Abstract
Calcifying nanoparticles (CNPs, previously called nanobacteria) are self-propagating, cultivable macromolecular complexes. Their extraordinary characteristic is that they can aggregate carbonate apatite on their envelope from soluble calcium and phosphorus at physiologic concentrations and display cytotoxic effects on murine and human fibroblast cell lines. The question arises whether CNPs contribute to the degeneration of pulp tissue and thus result in clinically significant human dental pulp stones as nidies. This study evaluates CNPs’ effects upon human dental pulp cells (HDPCs, the host cells in pulp tissue). We observed the ultrastructural variation of HDPCs attacked by CNPs. The spatial relationship of HDPCs and CNPs after coculture was also identified by immunofluroscence staining. Furthermore, it was verified by MTT viability assay that CNPs isolated from dental pulp stones exerted cytotoxic effect on HDPCs. Therefore, it could be concluded that the existence of CNPs might interfere with the normal physiologic function of the cells, and that might lead to dental pulp calcification. Elucidation of the cytotoxic characteristics of CNPs may offer a new perspective for understanding the etiology of human dental pulp stones.
Introduction
Calcifying nanoparticles (CNPs) are self-propagating, cultivable macromolecular complexes, previously called nanobacteria.Citation1,Citation2 The defining characteristics for CNPs are their ability to aggregate calcium and phosphate on their outer envelope at physiologic concentrations and conditions.Citation3 Therefore it precipitates CNPs as a potential etiological factor involved in various pathological calcification diseases in human beings, such as kidney stones,Citation4–Citation7 calcified arteries,Citation8–Citation10 human breast cancer,Citation11 and gallbladder stones.Citation12,Citation13
As there is a close relationship between CNPs and pathological calcification diseases in human beings, it is essential to clarify their effect on cultured mammalian cells. Çiftçioglu and KajanderCitation14 firstly reported that four out of six nanobacteria isolates from different sera exerted a cytotoxic effect on 3T6 fibroblasts verified by MTT viability assay, lactate dehygrogenase release, and direct microscopy. In nanobacteria-infected fibroblasts, electron microscopy revealed intra- and extracellular acicular crystal deposits, stainable with von Kossa staining and resembling calcospherules found in pathological calcification. Citation3 The authors suggested that the nanobacteria had a special way of invading mammalian cells and were an important cause of cell vacuolization and poor growth.Citation3,Citation14 In 2009, Zhang et alCitation15 claimed that gingival epithelial cells exposed to CNPs showed gross vacuolization and calcification occurring in intracellular vacuoles. This finding indicated CNPs’ role in pathologic calcification of primary cultured human gingival epithelial cells in vitro.
Pulp stones are discrete calcifications and are among changes that include more diffuse pulp calcifications such as dystrophic calcification.Citation16 Stones may exist freely within the pulp tissue or be attached to or embedded in dentine.Citation17 More often than not, the existence of pulp stones may lead to narrowing or obstruction of the access to the apical point in the root canal; this is one of the most important factors leading to the failure of root canal therapy and loss of the teeth. We have discovered the close relationship between CNPs and dental pulp calcification in a former investigation,Citation18 which indicated that CNPs might possibly play an important role in the formation of dental pulp stones. This finding significantly expanded our expectations to explain the formation of dental pulp calcification, of which the etiology until now was ambiguous.Citation19–Citation22 As far as we know today, pulp calcification is a type of chronic regressive degeneration of the pulp tissue; however, what leads to this devolution remains unclear. Dystrophic calcification is found to be of a variable degree, and this might originate from the impairment of the host cells.Citation16 Therefore, it could be hypothesized that CNPs might have particular potency on human dental pulp cells (HDPCs). Those cells suffered from the encroachment after accumulative effect and resulted in a progressive deposition of calcified masses. To better understand CNPs’ potential effect on cultured HDPCs based on this hypothesis, it is essential to evaluate the undermining mechanism of their co-interaction, qualitatively and quantitatively.
In this study, we observed the ultrastructural variation of HDPC attacked by CNPs through transmission electron microscopy (TEM) and the spatial relationship of HDPCs and CNPs after coculture by immunofluroscence staining. Furthermore, CNPs’ cytotoxic effect on HDPCs was verified by MTT viability assay. From these experiments, it could be concluded that CNPs might invade HDPCs and exert biological effects on the attacking cells that might result in pulp calcification.
Method and material
Culture of HDPCs
HDPCs (human dental pulp cells) were obtained from 22 healthy third molars (19- to 27-year-old patients) which were extracted during normal treatment at the Hospital of Stomatology, Sun Yat-sen University, Guangzhou, China. Protocol was reviewed and approved, and informed consents were obtained from the tissue donors with approval of the ethical committee of the Hospital of Stomatology, Sun Yat-sen University. The primary HDPCs were cultured as previously reported by Gronthos et al.Citation23 Teeth were placed into phosphate-buffered saline (PBS) and scored sagittally with a sterile diamond bur flushed with PBS. The pooling pulp tissue was collected and rinsed once in PBS and cut into 2 mm3 cubes using a dental surgical knife. Next, the fragmented pulp tissue was digested with 3 mg mL−1 type I collagenase (Sigma-Aldrich, St Louis, MO) and 4 mg mL−1 dispase (Sigma- Aldrich) for 30 minutes at 37°C. The resultant suspension was then centrifuged to collect the released cells. These cells were subsequently cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), at 37°C in a humidified atmosphere of 5% CO2. All experiments were performed on HDPCs between passages 5 and 8.
Culture of CNPs
CNPs were isolated from the collected dental pulp stones as described in previous studies.Citation4,Citation18 Samples were immersed in 0.3 μg mL−1 tetracycline and then demineralized in 1 mol L−1 HCl for 30 minutes, then powdered and neutralized with 0.5 mol L−1 Tris (pH 10.5; Sigma-Aldrich). Suspensions were centrifuged at 14,000 g for 15 minutes in a Minispin Centrifuge and sterile-filtered through 0.22 μm Millipore filters (Millipore, Billerica, MA). The filtrate was cultured in a flask containing DMEM with 10% FBS. Subcultures were carried out with a rubber scraper in serum-contained DMEM after 4 weeks of initial inoculation and subsequently after every day. Those after 2~3 subcultures were scraped and harvested by centrifugation at 20,000 g for 45 minutes at 4~C, washed with PBS (pH 7.2–7.4), and then resuspended by rigorous mixing for later use. The harvested CNP pallets were weighed and defined as the following concentration: 5.0 mg mL−1, 2.5 mg mL−1, 0.5 mg mL−1, attenuated by DMEM containing 2% r-FBS.
Invasion of CNPs on HDPCs
100 μL suspension of 2.5 mg mL−1 CNPs was applied to the flask containing HDPCs and cocultured for 48 hours in the conditions for culturing mammal cells. Cells were digested by 0.25% trypsin (Invitrogen, Carlsbad, CA) and 0.02% EDTA (Invitrogen) and collected by centrifugation to deposit cell pellets for the following analysis.
Immunofluorescence confocal microscope observation
Pallets of collected cells were fixed in 3.7% formaldehyde for 15 minutes, and then washed twice with 0.01 mol L−1 PBS (pH 7.2–7.4) for 5 minutes, and then one drop was applied to the coverlip. Endogenous peroxidase was blocked with 3% H2O2 in methanol for 15 minutes. After being blocked in 5% bovine serum albumin for 30 minutes, cells were incubated with IgG1 class anti-CNP mAbs, 8D10 (Nanobac Oy, Kuopio, Finland) overnight at 4°C and washed twice. The cells were then incubated in tris-buffered saline containing TRITC-labeled red-conjugated anti-mouse secondary antibodies (Wuhan Boshide, China) for 30 minutes at 37°C and washed twice. Subsequently, cells were applied with rabbit anti-human vimentin polyclonal antibody overnight and then with FITC-labeled secondary antibodies as above. Finally, coverlips were stained with 10 μg mL−1 Hoechst33258 (Sigma-Aldrich) for 10 minutes and then washed three times for 15 minutes in PBS. After being mounted, coverlips were viewed through LSM510 laser scanning confocal microscope (LSCM, Zeiss, Wetzlar, Germany) at a scanning thickness of 1 μm. Negative control went through the same process except that the coculture step was replaced with PBS.
Transmission electron microscope observation
Pellets were fixed in a 2.5% glutaraldehyde solution at 4°C for 24 hours, and then post-fixed in 1% osmium tetroxide for 1 hour and dehydrated in a graded series of ethanol. Cells were embedded in epon-araldite. Ultrathin sections were cut with ultramicrotomy (LKB, Bromma, Sweden) and collected onto copper grids and stained with 2.5% uranyl acetate in absolute ethanol and lead citrate. All sections were examined by TEM (Hitachi H-600; Hitachi, Tokyo, Japan).
Evaluation for cytotoxic effect
The defined concentrations of CNPs (5.0, 2.5, and 0.5 mg mL−1) were used to determine the cytotoxic effect on cell proliferation. Firstly, HDPCs were plated at 2×104 cell mL−1 in 96-well plates and washed five times with 500 μL PBS. Then cells were cultured again in a serum-free medium for at least 15 hours to remove any effect of the remaining steroid hormones. Secondly, media was replaced with fresh DMEM containing the indicated concentrations of CNPs through the 0.22-μm Millipore filter. Each concentration contained six replicate wells and a control well. Finally, after 24, 48, and 72 hours of coculture, 20 μL MTT (5 g L−1) (Sigma-Aldrich) was added to each well, and cells were incubated for 4 hours. Supernatant in each well was then removed and 150 μL dimethyl sulfoxide (Sigma-Aldrich) was added to dissolve the formed formazan crystals for 10 minutes. Cell proliferation was detected quantitatively using a microplate reader (Infinit, Tecan, Austria) on optical density (OD) value at 490 nm. After 72 hours’ coculture, HDPCs attacked by CNPs were viewed under inverted phase contrast microscope (Axiovert 40; Zeiss).
Descriptive data are given as means ± standard deviation. Data were analyzed using the SPSS statistical package (version 17.0; SPSS Inc., Chicago, IL). The multiple group means were compared by one-way analysis of variance (ANOVA), and differences were considered significant at P < 0.05 level.
Results
Immunostaining for the coculture
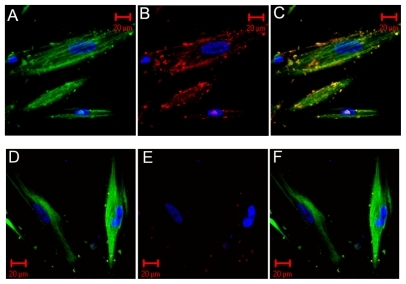
The topological distributions of red-labeled CNPs, greenlabeled HDPCs, and blue-labeled nuclei of the HDPCs were visualized distinctly (). The merged image () clearly reflects the physical initial between the HDPCs and CNPs. It shows the initial invasion of CNPs on HDPCs. Some particles stuck to the outer membranes of the cells or invaded a specific site in the cytoplasm, or even entered the nucleus of the cells. For the negative control (), green and blue fluorescence signals were visible, but the red-labeled CNPs were small and trivial and were distributed in the noncell region. The merged image () shows these red fluorescence signals had completely disappeared.
Figure 1 Immunofluorescent images. The first set is the positive immunofluorescent image for the invasion of CNPs and colocalization. The second set is the negative control. A, D) The green-conjugated secondary antibody recognized HDPCs and the Hoechst staining for blue-labeled nuclei of HDPCs. B, E) The blue-labeled nucleus of HDPCs and the red-conjugated secondary antibody recognized CNPs. C, F) The merged images of the above three colored signals. The invasion of CNPs on the cells is shown in C. The red-labeled particles stick to the outer membranes of the cells or invade a specific site in the cytoplasm, or even enter the nucleus of the cells. In the negative control (E), the red-conjugated CNPs were seldom seen and were distributed in the noncell region. The merged view of (F) shows the red signals had totally disappeared
Abbreviations: CNPs, calcifying nanoparticles; HDPC, human dental pulp cell.
Ultrastructural variation observation
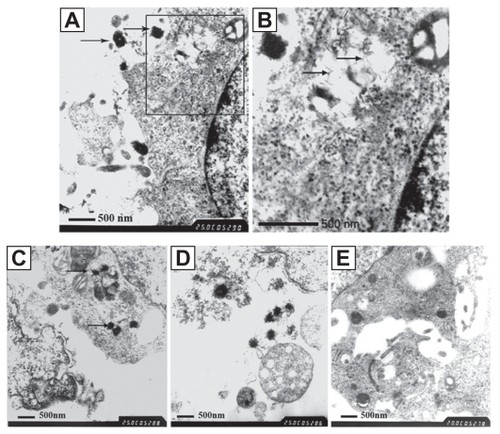
The ultrastructure of the attacked HDPCs was observed by TEM. We discovered round or oval-shaped CNPs of diameter 200–400 nm, with a needle-like crystal crust. Particles stuck to the outer membranes of the cells (). A vacuolus containing mineralization crystals and a swollen mitochondrion can be detected in the enlarged picture (). Some particles were also located in the cytolysosome, phagocytozed by the cells. The affected cells displayed swollen mitochondria and disordered arrangement of the mitochondrial lamellar body (). The cells surrounded by CNPs displayed a state of necrosis and disintegration. The swollen and vacuolized mitochondria were released by the affected cells (). While in the control group, the ultrastructure of the cells was normal and the CNPs could not be found, either in the interior or exterior area of the HDPCs ().
Figure 2 TEM images on the ultrastructure of the affected cells. A) The CNPs, diameter of 200–400 nm, with needle-like crystals, stuck to the outer membranes of the cells. B) The enlarged scale of , showing the vacuolus containing mineralization crystals and swollen mitochondrion (TEM × 25,000). C) Some particles located in the cytolysosome, phagocytosed by the HDPCs. The affected cells show swollen mitochondria and a disordered arrangement of the mitochondrial lamellar body. D) Cells surrounded by CNPs, showing necrosis and disintegration. Released vacuolized mitochondria can be seen (TEM × 25,000). E) The negative control, showing the normal ultrastructure of the cells (TEM × 20,000).
Abbreviations: CNPs, calcifying nanoparticles; HDPC, human dental pulp cell; TEM, transmission electron microscopy.
Cytotoxic effect of CNPs
Cytotoxic effect of CNPs towards HDPCs was quantitatively analyzed by MTT viability-measurement test. MTT results showed that the survival and growth of HDPCs was nearly the same after 24 hours’ coculture (P > 0.05), whereas these were obviously restrained after 48 hours’ invasion by CNPs of high and medium concentration (P < 0.05). The effect remained after 72 hours’ invasion for the cells subjected to high concentration of CNPs (P < 0.05) ().
Table 1 effect of various concentrations of CNPs on the growth and survival of HDPCs
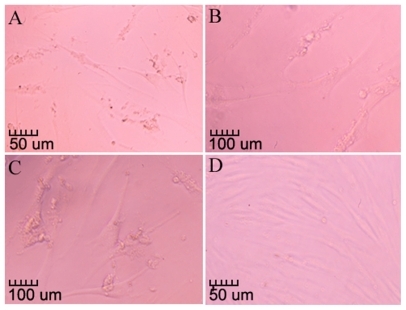
Phase contrast microscopic observation showed clearly that those cells attacked by the high concentrations of CNPs displayed lowered cell density and abnormal morphology. Noticeable cell contraction and vacuolization could be detected in the cytoplasm of the affected cells (), compared with the control ().
Figure 3 CNPs’ cytotox effect on the morphology of HDPCs. A) The lower cell density and abnormal morphology (100×). B, C) The enlarged details of the vacuolization in the cytoplasm of the attacked cells (200×). D) The negative control (100×).
Abbreviations: CNPs, calcifying nanoparticles; HDPC, human dental pulp cell.
Discussion
In previous research, we provided microscopic and immunological evidence on CNPs’ close relationship with dental pulp stones.Citation18 However, this did not explain the CNPs’ etiological role in the formation of the human dental pulp stones. In this study, we further investigated the biological effect exerted by CNPs on HDPCs to explain the undermining mechanism from a microcosmic point of view.
In this study, two to three subcultures of CNPs were chosen for two reasons. Firstly, subcultures increase the amount of the particles and therefore met the need for the whole experiment. Furthermore, this would not significantly affect the virulence characteristics for CNPs, for their reported cytotoxity decreases along with the subculture.Citation14 For the preparation of CNPs, they were first scraped, ultrasonic conditioned, and well distributed to verify the correct concentrations and intensify their biological characteristics. To prevent contamination by other bacteria or fungi, all the particle suspensions were filtered through 0.22 μm minispores in the coculture process.
It was reported that CNPs could infect several kinds of cells in human beings and adhere to 3T6 cells within 15 minutes after their addition. These particles appeared to be in intracellular vacuoles, possibly endosomes and lysosomes.Citation14 With the immunostaining technique, we constructed images to view cells, nuclei, and CNPs respectively by different fluorescence labels and furthermore used the merged view on CNP distribution in situ. It revealed for the first time the interaction between the CNPs and HDPCs. They could either stick to the outer membranes of the cells or invade a specific site in the cytoplasm, or even enter the nucleus of the cells. Immunofluorescent images proved the invasion of CNPs on HDPCs.
To identify the effect of this biological invasion, we observed the attacked cells through TEM. These round or oval-shaped particles, 200–400 nm in diameter, were seen. They closely resembled those found in other pathological calcification diseases.Citation24–Citation26 The attacked cells displayed abnormally, showing signs of vacuolar degeneration, membrane structural necrosis, mitochondrial swelling, etc. This indicated that the ultrastructure of the cells was severely damaged and physiologic function was in disorder after the infection. From the view of the particles located in the cytolysosome, it could be concluded that CNPs could invade HDPCs through a receptor-mediated endocytosis. There might exist a special cell adhesion molecule in the outer membrane of the CNPs that may induce the originally nonphagocytic human dental cells to become phagocytic and thus result in the impairment of the cells.Citation14 Interestingly, we also detected vacuoli containing mineralization crystals, which indicated the cells’ tendency for crystal deposits. It could be inferred that the existence of CNPs might promote the calcification as crystallization of the nuclei, which leads to the formation of biogenic apatite structures or mineralization degeneration of the cells. It reminded us that pathological calcification was not merely a passive consequence, but to some extent, occurred as a positive feedback loop of calcification and inflammation driving disease progression forward.
Quantitative assay of CNPs’ cytotoxic effect was followed by MTT test to measure cell viability. As the metabolic activity of the CNPs was about 100th of that of common organisms,Citation27 the environment for the coculture with different concentrations of CNPs could be regarded as similar and comparable. MTT results showed that the survival of HDPCs was obviously restrained after 48 hours’ coculture for high and medium concentrations, and the effect remained after 72 hours’ invasion by a high concentration of CNPs. This result was partially in agreement with the previous report that “the cytotoxity of CNPs was dependent on the concentration and the coculture time”.14 We proposed two possible reasons contributing to this result. Firstly, to prevent other contamination, the addition of CNPs was filtered, not directly applied. Positive pressure in the filter process might reduce their cytotoxic effect, and thus the difference in the 24-hour period could not be found. Secondly, as HDPCs are located in a nutritional environment, the OD value we measured was the result of the proliferation and the inhibition effect. After 48 hours’ coculture, the proliferation might outweigh the inhibition effect, so the difference was no longer significant for the cells cocultured with medium concentration of CNPs. Phase contrast microscopic observation on variations in the morphology and the amount of the cells proved once again the cytotoxic effect of CNPs on growing HDPCs.
To summarize, we report for the first time the interaction of CNPs with HDPCs. The presence of CNPs was proved as an active invader that interferes with the normal physiologic function of the cells and therefore might play a causative role in inducing calcification in the pulp tissue. However this is just a preliminary investigation; whether CNPs are nidies for calculi and contribute to the development of clinically significant human dental pulp stones needs to be further investigated, and more work is needed on whether it fulfills of Koch’s postulates.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No: 30471884).
Disclosure
The authors report no conflicts of interest in this work.
References
- KajanderEONanobacteria – propagating calcifying nanoparticlesLett Appl Microbiol200642654955216706890
- MartelJYoungJDPurported nanobacteria in human blood as calcium carbonate nanoparticlesProc Natl Acad Sci U S A2008105145549555418385376
- KajanderEOÇiftçiogluNNanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formationProc Natl Acad Sci U S A19989514827482799653177
- CiftçiogluNBjörklundMKuorikoskiKNanobacteria: an infectious cause for kidney stone formationKidney Int19995651893189810571799
- García CuerpoEOlavi KajanderECiftçiogluNNanobacteria. An experimental neo-lithogenesis modelArch Esp Urol200053429130310900759
- RyallRLThe future of stone research: rummagings in the attic, Randall’s plaque, nanobacteria, and lessons from phylogenyUrol Res2008362779718286270
- López-BreaMSelgasRNanobacteria as a cause of renal diseases and vascular calcifying pathology in renal patients (endovascular lithiasis)Enferm Infecc Microbiol Clin2000181049149211197997
- PuskásLGTiszlaviczLRázgaZDetection of nanobacteria-like particles in human atherosclerotic plaquesActa Biol Hung20055634233245
- AbulYAbulMHSilayMSThe role of nanobacteria in cardiac sarcoidosisMed Hypotheses2007685118017140741
- JelicTMChangHHRoqueRNanobacteria-associated calcific aortic valve stenosisJ Heart Valve Dis200716110110517315391
- AltundagKAltundagOAkyurekSPossible association between nanobacteria and malignant microcalcifications in breast cancerBreast J200612328716684338
- WangLShenWWenJAn animal model of black pigment gallstones caused by nanobacteriaDig Dis Sci20065161126113216865581
- WenYLiYGYangZLDetection of nanobacteria in serum, bile and gallbladder mucosa of patients with cholecystolithiasisChin Med J (Engl)2005118542142415780215
- ÇiftçiogluNKajanderEInteraction of nanobacteria with cultured mammalian cellsPathophysiology1998951482748279
- ZhangSMTianFJiangXQEvidence for calcifying nanoparticles in gingival crevicular fluid and dental calculus in periodontitisJ Periodontol20098091462147019722797
- GogaRChandlerNPOginniAOPulp stones: a reviewInt Endod J200841645746818422587
- JohnsonPLBevelanderGHistogenesis and histochemistry of pulpal calcificationJ Dent Res195635571472213367289
- ZengJYangFZhangWAssociation between dental pulp stones and calcifying nanoparticlesInt J Nanomedicine2010 In press
- HillmannGGeurtsenWLight-microscopical investigation of the distribution of extracellular matrix molecules and calcifications in human dental pulps of various agesCell Tissue Res199728911451549182609
- RobertsonALundgrenTAndreasenJOPulp calcifications in traumatized primary incisors. A morphological and inductive analysis studyEur J Oral Sci199710531962069249185
- SübayRKKayaHTarimBResponse of human pulpal tissue to orthodontic extrusive applicationsJ Endod200127850851111501587
- YamazoeTAokiKSimokawaHGene expression of bone matrix proteins in a calcified tissue appeared in subcutaneously transplanted rat dental pulpJ Med Dent Sci2002491576612160227
- GronthosSMankaniMBrahimJPostnatal human dental pulp stem cells (DPSCs) in vitro and in vivoProc Natl Acad Sci U S A20009725136251363011087820
- ZhouZHongLShenXDetection of nanobacteria infection in type III prostatitisUrology20087161091109518538692
- TsurumotoTZhuDSommerAPIdentification of nanobacteria in human arthritic synovial fluid by method validated in human blood and urine using 200 nm model nanoparticlesEnviron Sci Technol20084293324332818522113
- KajanderEOCiftciogluNAhoKCharacteristics of nanobacteria and their possible role in stone formationUrol Res2003312475412669155
- KajanderEOKuronenIKariKANanobacteria from blood: the smallest culturable autonomously replicating agent on EarthProc SPIE Int19973111420428