?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The best solvent type and ratio for grafting of poly-n-isopropylacrylamide (PNIPAAm) on the surface of polystyrene is obtained under ultraviolet radiation. In this study, the effects of solvents, such as water, methanol, and their combinations, under ultraviolet radiation were investigated successfully.
Method and results
Attenuated total reflection Fourier transform infrared analysis showed the existence of the graft PNIPAAm on the substrate for all samples resolved in solvents. The best solvent ratio and NIPAAm concentration for grafting was obtained with 40% NIPAAm concentrations resolved in a solvent of 9:1 (v/v) water/methanol (120%). Scanning electron microscopic and atomic force microscopic images clearly showed that a 10% increase of methanol to water would increase the amount of grafting. Surface topography and graft thickness in atomic force microscopic images of the grafted samples showed that the thickness of these grafts was about 600 nm. The drop water contact angles of the best grafted sample at 37°C and 4°C were 43.3° and 60.4°, respectively, which demonstrated the hydrophilicity and hydrophobicity of the grafted surfaces. Differential scanning calorimetric analysis also revealed the low critical solution temperature of the grafted sample to be 32°C. Thermoresponsive polymers were grafted to dishes covalently, which allowed epithelial cells to attach and proliferate at 37°C. The cells were also detached spontaneously without using enzymes when the temperature dropped below 4°C.
Conclusion
MTT analysis also showed good viability of cells on the grafted samples, suggesting that this type of grafted material had potential as a biomaterial for cell sheet engineering.
Introduction
Poly-n-isopropylacrylamide (PNIPAAm) and its copolymers, due to high-speed transition from liquid to solid phase and a critical dissolution temperature of 32°C, can be used in the field of medical science, as well as in drug delivery systems and tissue engineering. At temperatures higher than 32°C, the material exists in a solid and hydrophobic state, and at temperatures below 32°C the polymer shows fully hydrated and hydrophilic properties. PNIPAAm was synthesized in 1957 by Wooten.Citation1 This smart polymer enables surface modification of materials for use in cell sheet engineering.Citation2,Citation5 Different methods for surface modification of polymers, eg, polyethylene, polypropylene, polystyrene, and polyethylene terephthalate, with PNIPAAm grafting using chemical and physical methods, including gamma-ray exposure, plasma, ozone, and ultraviolet and electron beam can be used, each with advantages and disadvantages.Citation6,Citation9 In all such methods, a radical is created on the surface and, during collision with a monomer, the polymerization process occurs.
The ultraviolet irradiation method, in terms of its simplicity and low cost, could potentially be a suitable method for biomaterial surface modification.Citation10,Citation11 Factors such as radiation distance, absorption intensity, wavelength used appropriately to the initiator, thickness, and usual factors, including degassing, substrate, initiators, and sensitizers, contributed to the ultraviolet radiation delivery.Citation12 Of course, this method is required for optical sensitizers or initiators such as anthraquinoneCitation13 or benzophenone.Citation14 Selected solvents used in spectroscopy and polymerization with ultraviolet radiation are very important. Among the solvents used, water and ethanol are the most common organic solvents used in polymer chemistry. The hydrogen atoms of alcohols are transferred to radical substrates, and this can lead to competition between the monomers and alcohols with polymer radicals. One of the properties of alcohol as a solvent is constant chain transfer that is considered a very effective feature.Citation15 In one study, the effect of solvents such as methanol, acetone, and water on 2-hydroxyethyl methacrylate polymerization under ultraviolet light on the nylon substrate was evaluated.Citation15 The results showed that acetone, methanol, and water underwent the best grafting. The solvent effect may cause homopolymerization or grafting. Acetone, due to a lack of hydrogen, cannot donate a hydrogen atom to a substrate; hence, surface radicals can easily react with the monomer. However, there are problems with acetone, in particular, sensitivity to ultraviolet radiation (chromophore) and rapid evaporation during the process; thus, the amount of grafting is reduced.Citation15–Citation17 The negative effects of methanol as a solvent on acrylamide grafting to a polyethylene substrateCitation18 have been investigated. Methanol caused swelling and lack of movement of hydrogen atoms. The constant chain transfer of water was zero, but the combination of water and methanol increased the grafting of acrylamide onto cellulose, and then decreased it.Citation18 Methanol, due to its small molecular size, high swelling properties, and relatively low chain transfer constant (when its concentration in water was very low), showed more grafting than the heavier alcohols. When the concentration of methanol increased, the role of the chain transfer agent would be superior in the swelling process, and therefore grafting was reduced. Ethanol and propanol showed relatively reduced surface grafting due to weak swelling properties and a larger molecular size. The superiority of the stronger chain transfer agent with regard to swelling caused a sharp decrease in grafting. Alcohol solvents could cause solvent evaporation due to their better solubility in the monomer under radiation, and this is important with long-term radiation. On the other hand, the use of water as a solvent was problematic due to lower solubility of acrylamides in water than in alcohols. Thus, a mixture of solvents could potentially solve these problems.Citation15–Citation19 In this study, several important solvents, including water, ethanol, and dimethyl sulfoxide and their complexes were used for grafting PNIPAAm onto a polystyrene surface by ultraviolet radiation. Finally, a nanometric uniform thickness was obtained. Also, the nanometric thickness of the grafting was shown to have an important effect in the process of adhesion and separation of cells and cell sheets. In this research, technical knowledge was also used to achieve intelligent surfaces with nanometric thicknesses using this type of radiation and the best solvent type and ratio for the polymerization process.
Methods and materials
Polystyrene dishes (Orange County Industrial Plastics, Anaheim, CA) with dimensions of 1 × 1 cm and 1 mm thickness, ethanol and methanol (Merck Co, Tehran, Iran), NIPAAm (Sigma-Aldrich, Tehran, Iran), n-hexane (Merck Co), distilled water, polystyrene, and epithelial cells (Pastor Institute, Tehran, Iran) were used in this study. The polystyrene dishes were put in the solution of ethanol-methanol in a 50/50 ratio for 24 hours to dissolve impurities and oils existing on the surface of the dishes. The dishes were then removed from the solution and washed in distilled water. For recrystallization of NIPAAm, 10.3 g of NIPAAm (Sigma-Aldrich) were dissolved in n-hexane 125 mL, and the solution was put in a refrigerator prior to grafting.
Sample preparation
The nonirradiated polystyrene samples were irradiated at a dose of 40 kGy (60Co-γ-radiation source, supplied by Karaj Atomic Research Center, Karaj, Iran). The dose rate was 1 kGy/hour. Irradiation was carried out in air under ambient conditions. Monomers were dissolved in different percentages of solvents, including distilled water, ethanol, methanol, and acetone and their combinations, with a constant ratio of anthraquinone-2-sulfonic acid, and sodium salt monohydrate (3% w/v; Fluka Co, St. Louis, MO). The samples were degassed by nitrogen (2-bar mass flow rate) for 30 minutes. This process was used to increase the efficiency of free radical polymerization. The solutions were then poured in a plastic washer (diameter 1 cm and height 3 mm) attached to the polystyrene substrate. The samples in solution were exposed to an ultraviolet radiation source (black light, 160 W, 365 nm; Philips, Eindhoven, The Netherlands) for 30 minutes. Irradiation was carried out in air under ambient conditions. The samples were then removed and washed in distilled water, subsequently put in distilled water for 72 hours along with Soxhlet for removal of the ungrafted monomer, and then taken out for analysis. The effect of the solvents on the extent of grafting was measured using the following formula:
where w and w° indicate the grafted and ungrafted sample weight, respectively.
Attenuated total reflection Fourier transform infrared
The samples were examined by attenuated total reflection Fourier transform infrared (ATR-FTIR; Nexus; Thermo Niocolet, Waltham, MA) before and after adjustment, and then were put under the instrument for investigation.
Surface morphology study
The surface characteristics of various modified and unmodified films were studied by scanning electron microscopy (Cambridge Stereoscan, Model S-360; Cambridge Scientific Instruments, Cambs, UK) to measure changes in surface morphology. The films were first coated with a layer of gold (Joel Fine Coat, ion sputter for two hours) to provide surface conduction before scanning. Surface topology characteristics and the thickness of various modified and unmodified films were studied by atomic force microscopy (TMX 2010 and the Nanosurf® EasyScan 2 model) to study changes in surface topology.
Contact angle analysis
The static contact angle of the sample surfaces was investigated using the contact angle measuring device (G10; Krüss, Hamburg, Germany) following the sessile drop method. The contact angle formed would be the angle between the solid/liquid and the liquid/steam joint surface. In order to review the sample surface’s hydrophilic/hydrophobic behavior at high and low temperatures, a better sample was considered at two different temperatures of 4°C and 37°C, and the contact angles were measured at these temperatures.
Differential scanning calorimetry
Better samples were investigated by thermal analysis using a differential scanning calorimetry device (DSC 200 F3; Netzsch, Selb, Germany) at a heating rate of 5°C/min from 0°C to 60°C in a nitrogen gas atmosphere.
Biocompatibility study
Aliquots of cell suspension in RPMI medium including 300,000 SW742 epithelial cells were seeded on a 6-multiwell cell culture plate (Orange County Industrial Plastics) which was precoated with samples. The plate was put in an incubator (3°C, CO2) over three hours for cell attachment, followed by rinsing of the loosely attached cells with phosphate buffer solution, and adding 2 mL of fresh medium to the cell culture in the incubator for seven days. Proliferation of cells was determined from measurement of viable cell numbers by MTT assay. The MTT tetrazolium compound was reduced by living cells into a colored formazan product that was soluble in tissue culture medium. The quantity of formazan product was directly proportional to the number of viable cells in the culture. The assays were performed by adding 1 mL of MTT solution (Sigma-Aldrich) and 9 mL of fresh medium to each well after aspirating the spent medium, and incubating at 37°C for four hours with protection from light. The colorimetric measurement of formazan dyeing was performed at a wavelength of 570 nm using a microplate reader (Rayto, Shenzhen, People’s Republic of China).
For cell detachment, SW742 cells were seeded onto the samples at a density of 1,000,000 cells, and were cultured at 37°C under a humidified atmosphere of 5% CO2. Cell detachment was evaluated by incubating the cultures at 4°C for up to 60 minutes. The culture medium containing the detached cells was transferred to a new well. The numbers of detached cells and cells attached to the original well were determined by MTT assay.
Results and discussion
Grafting percentages
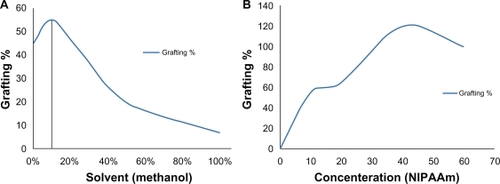
The grafted polystyrene samples with the different solvents under ultraviolet radiation were weighed at a constant temperature. and show that there was almost no grafting of samples using 100% solvents without water. More grafting is obtained with a methanol/water solvent of 10% (v/v). and show an increase in grafting with increased NIPAAm concentration in a methanol/water solvent of 10% (v/v). The peaks around 20% (g/v) and 40% (g/v) were probably due to the Trommsdorff effect. The small peak around 20% might be attributed to water, while the large one around 40% would be attributable to methanol.
Table 1 Grafting percentage of polymer on surface according to solvents used
Table 2 Grafting percentage of polymer on surface according to n-isopropylacrylamide concentration
Attenuated total reflection Fourier transform infrared analysis
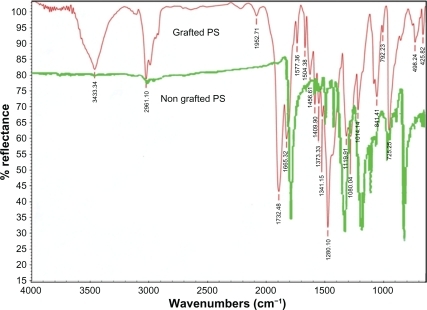
ATR-FTIR spectra results of the regular unadjusted and the ultraviolet radiation-adjusted polystyrene samples are shown in . The NIPAAm grafted by the ultraviolet-radiated polystyrene ATR-FTIR spectra are shown in . The PNIPAAm peak characteristics include 1601 cm−1 indicating –NH groups, 1730–1830 cm−1 indicating C=O groups, 3025 cm−1 indicating CH3 groups, and 3443 cm−1 indicating-NH groups in PNIPAAm. All these peaks were found in PNIPAAm-grafted polystyrene samples. These observations show that grafting between the PNIPAAm and the polystyrene surface occurs by activation of ultraviolet radiation coating.
Surface morphology
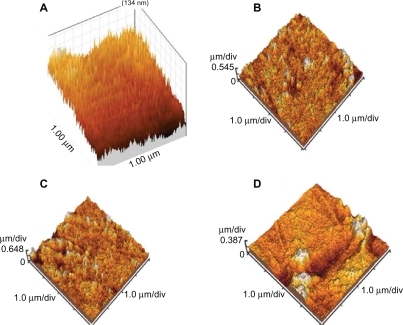
The microscopic images for investigating the adjusted samples through ultraviolet radiation have been shown in and . These images show PNIPAAm grafting onto the preirradiated polystyrene for different solvents. is the atomic force microscopic image obtained from the preirradiated polystyrene samples. The surface topography and the graft thickness created on the surfaces with different solvents are shown in the atomic force microscopic images. shows the surface topography for the grafted sample using the water solvent. shows the surface topography for the grafted sample in the solvent of 9:1 (v/v) water/methanol. is the atomic force microscopic image obtained from the grafted polystyrene samples in pure methanol. The mean graft thickness for the better sample grafting in the solvent of 9:1 (v/v) water/methanol and the pure water was about 600 nm, and the white spots indicate roughness created during radiation.
Figure 4 Atomic force microscopic image of grafted polystyrene with better grafting (120%) with 40% n-isopropylacrylamide concentration resolved in the solvent of 9:1 (v/v) water/methanol under ultraviolet radiation (scale 1 × 1 μm).
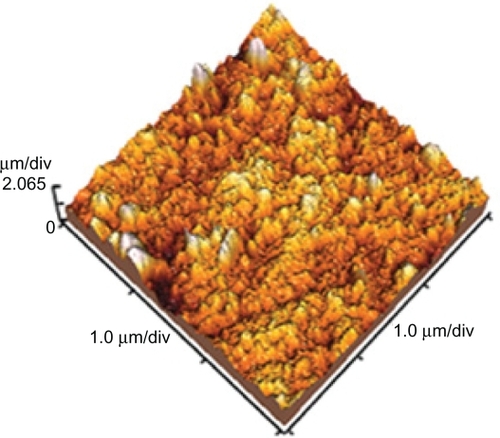
shows the surface topography and thickness of the grafted sample, with better grafting (120%) using the 40% NIPAAm concentration resolved in a solvent of 9:1 (v/v) water/methanol. The average graft thickness for this sample was about 2 μm, and the white spots indicated roughness created during radiation.
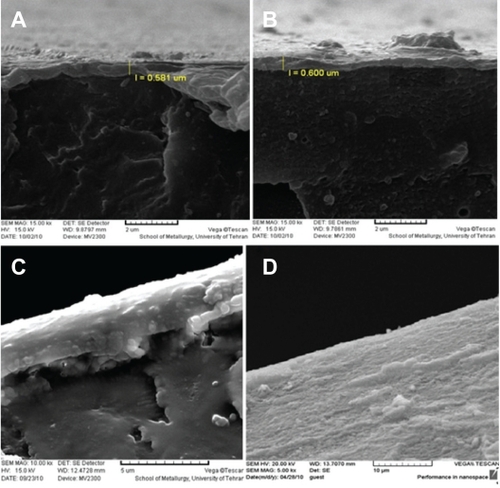
show the scanning electron microscopic images for the grafted polystyrene samples created using different solvents. The surface morphology clearly shows grafting and graft thickness on surfaces with different solvents. shows the surface morphology and thickness for the grafted sample in the water solvent. shows the surface morphology and thickness for the grafted sample in the solvent of 9:1 (v/v) water/methanol. shows the surface morphology and thickness for the grafted sample in the solvent of 9:1 (v/v) water/methanol with 40% of NIPAAm. The mean graft thickness for the better sample grafted in the solvent of 9:1 (v/v) water/methanol and with 40% of NIPAAm was about 600 nm and 2 μm. shows the surface morphology of the grafted sample in the solvent of 9:1 (v/v) water/methanol.
Figure 5 Scanning electron microscopy of grafted polystyrene under ultraviolet radiation. A) Grafted sample in water (cross section), B) Grafted sample in the solvent of 9:1 (v/v) water/methanol (cross section), C) Grafted sample in the solvent of 9:1 (v/v) water/methanol with 40% n-isopropylacrylamide (cross section), and D) Surface morphology of grafted sample.
Contact angle analysis
For contact angle measurement, the measured angle of normal polystyrene adjusted by ultraviolet-radiated PNIPAAm surface samples at 4°C and 37°C is shown in .
Table 3 Contact angles for normal and preirradiated and grafted samples
The average contact angles of 43.3° and 60.4° have been calculated for 4°C and 37°C temperatures, respectively. The results indicate a contact angle decrease below the temperature of 32°C (4°C), as well as hydrophilic surface features. When the contact angle increased above 32°C temperature (37°C), the hydrophobic surface feature was also seen.
Differential scanning calorimetry
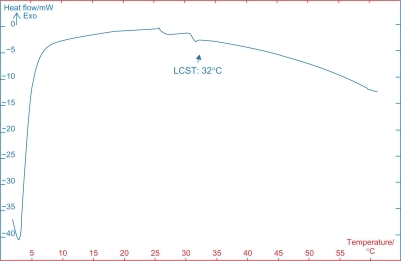
The samples were investigated by the differential scanning calorimetry device (Netzsch DSC 200 F3), with a heating rate of 5°C per minute from 0°C to 60°C in a nitrogen gas atmosphere. Review of the differential scanning calorimetry data for the grafted samples showed a critical temperature for the grafted PNIPAAm using a water/methanol ratio of 9/1. shows the differential scanning calorimetry thermogram in which a slope curve is obtained at 32°C. This shows no significant change in smart polymer critical temperature during the radiation and grafting process.
Biocompatibility results
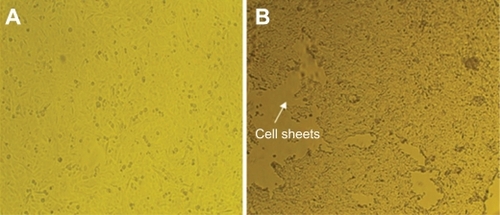
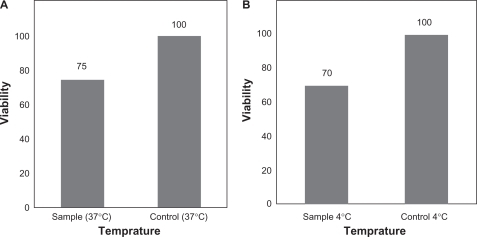
Biocompatibility data demonstrated that the grafted samples with better grafting (120%) with 40% of NIPAAm concentration resolved in the solvent of 9:1 (v/v) water/methanol under ultraviolet radiation supported epithelial cell adhesion and proliferation, and that the cells also maintained high viability (). After culture for seven days on grafted samples, almost all cells were alive, suggesting that the grafted samples were suitable for cell attachment and proliferation, and that the viability was about 75% (). When the cells were placed outside the incubator and the medium was cooled from 37°C to 4°C, almost all of them were alive () and the viability was as high as 70%.
Figure 7 Cell viability in grafted sample with 40% n-isopropylacrylamide concentration resolved in a solvent of 9:1 (v/v) water/methanol under ultraviolet radiation at physiological temperature (37°C, ) and detached cells on the sample surface after 60 minutes of incubation at 4°C () with cell viability on control tissue culture polystyrene dishes.
shows good cell growth on the surface of grafted samples at the physiological temperature of 37°C. shows spontaneous cell growth detached from the grafted sample surface, in the absence of enzymes (trypsin/ethylenediamine tetra-acetic acid). Cell detachment efficiency from the grafted samples was high. In contrast, cell growth on the tissue culture polystyrene dishes did not show any temperature-dependent cell sheet detachment. After a longer period of cell cultivation (for seven days), confluent cells formed a continuous monolayer cell sheet on the surface of the grafted samples. The cell sheet was spontaneously detached from the surface of the thermoreversible grafted samples when cooled to 4°C without treating by any enzymes. As shown in , cell detachment started from the edge of the cell monolayer. After 60 minutes of incubation at 4°C, a monolayer cell sheet could be lifted up from the edge upon mild perturbation of the medium. A living cell sheet, completely detached from the culture surface, could be obtained within 60 minutes. Such results demonstrated that cold treatment effectively released the cell sheet from the plate without damaging cell–cell connections.
Conclusion
The effect of solvents on polymer grafting in a polystyrene sample dish under ultraviolet radiation was studied. The ATR-FTIR spectrum showed formation of the grafted polymer on polystyrene surfaces. The chain transferring constant and molecular weight effect of solvents and their penetration into the materials led to competition between the solvents for grafting. The chain transfer constancy of water was nearly zero, but the monomer NIPAAm did not solve well. In contrast, the monomer was well solved in alcohol, but the chain transfer constancy increased with an increase in the molecular weight of the alcohol. Water combined with a low chain transfer constant and an alcohol of low molecular weight could be a good solvent for grafting. Imaging and gravimetric analysis of the grafting quantity with different solvents indicated an increase in the grafting quantity by adding 10% methanol to water with 40% of NIPAAm concentration. The scanning electron microscopic images showed the grafted surface morphology for different solvents, and we could clearly observe and compare our increases in graft increasing. The surface topology shown in the atomic force microscopic images confirmed these results. The graft thickness of the best samples of solvent in this study was about 600 nm. The contact angles 43° and 60° obtained at temperatures of 4°C and 37°C, as well as polymer critical temperature constancy (32°C) measured by the differential scanning calorimetry method indicated that grafting caused no change in PNIPAAm operation and function. Thermoresponsive polymers were grafted to the dishes covalently, which allowed epithelial cells to attach and proliferate at 37°C. Cells from the sheet also detached spontaneously when the temperature decreased below 32°C, without using enzymes. MTT analysis also showed good viability of the grafted samples. These characteristics suggest that these types of grafted materials have potential as biomaterials for cell sheet engineering.
Disclosure
The authors report no conflicts of interest in this work.
References
- HeskinsMGuilletJESolution properties of poly (N-isopropylacrylamide)J Macromol Sci Chem1968A2814411455
- Da SilvaRMManoJFReisRLSmart thermoresponsive coatings and surfaces for tissue engineering: Switching cell-material boundariesTrends Biotechnol20072557758317997178
- YakushijiTSakaiKKikuchiAAoyagiTSakuraiYOkanoTGraft architectural effects on thermo-responsive wettability changes of poly(N-isopropylacrylamide)-modified surfacesLangmuir19981446574662
- KikuchiAOkanoTNanostructured designs of biomedical materials applications of cell sheet engineering to functional regenerative tissues and organsJ Control Release2005101698415588895
- GalaevIYMattiassonBStimulus responsive surfaces: Possible implication for biochromatography, cell detachment and drug deliveryLandes Bioscience20036116129
- BiazarEZeinaliRMontazeriNCell engineering: Nanometric grafting of poly-N-isopropylacrylamide onto polystyrene film by different doses of gamma radiationInt J Nanomedicine2010554955620957116
- CurtiPSDe MouraMRRadovanovicERubiraAFMunizECSurface modification of polystyrene and poly(ethylene terephtalate) by grafting poly (N-isopropylacrylamide)J Mater Sci Mater Med2002131175118015348662
- JoengBGutowskaALessons from nature: Stimuli-responsive polymers and their applicationsTrends Biotechnol20022030531012062976
- AkiyamaYKikuchiAYamatoMOkanoTUltrathin poly (N-isopropylacrylamide) grafted layer on poly(styrene) surfaces for cell adhesion/detachment controlLangmuir2004205506551115986693
- DelivopoulosEMurrayAFCurtisJCEffects of parylene-C photo-oxidation on serum-assisted glial and neuronal patterningJ Biomed Mater Res A201094475820091707
- DelivopoulosEMurrayAFMacLeodNKCurtisJCGuided growth of neurons and glia using microfabricated patterns of parylene-C on a SiO2 backgroundBiomaterials2009302048205819138795
- DengJWangLLiuLYangWDevelopments and new applications of UV-induced surface graft polymerizationsProg Polym Sci200934156193
- GeuskensGEtocADi MichelePSurface modification of polymers VII: Photochemical grafting of acrylamide and N-isopropylacrylamide onto polyethylene initiated by anthraquinone-2-sulfonate adsorbed at the surface of the polymerEur Polym J200036265271
- BousquetJAHaidarBFouassierJPVidalAPhotocrosslinking of elastomer systems – III Ultraviolet light induced reactions in EPDM and EPR blended with or modified by grafting of derivatives of benzophenoneEur Polym J198319135142
- NandiUSSinghMChain transfer of alcohols in the polymerization of acrylic estersMakromolekulare Chemie198218314671472
- SasakiTRatnerBDHoffmanASRadiation-induced co-graft polymerization of 2-hydroxyethyl methacrylate and ethyl methacrylate onto silicone rubber filmsHydrogels for Medical and Related Applications, ACS Symposium Series19763283294
- LuJLiJYiMHaHSolvent effect on grafting polymerization of NIPAAm onto cotton cellulose via G-preirradiation methodRadiat Phys Chem200160625628
- OgiwaraYTakumiMKubotaHPhotoinduced grafting of acrylamide onto polyethylene film by means of two-step methodJ Appl Polym Sci19822737433750
- RanogajecFMlinac-MiakMImprovement of the polymer stability by radiation graftingRadiat Phys Chem200471229233