Abstract
Background
Candida albicans is a major opportunistic fungal pathogen. One of the most important virulence factors that contribute to the pathogenesis of candidiasis is its ability to form biofilms. A key characteristic of Candida biofilms is their resistance to antifungal agents. Due to significant morbidity and mortality rates related to biofilm-associated drug resistance, there is an urgency to develop novel nanotechnology-based approaches preventing biofilm-related infections.
Methods
In this study, we report, for the first time, the synthesis of selenium nanoparticles by irradiating selenium pellets by nanosecond pulsed laser ablation in liquid chitosan as a capping agent. Synergy of the fungicidal effect of selenium nanoparticles and chitosan was quantified by the combination index theorem of Chou–Talalay.
Results
This drug combination resulted in a potent fungicidal effect against a preformed C. albicans biofilm in a dose–response manner. By advanced electron microscopy techniques, we documented the adhesive and permeabilizing properties of chitosan, therefore allowing selenium nanoparticles to enter as the cell wall of the yeast became disrupted and distorted. Most importantly, we demonstrated a potent quantitative synergistic effect when compounds such as selenium and chitosan are combined.
Conclusion
These chitosan-stabilized selenium nanoparticles could be used for ex vivo applications such as sterilizers for surfaces and biomedical devices.
Introduction
Candida albicans biofilm
C. albicans is a major opportunistic fungus capable of causing a broad spectrum of diseases. Candidiasis is the fourth most common hospital-acquired bloodstream infection (BSI) in the USA causing severe sepsis.Citation1 One of the most important factors that contribute to the pathogenesis of candidiasis is its ability to form biofilm formation.Citation2 Indwelling prosthetic materials and most catheters represent ideal surfaces for the adherence and growth of Candida biofilms. Therefore, Candida is the most frequently isolated fungal pathogen in catheter-related BSIs (CRBSIs) resulting in high morbidity and mortality rates in hospitalized patients.Citation3 Candida biofilms are enclosed by an exopolymeric substance or extracellular polymeric substance (EPS) matrix which protects the pathogen from adverse exposure to environmental conditions,Citation4 the host’s immune defense and fungicides. Furthermore, detached yeasts from the biofilm are the source of infection by spreading the BSI.Citation5 Morphogenetic conversions between yeast and hyphae have a key role in biofilm formation and represent an important virulence factor for disease pathogenesis.Citation6 The main characteristic of Candida biofilm is the high level of drug resistance that can endure up to 1,000-fold as compared to their planktonic (freely suspended) counterparts.Citation7 As Candida biofilms have reduced susceptibility against currently existing antifungals,Citation7,Citation8 there is an urgent need to develop novel combination nanotherapeutic approaches for Candida biofilm treatment.Citation9
Combination drug therapy and synergy
Combination drug therapy is the use of two or more pharmacological agents; this is a standard clinical practice in the treatment of antibiotic-resistant infections.Citation10 This combination therapy is designed to achieve synergy,Citation11 defined as the interaction of two or more drugs to accomplish a combined effect greater than the sum of their separate effects.Citation12 Synergy can be demonstrated doing an analysis of the inhibitory assay by the CompuSyn software showing the combination index (CI) with a quantitative definition for additive effect (CI=1), synergism (CI<1), and antagonism (CI>1). The theorem of Chou–Talalay also provides algorithms for automated computer simulation for synergism, as shown in the CI plot.Citation13 The mass–action lawCitation13–Citation15-based software CompuSynCitation16 is one of the most cited software packages.Citation17
Selenium nanoparticles (SeNPs)
As selenium (Se) sulfide is used in the treatment of superficial mycoses in the form of a topical antifungal shampooCitation18 and SeNPs have demonstrated fungicidal effects against C. albicans biofilms,Citation19 we decided to test pure SeNPs in combination with chitosan (CS) or alone against a preformed C. albicans biofilm. Se is a naturally occurring metalloid trace element that is vital as nutrient with important human health benefits due to its dynamic role inhibiting the formation of free radicals; therefore, Se prevents oxidative stress, a major source of age-related diseases;Citation20 there are approximately 25 known selenoproteins incorporated in the human genome;Citation21 however, in high quantities, Se becomes toxic showing a narrow margin between beneficial and toxic effects.Citation22 Yeasts of the genus Candida have the ability to accumulate (within the cell) extensive quantities of trace elementsCitation21 incorporated as organic compounds.Citation23 The mechanisms of accumulation and transformation of Se into the cell wall (CW) architecture of C. albicans remain elusive.Citation23 Se enters the yeast by chemisorption with the formation of ionic bonds by the CW polymers.Citation23–Citation25 The fungicidal effect may be due to the mixing of Se with cell proteins in which Se displaces sulfur (due to the chemical analogy of both elements), for sulfur-containing amino acids such as cysteine (Cys) and methionine (Met).Citation23 Yeasts absorb Se into the cytosol, using transporters such as sulfate permeases Sul1 and Sul2.Citation26 Selenoproteins in excess generate reactive oxygen species (ROS) causing DNA strand breaks;Citation27 this process can lead to changes in protein misfolding, stability, structural changes, and enzyme dysfunction.Citation21,Citation23–Citation25 Toxic activity of inorganic Se compounds in the yeasts involves the reaction of selenites with thiol-containing compounds.Citation25 Therefore, SeNPs have demonstrated an anti-Candida biofilm effect.Citation19
CS
Another compound tested in this study alone or in combination with SeNPs was CS, an effective capping and stabilizing agent of SeNPsCitation28,Citation29 with fungicidal effects.Citation30–Citation32 CS is a polysaccharide derivative of chitin, extracted from fungi, arthropods such as crustaceans and insects. It is a biocompatible, natural, biodegradable, bioadhesive, and positively charged polymer with low cytotoxicity.Citation33 Microbicidal and fungicidal properties of CS are well established.Citation30 The polycationic agents of the polymer interact with anionic components of the yeast reducing the negative surface charge of the outer CW ensuing in a strong attachment of the biopolymer, permeabilizing the CW as a result.Citation30,Citation34 CS-decorated SeNPs (CS-SeNPs) synthesized by chemical methods have been previously studied in cancer research.Citation28,Citation29
Nanotechnology
Nanotechnologies have demonstrated a remarkable potential against infections;Citation35 therefore, there is an urgency to develop novel nanotechnology-based drugs alone and in combination to overcome biofilm drug resistance.Citation36,Citation37 C. albicans biofilm inhibition by silver nanoparticles (AgNPs)Citation38,Citation39 or SeNPs has been previously reported;Citation40–Citation42 our group previously described the inhibition of a preformed biofilm by SeNPs synthesized by a femtosecond laser, and we demonstrated that crystallinity and size are the two main physical parameters of SeNPs that affect the viability of C. albicans biofilms.Citation19
CS-SeNPs synthesized by nanosecond laser ablation in liquids
In this study, we report, for the first time, the synthesis of SeNPs by irradiating Se pellets submerged in deionized (DI) water consisting of CS at 0.25% (CS-SeNPs). The irradiation was achieved by using a pulsed nanosecond neodymium-doped yttrium aluminum garnet (Nd:YAG) laser. The ability to synthesize contamination-free pure nanoparticles is essential for biomedical applications. To the best of our knowledge, this is the first time that CS-SeNPs were tested against a preformed mature C. albicans biofilm, also showing that the combination of SeNPs and CS has a potent fungicidal effect in a dose-dependent manner measured by a well-established phenotypic assay,Citation43–Citation45 and most importantly, we demonstrated a potent synergistic fungicidal effect with the combination of CS with SeNPs against the C. albicans biofilms. Synergy was analyzed by the CompuSyn softwareCitation46 to quantitatively determine the synergistic effect;Citation13,Citation15 we analyzed the CI with the data of the nonlinear dose–effect curves (effect-based approaches).Citation12 Finally, we showed by advanced electron microscopy (EM) that CS-SeNPs permeabilize the outer CW due to CS properties, allowing Se to enter. The change of the characteristic spherical structure of the Candida cells is due to protein misfolding that causes a change of structure induced by Se toxicity.Citation21,Citation23–Citation26 We conclude that CS-SeNPs have a synergistic effect with an effective dose–response inhibition of the preformed mature biofilm in vitro. More studies are warranted to understand the synergistic effect of the combination of Se and CS against the Candida biofilm.
Materials and methods
Materials
Se pellets (Se, <5 mm, ≤99.999% trace metals), CS (low molecular weight), sodium hydroxide (NaOH, American Chemical Society [ACS] reagent, ≥97.0%, pellets), and acetic acid (ACS reagent, ≥99.7%) were purchased from Sigma-Aldrich (St Louis, MO, USA). Molecular biology grade water (MT46000CM) was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Nanoparticle synthesis
The synthesis of SeNPs and CS-SeNPs was performed using a nanosecond laser source by placing 0.35 g of Se pellets (purity 99.9%) deposited as target at the bottom of a 1.7 mL microcentrifuge tube that contained 0.5 mL of DI water or 0.25% (wt/vol) CS solution. The laser used for the irradiation (Ekspla, NT342A, Vilnius, Lithuania) was the Nd:YAG with a laser pulse duration of 3.6 ns at a wavelength of 1,064 nm and a repetitive rate of 20 Hz. The laser beam was aimed at the target vertically from above into the open microcentrifuge tube where the beam focused onto the pure Se pellets placed at the bottom. The target was irradiated for 15 min producing a red–orange color in the solution that was extracted for further analysis. The SeNPs produced in the 0.25% (wt/vol) CS solution were washed to remove the excess CS from the sample. An acid wash was performed using a 50/50 solution of acetic acid and DI water which was added to the sample and then centrifuged to produce a pellet resuspended in phosphate-buffered saline (PBS) solution.
Characterization
The SeNPs were produced by irradiating Se pellets using the nanosecond laser pumped by a 20 Hz Q-switched Nd:YAG laser powered at 20 mJ. The determination of the concentration of the solution was performed using atomic absorption spectroscopy (AA-6200; Shimadzu, Kyoto, Japan) with an Se lamp (L2433-34NQ; Hamamatsu, Boston, MA, USA). Hydrodynamic size and zeta potential (ZP) of SeNPs were characterized using the dynamic light scattering (DLS) system (Zetasizer Nano ZS; Malvern Instruments, Malvern, UK) at 25°C. The high-resolution transmission electron microscope (HRTEM) (JOEL 2010F) and atomic resolution microscope (ARM, JOEL ARM 200F) were used to acquire images of the SeNPs to determine the size and shape of the nanoparticles using the Cs probe at a voltage of 200 kV with a spatial resolution of 0.75 Å. The specially resolved elemental analysis was performed by X-ray emission spectroscopy attachment to the HRTEM.
Strain, media, and culture conditions
The wild-type C. albicans clinical strain SC5314Citation47 was used in all experiments as a control standard. In addition, we used TW1Citation48 and two C. albicans clinical isolates resistant to fluconazole, named TW17Citation48,Citation49 and 6486Citation50,Citation51 strains as previously described. Frozen cells from stocks stored at −80°C were propagated overnight in yeast–peptone–dextrose (YPD) agar plates. Flasks of YPD liquid media were inoculated with a loopful of Candida growth and incubated in an orbital shaker (180 rpm) at 30°C and grown for 14–16 h. Biofilms were assessed using the 96-well microtiter plate-based method as previously reported.Citation43
Activity against a preformed C. albicans biofilms
Biofilms were assessed using a well-known 96-well microtiter plate-based method.Citation43 Briefly, yeast cells collected from overnight cultures were washed in sterile PBS and resuspended at a final concentration of 1.0×106 cells/mL in Roswell Park Memorial Institute (RPMI) medium-1640. Biofilms were formed on tissue culture-treated, 96-well microtiter plates (Corning Incorporated) incubated at 37°C for 24 h. The biofilms formed and attached to the flat bottom of the wells were washed twice with PBS. CS-SeNPs, SeNPs, and CS were assessed against the preformed biofilm for their fungicidal activity at concentrations ranging from 5 to 2,500 and 0.05 to 25 ppm for CS and SeNPs, respectively, in serial twofold dilutions as previously described.Citation43,Citation44,Citation58 The plates were covered with parafilm and incubated for another 24 h. Then, plates were carefully washed twice, and the biofilms were quantified using the tetrazolium salt (2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide [XTT]) reduction assay to test the efficacy of the nanoparticle preparations. All tests were performed in duplicate and were repeated at least three times in independent experiments. The IC50 was performed with the dose–response fits calculated using Origin 9 software (OriginLab Corporation, Northampton, MA, USA).Citation59
Cytotoxicity assay
A stock solution of CS-SeNPs was diluted to the desired concentrations ranging from 50 to 1 ppm in growth medium and subsequently added into a 96-well plate containing human retinal pigment epithelial cell line ARPE-19 (5×104 cells/well). Microtiter plates were incubated at 37°C in a 5% CO2 air humidified atmosphere for 24 h. The cell experiments were performed in triplicate, and readings of the cell viability using a luminescent cell viability assay (CellTiter-Glo; Promega Corporation, Madison, WI, USA) were performed after 24 h.
Visualization by SEM of the effects of nanoparticles on preformed C. albicans biofilms
For SEM ultrastructural observation of the mature biofilms cultured on 48-well plates for 24 h treated with CS-SeNPs (3.5 ppm), SeNPs (21.7 ppm), and CS (25,000 ppm), we gently washed the treated biofilm twice with PBS and fixed with 4% formaldehyde and 1% glutaraldehyde (GA) for 1 h at room temperature. The fixed samples were washed with PBS and then stained in 1% osmium tetroxide (OsO4) solution buffered with PBS for 1 h. The samples were dehydrated with a series of ethanol solutions (25% for 10 min, 50% for 10 min, 70% for 10 min, 95% for 10 min, and absolute alcohol for 20 min).Citation60 The dehydrated specimens were then transferred to 300-mesh carbon-coated copper grids to be observed by SEM in a Hitachi S-5500 (Hitachi Ltd., Tokyo, Japan).
Visualization by TEM of the effects of nanoparticles on preformed C. albicans biofilms
For ultrastructural TEM imaging, Candida biofilms were grown for 24 h and then treated with CS-SeNPs (3.5 ppm). The treated mature biofilm was then centrifuged at 3,500 rpm for 10 min. After washing two times with PBS, cells were fixed in 1 mL of 4% formaldehyde and 1% GA for 2 h. The fixed samples were stained with 1% OsO4 for 1 h. After washing the Candida biofilms with PBS to eliminate the heavy metal stain, a dehydration series was performed with 25, 50, 75, 95, and 100% ethanol diluted in dH2O. The absolute dehydration was assured with propylene oxide before embedding in an epoxy resin LX-112 (Ladd Research Industries, Williston, VT, USA), and the resin was left 48 h at 60°C to harden. The epoxy resin-embedded sections were cut (90 nm thick) using an ultramicrotome (Leica Microsystems, Wetzlar, Germany) and a 45° angle diamond knife as previously described.Citation61 Ultrathin sections were mounted on an uncoated copper mesh grid and visualized using JEOL JEM-2010F.
CI analysis
CI theorem of Chou–Talalay analysesCitation13,Citation14 was calculated using the computer software CalcuSynCitation15,Citation16 version 2.0 (Biosoft, Cambridge, UK).Citation46 We analyzed the CI based on the nonlinear dose–effect curves (effect-based approaches).Citation12 To analyze the dose–effect parameters (% inhibition of the biofilm) of each compound alone (CS and SeNPs) as well as in combination (CS-SeNPs) and calculate the CI value, the parameters can be automatically determined from the median-effect equation.Citation13 Comprehensive procedures of automated dose–effect dynamic analysis via mathematical induction for quantization of synergism in drug combination studies are given in the user’s manual for CompuSyn software.Citation14 Briefly, the CI-isobologram equation was used, where CI values around 1 demonstrate additive effects of the two drugs tested. CI<1 indicates a synergistic effect of the two drugs combined, and CI>1 indicates an antagonistic effect. We need to input each dose–response result as shown in .
Table 1 Synergy based on the nonlinear dose–effect curves
Statistical analyses
The IC50 was calculated by dose–response fitting using Origin 9 software. The graphs show the mean ± standard error of the mean from three separate experiments performed in duplicate. For quantitative assays, statistical analyses were performed with Student’s t-test. Statistical significance was accepted at a P-value of <0.05.
Results
Characterization
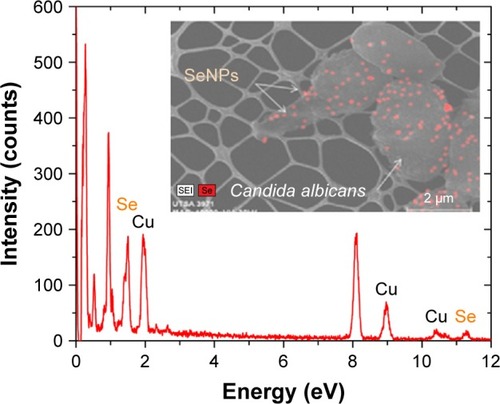
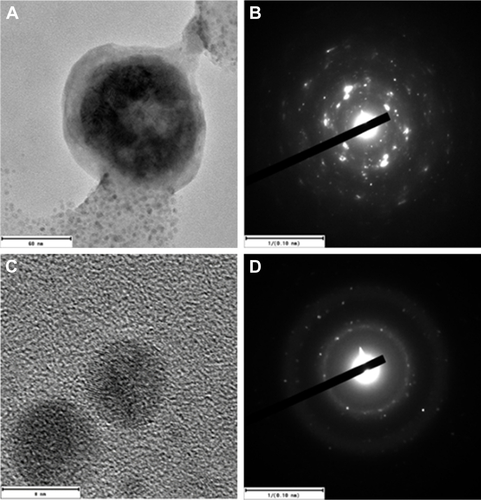
The average hydrodynamic diameter of the CS-SeNPs dispersed in DI water was around 96 nm determined by DLS (). The ZP had a negative charge of about −35.7 mV (). The concentration of the solution determined by atomic absorption spectroscopy (AAS) was 25.5±0.5 ppm. Energy-dispersive spectroscopy (EDS) spectra of CS-SeNPs demonstrate the presence of Se signal in the sample by spectral mapping acquisition (). SeNPs were detected on the outer CW of the C. albicans (red dots) by EDS mapping. For the nanoparticles produced in the presence of CS (CS-SeNPs), HRTEM images show spherical shape of the nanoparticles, and the diffraction pattern of small and large nanoparticles indicates that both are crystalline in nature. The CS matrix surrounding the nanoparticles can be observed (); this CS matrix enhances the crystalline nature of the nanoparticle compared to the amorphous nature of SeNPs produced in DI water. The stability of the nanoparticles due to the CS was demonstrated by ZP which had a negative charge of about −35.7 mV, where the binding effects of the CS to the nanoparticle maintain the stability of the nanoparticles.
Figure 1 DLS and ZP.
Notes: (A) Hydrodynamic diameters of the synthesized SeNPs stabilized in bovine serum albumin and dispersed in DI water; the average size of the nanoparticles is 96.3 nm. (B) The ZP showed a negative charge of −35.7 mV.
Abbreviations: DI, deionized; DLS, dynamic light scattering; SeNPs, selenium nanoparticles; ZP, zeta potential.
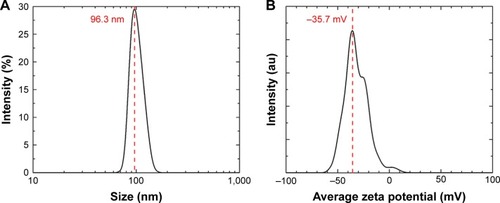
Figure 2 EDS spectrum of SeNPs.
Notes: Se EDS peaks are labeled. Strong signals from the atoms in the SeNPs observed in the spectrum confirm Se metalloid nanoparticles on the yeast outer CW by spectral mapping acquisition.
Abbreviations: CW, cell wall; EDS, energy-dispersive spectroscopy; Se, selenium; SeNPs, selenium nanoparticles; UTSA, University of Texas at San Antonio.
Inhibition of preformed biofilms
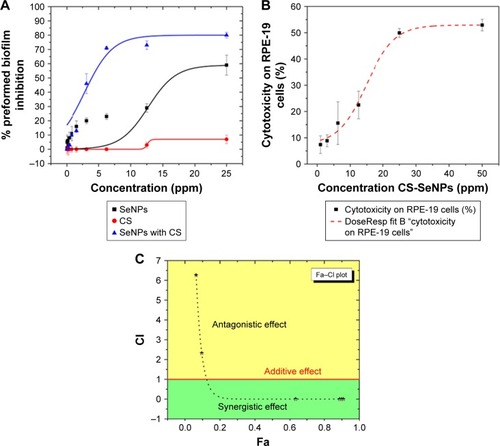
In this study, we used a well-established phenotypic assay as reported by Pierce et alCitation44 and Pierce and Lopez-Ribot.Citation45 Results indicated an inhibitory effect of SeNPs in a dose-dependent manner on the preformed C. albicans biofilm, with a calculated IC50 of 21.7 ppm. CS alone had an inhibitory effect on the biofilm obtaining a 7% inhibition at 25 ppm. CS-SeNPs showed the most potent inhibition against preformed biofilms in a dose–response manner with an IC50 of 3.5 ppm, which pointed to a strong synergistic effect when compared to both compounds alone (). We also compared the activity of CS-SeNPs on preformed biofilms of two drug-sensitive strains (SC5314Citation47 and TW1Citation48) and two drug-resistant C. albicans strains (TW17Citation48,Citation49 and 6486Citation50,Citation51). No statistical difference in dose–response curves was observed between sensitive and resistant clinical strains. Statistical significance was accepted at a P-value of <0.05.
Figure 3 Dose–response curves.
Notes: (A) Dose–response curves for the activity of SeNPs, CS, and CS-SeNPs against preformed C. albicans biofilms. The half maximal inhibitory concentration (IC50) values were calculated as SeNPs (21.7 ppm) and CS-SeNPs (3.52 ppm). Values are the mean ± SEM (error bars) from independent experiments performed in duplicate. (B) Cytotoxicity of CS-SeNPs in RPE-19 cells, the 50% cytotoxic concentration (CC50) is 26.3 ppm. Cytotoxicity of CS-SeNPs was quantified by a Luciferase assay after 24 h of compound incubation. X-axis, concentration in ppm; Y-axis, percentage of cell cytotoxicity. Dose–response curves were plotted using Origin 9 curve fit. Each data point represents the mean ± SEM of three independent experiments performed on different days. (C) The CI was determined. A horizontal red line marks CI=1. The data are the mean values from three independent experiments. Combined doses of CS and SeNPs from 160–1.6 to 25,000–25 ppm, respectively, resulted in Fa from 0.6 to 0.9 with a CI<1 (synergy). Doses under 3.5–35 ppm of CS-SeNPs, respectively, were antagonistic.
Abbreviations: C. albicans, Candida albicans; CI, combination index; CS, chitosan; CS-SeNPs, CS-decorated SeNPs; Fa, fraction affected; SEM, standard error of the mean; SeNPs, selenium nanoparticles.
Cytotoxicity assay
The CS-SeNP concentrations showing potent fungicidal effects against mature C. albicans biofilms (3.5 ppm) are lower than those at which they exhibit cytotoxicity, as demonstrated by a cytotoxicity assay using human ARPE cells (obtained from the American Type Culture Collection, Manassas, VA, USA), resulting in a 50% cytotoxicity concentration (CC50) of 26.3 ppm ().
Visualization by scanning EM (SEM) of the effects of nanoparticles on preformed C. albicans biofilms
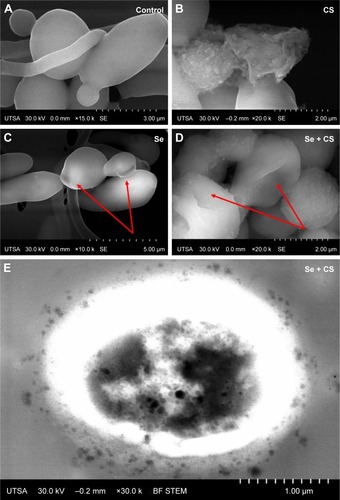
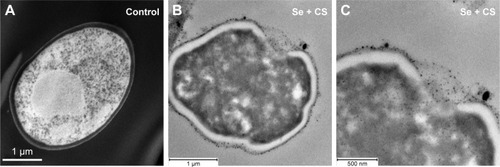
The ultrastructural changes were imaged under SEM after treatment with CS, SeNPs, and CS-SeNPs. shows untreated C. albicans SC5314 biofilms with a dense network of yeast cells and hyphae; yeast cells display a characteristic ovoid morphology. shows C. albicans biofilms treated with CS at 25,000 ppm; yeast cells became enlarged and deformed; CS adheres to the outer CW of the cells. C. albicans treated with SeNPs at 21.7 ppm () has changes in morphology (red arrows). Yeasts treated with CS-SeNPs at 3,500–3.5 ppm, respectively, show major changes in morphology and structure. Red arrows show major deformation of the yeast cells and the polycationic polymer adhered to the negatively charged outer CW (). Bright-field (BF) scanning transmission electron microscopy (STEM) image shows CS-SeNPs entering the yeast (), CS adhered to the outer CW, with SeNPs (darker dots).
Figure 4 SEM.
Notes: (A) Untreated C. albicans biofilms show a characteristic ovoid morphology, and a dense network of yeast cells and hyphae (B) biofilms treated with CS at 25,000 ppm showed enlarged and deformed yeast cell, (C) C. albicans treated with SeNPs at 21.7 ppm shows changes in morphology (red arrows) and (D) C. albicans treated with CS-SeNPs at 3.5 ppm has major changes in morphology and structure (red arrows), the polymer adheres to the CW. (E) BF STEM image of a C. albicans biofilm treated with CS-SeNPs. Throughout the CW, nanoparticles can be observed entering and the CS biopolymer containing SeNPs adheres to the outer CW. CS-SeNPs, CS-decorated SeNPs.
Abbreviations: BF, bright-field; C. albicans, Candida albicans; CS, chitosan; CW, cell wall; SEM, scanning electron microscopy; SeNPs, selenium nanoparticles; STEM, scanning transmission electron microscopy; UTSA, University of Texas at San Antonio.
Visualization by transmission EM (TEM) of the effects of nanoparticles on preformed C. albicans biofilms
In TEM image, we observed the C. albicans yeast cell after thin sectioning; cells without treatment showed a characteristic ovoid morphology and intact CW (). After 24 h treatment with CS-SeNPs (3.5 ppm), a major distortion of the ovoid morphology of the cell is shown and CS-SeNPs are observed adhering to the outer CW (), metalloid nanoparticles enter the cell by disruption of the CW and the cell membrane. Ultrathin-section electron micrographs of the yeast cells document intracellular accumulation of the nanoparticles.
Figure 5 TEM.
Notes: (A) Ultrastructure of untreated C. albicans yeast cell after thin sectioning shows a characteristic ovoid morphology. (B and C) After 24 h treatment with CS-SeNPs (3.5 ppm), the yeast cell loses the characteristic morphology as the cell distorts, the CW disrupts, and the CS-SeNPs enter the cell. CS-SeNPs, CS-decorated SeNPs.
Abbreviations: C. albicans, Candida albicans; CS, chitosan; CW, cell wall; SeNPs, selenium nanoparticles; TEM, transmission electron microscopy.
Synergy
CalcuSynCitation16 generated CI values according to the Chou–Talalay method.Citation15 The CS-SeNP combination treatment was synergistic when CS (2,500–160 ppm) combined with SeNPs (25–1.6 ppm), resulting in CI values of <1; for lower doses, the effect was antagonistic with CI values of >1 as shown in . The additive effect of drug combination depends on the individual dose–effect curves and enables the formulation of synergy, additivity, or antagonism. The dose–effect-based methods depend on the Loewe additivity model.Citation12
Discussion
A wild-type C. albicans strain SC5314 was used in this study; although this is a “susceptible” strain under normal planktonic (free-floating) growing conditions, it has been demonstrated that biofilms formed by this strain display high level of resistance against most clinically used antifungals.Citation43 The antifungal properties of CSCitation30 and SeNPs alone had been previously reported;Citation40 our group demonstrated that SeNPs bind and get adsorbed into the yeast cell, and the main physical properties of SeNPs against the Candida biofilm are size and crystallinity of the nanoparticles.Citation19
In this study, we demonstrated the first synthesis of pure, stable, and crystalline nanoparticles by pulsed laser-ablation in liquids (PLAL) with a nanosecond laser. This laser synthesis is well adapted for biomedical applications because the surface of the produced nanoparticles is free of any chemical byproducts or surfactants.Citation52 Therefore, the biological interaction between the yeast cell and the nanoparticle is not altered by any contaminants. To achieve this, the CS-SeNPs were synthesized by PLAL in DI water containing 0.25% of CS resulting in SeNPs wrapped within a CS shell, shown by HRTEM;Citation53 this CS wrapping is most likely due to the amino acid groups in CS interacting with the surface of the SeNPs, where the coordination behaviors of the nanoparticles maintain the nanoparticle stability and enhance the crystallinity.Citation54 The average size of these wrapped nanoparticles and the electric potential surrounding the particle, called ZP, were measured by DLS. The particles were around ~100 nm in size with an ZP equal to −35.7 mV; this large negative value confirms that the CS-SeNPs are stable in solution due to the polycationic surface charge of the CSCitation30,Citation32,Citation55 (). Furthermore, the slight increase in the value of the ZP of the CS-SeNPs when compared to the SeNPs can be attributed to the polycationic surface charge of the CS and suggests that CS is coating the SeNPs.
The CS and SeNPs alone or combined were tested by a standardized method for in vitro antifungal susceptibilityCitation43,Citation56 against mature C. albicans biofilms which are known to be very difficult to inhibit because sessile cells become shielded inside an exopolymeric matrix of the biofilm displaying intrinsic resistance to most conventional antifungals.Citation4,Citation36 Taken separately, SeNPs (IC50 21.7 ppm) and CS have shown fungicidal properties, but when combined (CS-SeNPs) achieved a potent inhibitory effect (IC50 3.5 ppm) against the mature biofilm in a dose–response manner (). To quantify the synergistic effect of the synthesized CS-SeNPs, we used the Chou–Talalay methodCitation15 specifically designed for drug combination. This theory uses algorithms developed by CompuSyn softwareCitation13 to demonstrate synergy, additivity, or antagonism, as shown in the fraction affected (Fa)–CI plotCitation57 (). We analyzed the dose–effect parameters of each compound alone (CS and SeNPs) as well as in combination, and thus obtained the CI value.Citation12,Citation13 With higher doses of CS-SeNPs, a stronger synergistic interaction was obtained as expected, and doses below the IC50 became antagonistic ().
This is the first reported case, to the best of our knowledge, that proved the synergistic fungicidal effect of CS-SeNPs against preformed C. albicans biofilms.
We theorize that as the bioadhesive polycationic polymer CS strongly binds and permeates the CW of the C. albicans yeast (by reducing the negative surface charge of the CWCitation30,Citation34) this permeabilization enables SeNPs to straightforwardly enter the yeast in excess. Inside the cell, Se competes by affinity with sulfurCitation21,Citation23–Citation25 producing an oxidative stressCitation23 resulting in DNA strand breaks,Citation25 therefore, creating changes in the morphology of the yeast cellCitation19 as documented by advanced EM ( and ).
The dose of CS-SeNPs required to inhibit the mature biofilm (IC50 3.5 ppm) is within the noncytotoxic range on human cells (CC50 26.3 ppm); thus CS-SeNPs could be a good choice for antibiofilm coatings. Future research is warranted to determine the complete mechanism of action and toxicity.
Conclusion
Applying nanosecond PLAL, we obtained, for the first time, the synthesis of CS-stabilized pure SeNPs free of contaminants. To the best of our knowledge, this is the first time that CS-SeNPs have demonstrated efficient dose-dependent activity against preformed C. albicans biofilms with an IC50 of 3.5 ppm of CS-SeNPs. By EM, we observed the fungicidal effect of CS-SeNPs by permeabilization of the outer CW due to the CS properties, and the changing of the structure of C. albicans was due to SeNP effect, documenting the entry of SeNPs in excess into the yeast cell. Most importantly, the results of the CI show a potent synergistic effect.
Acknowledgments
HHL and JLLR would like to acknowledge the National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health, the National Science Foundation Partnerships for Research and Education in Materials (NSF-PREM) (DMR-0934218), and the Welch Foundation (AX-1615). GG and KLN would like to acknowledge grant support from the Biomedical Research Grants Program of the San Antonio Area Foundation. BAV, LCM, and JM would like to acknowledge support from the Air Force Office of Scientific Research (FA9550-15-1-0109).
Supplementary material
Figure S1 HRTEM images of the CS-SeNPs.
Notes: Electron diffraction pattern of (A and B) large and (C and D) small nanoparticles indicates that both are crystalline in nature. CS-SeNPs, CS-decorated SeNPs.
Abbreviations: CS, chitosan; HRTEM, high-resolution transmission electron microscope; SeNPs, selenium nanoparticles.
Disclosure
The authors report no conflicts of interest in this work.
References
- DelaloyeJCalandraTInvasive candidiasis as a cause of sepsis in the critically ill patientVirulence20145116116924157707
- HarriottMMNoverrMCImportance of Candida-bacterial polymicrobial biofilms in diseaseTrends Microbiol2011191155756321855346
- GahlotRNigamCKumarVYadavGAnupurbaSCatheter-related bloodstream infectionsInt J Crit Illn Inj Sci20144216216725024944
- MathéLVan DijckPRecent insights into Candida albicans biofilm resistance mechanismsCurr Genet201359425126423974350
- ChatterjeeSMaitiPDeyRKunduADeyRBiofilms on indwelling urologic devices: microbes and antimicrobial management prospectAnn Med Health Sci Res20144110010424669340
- MayerFLWilsonDHubeBCandida albicans pathogenicity mechanismsVirulence20134211912823302789
- TaffHTMitchellKFEdwardJAAndesDRMechanisms of Candida biofilm drug resistanceFuture Microbiol20138101325133724059922
- KuhnDMGeorgeTChandraJMukherjeePKGhannoumMAAntifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandinsAntimicrob Agents Chemother20024661773178012019089
- LiuSYueLGuWLiXZhangLSunSSynergistic effect of fluconazole and calcium channel blockers against resistant Candida albicansPLoS One2016113e015085926986478
- LeekhaSTerrellCLEdsonRSGeneral principles of antimicrobial therapyMayo Clin Proc201186215616721282489
- MaLKohliMSmithANanoparticles for combination drug therapyACS Nano20137119518952524274814
- FoucquierJGuedjMAnalysis of drug combinations: current methodological landscapePharmacol Res Perspect201533e0014926171228
- ChouT-CDrug combination studies and their synergy quantification using the Chou-Talalay methodCancer Res201070244044620068163
- ChouT-CTheoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studiesPharmacol Rev200658362168116968952
- ChangTTChouTCRational approach to the clinical protocol design for drug combinations: a reviewActa Paediatr Taiwan200041629430211198934
- BijnsdorpIVGiovannettiEPetersGJAnalysis of drug interactionsMethods Mol Biol201173142143421516426
- YadavBWennerbergKAittokallioTTangJSearching for drug synergy in complex dose-response landscapes using an interaction potency modelComput Struct Biotechnol J20151350451326949479
- DiasMFBernardes-FilhoFQuaresma-SantosMVAmorimAGSchechtmanRCAzulayDRTreatment of superficial mycoses: review. Part IIAn Bras Dermatol201388693794424474103
- GuisbiersGLaraHHMendoza-CruzRInhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquidsNanomedicine20171331095110327793789
- TinggiUSelenium: its role as antioxidant in human healthEnviron Health Prev Med200813210210819568888
- KieliszekMBłażejakSKurekEBinding and conversion of selenium in Candida utilis ATCC 9950 yeasts in bioreactor cultureMolecules2017223352
- RaoLMaYZhuangMLuoTWangYHongAChitosan-decorated selenium nanoparticles as protein carriers to improve the in vivo half-life of the peptide therapeutic BAY 55-9837 for type 2 diabetes mellitusInt J Nanomedicine201494819482825378923
- KieliszekMBłażejakSGientkaIBzducha-WróbelAAccumulation and metabolism of selenium by yeast cellsAppl Microbiol Biotechnol201599135373538226003453
- KieliszekMBłażejakSPłaczekMSpectrophotometric evaluation of selenium binding by Saccharomyces cerevisiae ATCC MYA-2200 and Candida utilis ATCC 9950 yeastJ Trace Elem Med Biol201635909627049131
- KieliszekMBłażejakSBzducha-WróbelAKurczAEffects of selenium on morphological changes in Candida utilis ATCC 9950 yeast cellsBiol Trace Elem Res2016169238739326166197
- HerreroEWellingerREYeast as a model system to study metabolic impact of selenium compoundsMicrob Cell20152513914928357286
- LetavayováLVlasákováDSpallholzJEBrozmanováJChovanecMToxicity and mutagenicity of selenium compounds in Saccharomyces cerevisiaeMutat Res2008638111017900630
- ZhaiXZhangCZhaoGStollSRenFLengXAntioxidant capacities of the selenium nanoparticles stabilized by chitosanJ Nanobiotechnology2017151428056992
- ZhangSLuoYZengHEncapsulation of selenium in chitosan nanoparticles improves selenium availability and protects cells from selenium-induced DNA damage responseJ Nutr Biochem201122121137114221292467
- PeñaASánchezNSCalahorraMEffects of chitosan on Candida albicans: conditions for its antifungal activityBiomed Res Int2013201352754923844364
- KvasničkováEPaulíčekVPaldrychováMJeždíkRMaťátkováOMasákJAspergillus fumigatus DBM 4057 biofilm formation is inhibited by chitosan, in contrast to baicalein and rhamnolipidWorld J Microbiol Biotechnol2016321118727660214
- PuYLiuAZhengYYeBIn vitro damage of Candida albicans biofilms by chitosanExp Ther Med20148392993425120626
- CheungRCFNgTBWongJHChanWYChitosan: an update on potential biomedical and pharmaceutical applicationsMar Drugs20151385156518626287217
- DaiTTanakaMHuangY-YHamblinMRChitosan preparations for wounds and burns: antimicrobial and wound-healing effectsExpert Rev Anti Infect Ther20119785787921810057
- ZhuXRadovic-MorenoAFWuJLangerRShiJNanomedicine in the management of microbial infection – overview and perspectivesNano Today20149447849825267927
- PierceCGSrinivasanAUppuluriPRamasubramanianAKLópez-RibotJLAntifungal therapy with an emphasis on biofilmsCurr Opin Pharmacol201313572673024011516
- SinghRSmithaMSSinghSPThe role of nanotechnology in combating multi-drug resistant bacteriaJ Nanosci Nanotechnol20141474745475624757944
- MonteiroDRGorupLFSilvaSSilver colloidal nanoparticles: antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrataBiofouling201127771171921756192
- SilvaSPiresPMonteiroDRThe effect of silver nanoparticles and nystatin on mixed biofilms of Candida glabrata and Candida albicans on acrylicMed Mycol201351217818422803822
- ShakibaieMSalari MohazabNAyatollahi MousaviSAAntifungal activity of selenium nanoparticles synthesized by Bacillus species Msh-1 against Aspergillus fumigatus and Candida albicansJundishapur J Microbiol201589e2638126495111
- KheradmandERafiiFYazdiMHSepahiAAShahverdiAROveisiMRThe antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against Candida albicansDaru2014224824906455
- CremoniniEZonaroEDoniniMBiogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblastsMicrob Biotechnol20169675877127319803
- PierceCGUppuluriPTristanARA simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testingNat Protoc2008391494150018772877
- PierceCGUppuluriPTummalaSLopez-RibotJLA 96 well microtiter plate-based method for monitoring formation and antifungal susceptibility testing of Candida albicans biofilmsJ Vis Exp Epub20101021
- PierceCGLopez-RibotJLCandidiasis drug discovery and development: new approaches targeting virulence for discovering and identifying new drugsExpert Opin Drug Discov2013891117112623738751
- BijnsdorpIVGiovannettiEPetersGJAnalysis of drug interactionsMethods Mol Biol201173142143421516426
- GillumAMTsayEYKirschDRIsolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutationsMol Gen Genet198419821791826394964
- SinghAYadavVPrasadRComparative lipidomics in clinical isolates of Candida albicans reveal crosstalk between mitochondria, cell wall integrity and azole resistancePLoS One201276e3981222761908
- LohbergerACosteATSanglardDDistinct roles of Candida albicans drug resistance transcription factors TAC1, MRR1, and UPC2 in virulenceEukaryot Cell201413112714224243794
- PierceCGChaturvediAKLazzellALA novel small molecule inhibitor of Candida albicans biofilm formation, filamentation and virulence with low potential for the development of resistanceNPJ Biofilms Microbiomes2015111501226691764
- VilaTVMChaturvediAKRozentalSLopez-RibotJLIn vitro activity of miltefosine against Candida albicans under planktonic and biofilm growth conditions and in vivo efficacy in a murine model of oral candidiasisAntimicrob Agents Chemother201559127611762026416861
- MatsumotoATamuraAHondaTTransfer of the species dissolved in a liquid into laser ablation plasma: an approach using emission spectroscopyJ Phys Chem2015119472650626511
- GuisbiersGWangQKhachatryanEAnti-bacterial selenium nanoparticles produced by UV/VIS/NIR pulsed nanosecond laser ablation in liquidsLaser Phys Lett201512116003
- YuBZhangYZhengWFanCChenTPositive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticlesInorg Chem201251168956896322873404
- MartinezLRMihuMRTarMDemonstration of antibiofilm and antifungal efficacy of chitosan against candidal biofilms, using an in vivo central venous catheter modelJ Infect Dis201020191436144020331379
- SilesSASrinivasanAPierceCGLopez-RibotJLRamasubramanianAKHigh-throughput screening of a collection of known pharmacologically active small compounds for identification of Candida albicans biofilm inhibitorsAntimicrob Agents Chemother20135783681368723689719
- YuDKahenECubittCLIdentification of synergistic, clinically achievable, combination therapies for osteosarcomaSci Rep201551699126601688
- LaraHHRomero-UrbinaDGPierceCLopez-RibotJLArellano-JiménezMJJose-YacamanMEffect of silver nanoparticles on Candida albicans biofilms: an ultrastructural studyJ Nanobiotechnology2015139126666378
- Di VeroliGYFornariCGoldlustIAn automated fitting procedure and software for dose-response curves with multiphasic featuresSci Rep201551470126424192
- FischerERHansenBTNairVHoytFHDorwardDWScanning electron microscopyCurr Protoc Microbiol2012 Chapter 2:Unit2B.2
- KuwajimaMMendenhallJMLindseyLFHarrisKMAutomated transmission-mode scanning electron microscopy (tSEM) for large volume analysis at nanoscale resolutionPLoS One201383e5957323555711
