using in vitro (Caco-2 cells) and ex vivo (excised rat jejunum) modelsEur J Pharm Biopharm2007651687716962751
- SubbiahRRamalingamPRamasundaramSN,N,N-Trimethyl chitosan nanoparticles for controlled intranasal delivery of HBV surface antigenCarbohydr Polym20128941289129724750944
- AmidiMRomeijnSGVerhoefJCN-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse modelVaccine200725114415316973248
- MohananDSlutterBHenriksen-LaceyMAdministration routes affect the quality of immune responses: a cross-sectional evaluation of particulate antigen-delivery systemsJ Control Release2010147334234920727926
- BalSMSlutterBVerheulRBouwstraJAJiskootWAdjuvanted, antigen loaded N-trimethyl chitosan nanoparticles for nasal and intra-dermal vaccination: adjuvant- and site-dependent immunogenicity in miceEur J Pharm Sci201245447548122009113
- BalSMSlutterBJiskootWBouwstraJASmall is beautiful: N-trimethyl chitosan-ovalbumin conjugates for microneedle-based transcutaneous immunisationVaccine201129234025403221443959
- PasquevichKACoriaLMSamartinoCGAn oral vaccine based on U-Omp19 induces protection against B. abortus mucosal challenge by inducing an adaptive IL-17 immune response in micePLoS One201161e1620321264260
- GoldingBScottDEScharfOImmunity and protection against Brucella abortusMicrobes Infect200131434811226853
- BoonthaSJungingerHEWaranuchNPolnokAPitaksuteepongTChitosan and trimethyl chitosan particles as oral vaccine delivery systems: comparison of the potential to initiate immune responsesJournal of Metals, Materials and Minerals20112114347
- ZhanYCheersCEndogenous gamma interferon mediates resistance to Brucella abortus infectionInfect Immun19936111489949018406893
- VitryMADe TrezCGorielySCrucial role of gamma interferon-producing CD4+ Th1 cells but dispensable function of CD8+ T cell, B cell, Th2, and Th17 responses in the control of Brucella melitensis infection in miceInfect Immun201280124271428023006848
- SaraivaMChristensenJRVeldhoenMMurphyTLMurphyKMO’GarraAInterleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen doseImmunity200931220921919646904
- CassataroJVelikovskyCADe La BarreraSA DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic responseInfect Immun200573106537654616177328
- CassataroJVelikovskyCABrunoLImproved immunogenicity of a vaccination regimen combining a DNA vaccine encoding Brucella melitensis outer membrane protein 31 (Omp31) and recombinant Omp31 boostingClin Vaccine Immunol200714786987417428946
- AbkarMLotfiASAmaniJSurvey of Omp19 immunogenicity against Brucella abortus and Brucella melitensis: influence of nanoparticulation versus traditional immunizationVet Res Commun201539421722826395469
- KumarPChenKKollsJKTh17 cell based vaccines in mucosal immunityCurr Opin Immunol201325337338023669353