Abstract
Background
Intravenously (IV)-injected gold nanoparticles (AuNPs) powerfully enhance the efficacy of X-ray therapy of tumors including advanced gliomas. However, pharmacokinetic issues, such as slow tissue clearance and skin discoloration, may impede clinical translation. The direct infusion of AuNPs into the tumor might be an alternative mode of delivery.
Materials and methods
Using the advanced, invasive, and difficult-to-treat F98 rat glioma model, we have studied the biodistribution of the AuNPs in the tumor and surrounding brain after either IV injection or direct intratumoral infusion by convection-enhanced delivery using light microscopy immunofluorescence and direct gold visualization.
Results
IV-injected AuNPs localize more specifically to intracerebral tumor cells, both in the main tumor mass and in the migrated tumor cells as well as the tumor edema, than do the directly infused AuNPs. Although some of the directly infused AuNPs do access the main tumor region, such access is largely restricted.
Conclusion
These data suggest that IV-injected AuNPs are likely to have a greater therapeutic benefit when combined with radiation therapy than after the direct infusion of AuNPs.
Introduction
Every year >22,000 people in the USA are diagnosed with primary brain tumors, with 13,000 deaths; the 5-year survival rate is 24%. For glioblastoma (GBM) multiforme, <10% of patients survive for >3 years.Citation1 Primary brain tumors also account for 20% of cancers in children. More effective treatments of malignant brain tumors are desperately needed.
Urgent neurosurgery is typically performed to remove the primary brain tumor and relieve dangerous intracranial pressure. However, recurrence of brain tumors is generally inevitable. The problem is that at the time of diagnosis, tumor cells have already migrated as much as 4 cm from the main tumor mass.Citation2–Citation4 Unfortunately, the migrated tumor cells cannot be removed by surgery and effective radiotherapy cannot be applied over such a large volume. Further chemotherapy agents are largely ineffective in preventing recurrence. Here, we propose that gold nanoparticles (AuNPs) are targeted to migrating tumor cells, thereby selectively enhancing radiotherapy.
Hainfeld et alCitation5–Citation8 pioneered the use of AuNPs to enhance the radiotherapy of tumors. In previous work, we showed that nanogold-enhanced radiosurgery of a very aggressive orthotopic glioma in mice resulted in 50% long-term survival (>1 year) compared to 0% with radiosurgery alone.Citation8 In those studies, the AuNPs were intravenously (IV) administered and accumulated preferentially in the tumors; ratios of gold in tumor to normal brain werê19:1. To load tumors with enough gold to obtain these extraordinary dose enhancements (~400%), large quantities of AuNPs need to be injected. While the AuNPs used have shown no signs of toxicity to date, the injection of such large quantities of AuNPs presents a number of pharmacokinetic challenges including poor whole-body clearance, cost, and cosmetic issues (long-term skin discoloration). The direct introduction of AuNPs into the tumor region would solve many of these issues. The goal of this study is to compare the distribution of IV-injected AuNPs with AuNPs infused intratumorally by convection-enhanced delivery (CED).
A number of studies have shown that escaping tumor cells migrate into the peritumor edema, often along blood vessels (BVs) and white matter tracts.Citation9,Citation10 In this study, we investigated the hypothesis that AuNPs could travel in the peritumor edema after they are infused into the primary tumor site and reach disseminated tumor cells. The AuNPs, because of their smaller size, might “catch up” to and surround migrating tumor cells. If the gold distribution was specific to the tumor, gold concentrations might be administered that would lead to significantly enhanced radiotherapy. A radiotherapy dose enhancement factor of ~4 (boosting 25 to 100 Gy) has been calculated to be possible if the gold concentration was 2% by weight.Citation11 Thus, the normal brain would be spared, since the electrons ejected from the gold only travel a short distance and tightly confine the boosted radiation dose.Citation12 Moreover, this approach would cause radiation-resistant cancer stem cells as well as dormant and drug-resistant tumor cells to be killed, since the targets of the ejected electrons and the free radicals produced are the DNA of the tumor cells and other sensitive cell machinery, rather than any particular metabolic pathway. This approach overcomes previous concerns, including the cost of an IV injection of gold, the loading of other organs and tissues, and whole-body clearance, as a much smaller amount of gold is needed locally; the gold will no longer have to pass through a leaky endothelium – it is administered directly to the edematous space in which the tumor cells reside.
F98 rat glioma cells first described by Kobayashi et alCitation13 were transduced with mCherry red fluorescent protein, and tumors were orthotopically grown in brains of isogeneic rats. IV-injected AuNPs were compared with AuNPs directly infused into F98 gliomas growing in the rat brain by CED. CEDCitation14–Citation16 is a very slow injection via a pump that better allows the injectate to travel in the interstitial space or edema than a more rapid injection. The small AuNPs were visualized in the light microscope after gold enhancement, and the coincidence of AuNPs and tumor cells was studied histologically. Edema was visualized with fluorescein isothiocyanate (FITC)-labeled anti-albumin.Citation17
The results show that IV-injected AuNPs localize more specifically to intracerebral tumor cells and to the tumor edema than do the directly infused AuNPs; this is likely due to the ability of IV-injected AuNPs to better target tumor cells through a disrupted endothelial barrier, whereas directly infused AuNPs appear to have less-specific localization to the tumor cells due to disrupted generalized diffusion possibly due to encountering diffusion barrier material in the interstitial space around tumor cells.
Materials and methods
All the work and study protocols performed were approved by the University of Connecticut Health Center’s Animal Care and Use Committee and adhered to the rules set forth in the Guide for the Care and Use of Laboratory Animals.
F98 cell line
The F98 cell line was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA).
Rat anesthesia
Rat anesthesia for surgical procedures was an intramuscular (im) injection of 54 mg/kg ketamine and 5 mg/kg xylazine.
Transduction of F98 glioma cells stably expressing mCherry red (F98CR)
One milliliter of 9 L medium (Dulbecco Modified Eagle’s Medium [DMEM] + 10% Penicillin/Streptomycin [Penn/Strep] + fungizone) containing 6 μM polybrene + 1/100 dilution of virus stock (107/mL) was added to 80% confluent F98 cells. After ~3 h, the wells were replaced with 9 L medium. This procedure was repeated on day 2. On day 3, the confluent cells were harvested and plated at ~1 cell/well (400 wells at 100 μL/well). Wells were screened by fluorescence microscopy. Highly expressing cells were cloned and used for the experiments below.
Stereotactic implantation of F98CR intracerebrally in syngeneic rats
After a midline incision to expose the scalp, a 0.6 mm burr hole was drilled at a point ~4 mm to the left of the midline along the serrated coronal suture of anesthetized Fisher 344 rats. The rats were then mounted on a David KOPF Instruments (KOPF) stereotactic frame and the head aligned and coordinates recorded such that the bregma and lambda sutures were in the same horizontal plane. A 27 G needle mounted vertically on the frame (Z = 0 when just touching both the bregma and lambda sutures) was then manually adjusted until it could be inserted into the burr hole and used to deliver 10,000 F98CR cells in 1 μL, 4.5–5 mm beneath the skull into the left striatum, over ~60 s. After an additional 30 s, the needle was removed slowly over 1 min. The wound on the top of the head was repaired with Vet Bond.
Direct stereotactic intratumoral infusion of AuNPs
Approximately 3 weeks after the implantation of 10,000 F98CR cells, the anesthetized rats were similarly positioned on the stereotactic frame using the same strategy and coordinates as described (Stereotactic implantation of F98CR intracerebrally in syngeneic rats). The AuNPs were delivered at the same depth as described (Stereotactic implantation of F98CR intracerebrally in syngeneic rats) also using a 27 G needle mounted on the stereotactic frame and attached to a BS800 syringe pump (Braintree Scientific, Braintree, MA, USA) set to deliver 10 μL of AuNPs (AuroVist 15 nm, 200 mg Au/mL; Nanoprobes, Inc, Yaphank, NY, USA) over 30 min. The needle was removed slowly over 1 min, and the skin wound was repaired with Vet Bond. AuNP infusions into the left hemisphere of control rat brains without brain tumors were performed similarly.
Femoral vein injection of AuNPs
Anesthetized Fisher 344 rats with advanced F98CR tumors were placed on a surgical board (Germfree Labs, Miami, FL, USA). A small, 3 mm incision was made in the groin region of appropriately positioned rats. A hemostat was used to tease apart soft tissue exposing the femoral vein, and ~80 mg of 15 nm AuNPs (AuroVist 15 nm) were injected IV in 0.4 mL.
Perfusion fixation of rats
Deeply anesthetized rats were perfused transcardially with normal saline followed by 4% buffered formalin pH 7.4 (Fisher Healthcare, Pittsburgh, PA, USA) 24 h after IV injection and direct intratumoral infusion of AuNps.
Rat euthanasia
The euthanasia procedure, CO2 narcosis, is done using the Euthanex system, a method approved in the Guidelines on Euthanasia by the American Veterinary Medical Association and by the University of Connecticut Health Center’s Animal Care and Use Committee.
Cryosections of rat brains
Formalin-perfused/fixed rat brains were excised and incubated in 4% buffered formalin for 4 h at 4°C. The brains were then transferred to 30% sucrose in PBS for 24 h for cryopreservation. The brains were cut coronally through the site of tumor implantation and arranged tumor-face down in cassettes containing Cryomatrix (Thermo Fisher Scientific, Waltham, MA, USA) prior to rapid freezing in 2-methylbutane cooled in dry ice. The cassettes were stored at −20°C prior to sectioning. Brain tissues were cut into 7-μm-thick sections using a Cryostat (cat #: CM 3050S; Leica Microsystems, Wetzlar, Germany) at approximately −22°C–26°C, and adhesive tape (cat #: 62800-72; CryoJane) was used to transfer the sections onto adhesive slides (cat #: 62800-X; CryoJane). Sections were cross-linked to the slides with a UV-light source (cat #: CM 3050S-3-1; Leica Microsystems). Slides were stored at 4°C overnight prior to antibody staining.
Immunofluorescence
Slides were rehydrated in PBS for 10 min at room temperature (RT) and blocked with 200 μL PowerBlock for 30 min prior to the addition of primary antibodies and then incubated overnight at 4°C in a humidified chamber. Primary antibodies used were as follows: A: sheep antirat albumin: FITC, polyclonal immunogloubin G (IgG) (1:100, cat #: 0220-2424; Bio-Rad Laboratories Inc., Hercules, CA, USA), B: rabbit anti-Iba-1 (1:500, cat #: 019-19741; Wako Pure Chemical Industries, Ltd., Osaka, Japan), C: mouse antirat TUJ1 (1:1,000, cat #: MO15013; Neuromics), D: anti-GFAP (1:500, cat #: Z0334; Dako Denmark A/S, Glostrup, Denmark), and E: goat antimouse/rat CD31 polyclonal IgG (1:100, cat #: AF3628-SP; R&D Systems, Inc., Minneapolis, MN, USA). Slides were washed 3× with PBS for 5 min each prior to the addition of secondary antibodies for 60 min at RT. Secondary antibodies used were as follows: I: goat antirabbit IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor 488 (1:500, cat #: A-11008; Thermo Fisher Scientific), and II: goat antimouse IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor 647 (1:200, cat #: A-21235; Thermo Fisher Scientific). The primary and secondary antibodies were paired as follows: A, not paired, edema; B:I, microglia; C:II, neurons; D:I, astrocytes; and E:II, endothelial cell. Finally, slides were washed 3× for 5 min each with PBS; coverslips were mounted in glycerol:PBS (1:1) containing a 1:1,000 dilution of Hoechst (DAPI) (cat #: 62249; Thermo Fisher Scientific).
Gold enhance
Gold enhance (GoldEnhance™ LM; Nanoprobes, Inc) was performed according to manufacturer’s recommendations GoldEnhance catalytically deposits additional gold on the small AuNPs so that their location can be seen in the light microscope. Background was reduced by transferring water-washed slides (~5 min) into 15% sodium sulfite solution (~5 min) and then water washed prior to cover slip mounting.
Fluorescent microscopy
Images were first taken of regions with red and green fluorescence using a Zeiss Axio Scan.Z1 microscope using the Zen 2.3 software. The slides were then gold enhanced and reimaged. The fluorescent- and gold-enhanced images were then aligned as individual layers in Photoshop, but most analyses were done on side-by-side comparisons of fluorescent- and gold-enhanced images.
Data analysis
Migration of tumor cells away from the main tumor mass
A straight line was drawn in Photoshop between the center of each migrating cell and the nearest defined edge of the tumor mass. The number of pixels in the new line was then proportionally divided by the number of pixels in the scale bar to calculate the metric migration distance.
Diameters of BVs containing tumor cells
Diameters were measured by drawing straight lines across the largest and the smallest diameters of each eligible BV. For each distinct BV, the number of pixels in each of its two diameter lines was averaged, and the average diameter was then proportionally divided by the number of pixels in the scale bar to calculate its metric diameter.
AuNP content of F98-containing BVs
F98-containing BVs were scored semiquantitiatively for AuNP content after gold enhancement. The level of AuNP observed was classified as negative (−), relatively low (+), and relatively high (++).
Results
F98 rat gliomas were confirmed to be invasive: tumor cells were found far from the main tumor mass, usually in association with BVs and peritumor edema
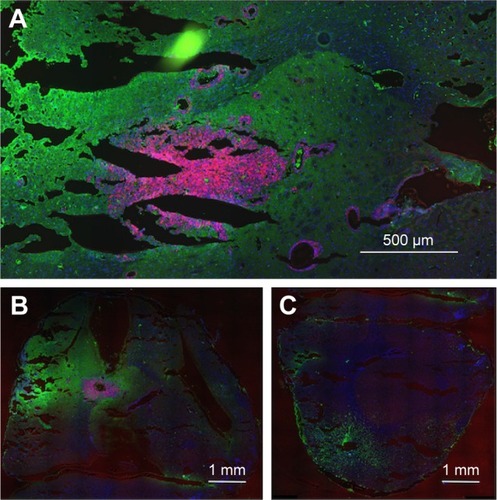
shows that large numbers of tumor cells (red) were found distal to the margins of the main tumor mass, usually around structures that resembled BVs. The tumor () measures ~1,100 × 600 μm. Tumor cells lining BV could be seen up to 1,100 μm away from the margins of the main tumor mass. Individual mCherry positive tumor cells could also be seen in these regions. In general, the tumor cells lining the BV were two to six cells thick, as determined by counting DAPI-stained nuclei. A total of 85% of the BV lined by tumor cells had areas ranging from 100 to 2,000 μm2 (median 363 μm), 13.5% were larger, between 2,000 and 10,000 μm2, and 1.5% were associated with structures up to 150,000 μm2. shows that the tumor containing left hemisphere had more FITC albumin stain (edema) than the right hemisphere () that is devoid of tumor cells. Bright FITC staining could be seen around the central tumor mass and the region around the tumor insertion needle track.
IV-injected AuNPs track with tumor
is the low power image of left hemispheres containing mCherry red expressing F98 tumors (two separate rats) and is the corresponding image, after gold enhancement. IV-injected AuNPs were found in the same brain areas as tumor and peritumor edema. The corresponding right hemisphere images () are devoid of both tumor and AuNPs. Left and right hemispheres are devoid of enhanced gold in saline-injected rats ().
Figure 2 IV-injected AuNPs colocalize with F98 tumors. (A) Rat 1, left hemisphere, fluorescence; (B) Rat 1, left hemisphere, gold enhance; (C) Rat 1, right hemisphere, fluorescence; (D) Rat 1, right hemisphere, gold enhance; (E) Rat 2, left hemisphere, fluorescence; (F) Rat 2, left hemisphere, gold enhance; (G) Rat 2, right hemisphere, fluorescence; and (H) Rat 2, right hemisphere, gold enhance. (A–G) mCherry red (tumor, red), anti-albumin (edema, green), DAPI (nuclei, blue), and anti-CD31 (blood vessels, white) and (B–F) gold enhanced (gold stained, black).
Abbreviations: AuNPs, gold nanoparticles; CD31, cluster of differentiation 31; IV, intravenous.
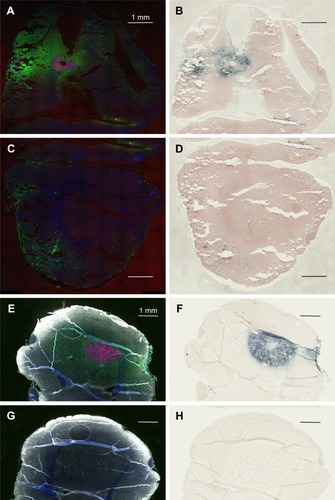
Figure 3 Complete lack of gold enhancement in saline-injected rats.
Notes: (A) Left hemisphere, fluorescence; (B) left hemisphere, gold enhance; (C) right hemisphere, fluorescence; and (D) right hemisphere, gold enhance. (A and C) mCherry red (tumor, red), anti-albumin (edema, green), DAPI (nuclei, blue), and anti-CD31 (blood vessels, white) and (B and D) gold enhanced (gold stained, black).
Abbreviation: CD31, cluster of differentiation 31.
IV-injected AuNPs track with tumor cells and tumor edema
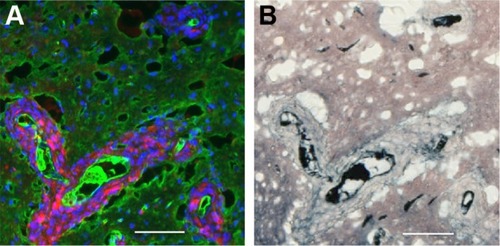
Tumor cells that have migrated away from the main tumor mass are usually seen to have gold associated with them after IV injection of AuNPs (). Interestingly, the gold is often seen lining the perimeter of the tumor cells creating a cobblestone-like appearance. This gold labeling is specific, as it is not seen in saline-injected controls (). shows tumor cells typically surrounding a BV that is clearly stained white with the endothelial marker anti-CD31. The most intense gold stain leaks out where there is edema. FITC-albumin staining (edema) is typically brightest around BVs associated with migrating tumor cells. This is shown in .
Figure 4 IV-injected AuNPs are associated with tumor cells lining blood vessels far from the main tumor mass.
Notes: Region far from the central tumor: (A) fluorescence and (B) gold enhanced. Saline control far from the central tumor: (C) fluorescence and (D) gold enhanced. (A and C) mCherry red (tumor, red), anti-albumin (edema, green), DAPI (nuclei, blue), and anti-CD31 (blood vessels, white) and (B and D) gold enhanced (gold stained, black).
Abbreviations: AuNPs, gold nanoparticles; CD31, cluster of differentiation 31; IV, intravenous.
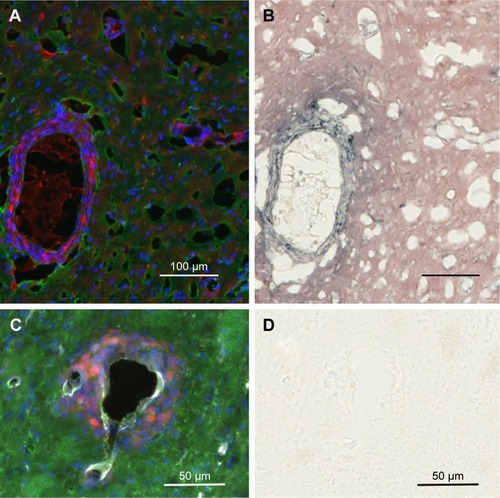
Figure 5 IV-injected AuNPs are found around tumor cells lining blood vessels and edema in peritumor region.
Notes: Peritumor region: (A) fluorescence, mCherry red (tumor, red), anti-albumin (edema, green), DAPI (nuclei, blue), and anti-CD31 (blood vessels, white) and (B) gold enhanced (gold stained, black). Scale bar, 50 μm.
Abbreviations: AuNPs, gold nanoparticles; CD31, cluster of differentiation 31; IV, intravenous.
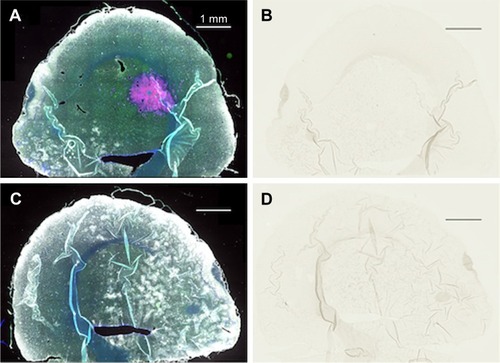
Direct stereotactic infusion of AuNPs into the established brain tumor
Inspection of the brains revealed that much of the infused dose could be found along the corpus callosum and a white matter tract called the external capsule, which is lateral to the corpus striatum (). The corresponding right hemisphere that did not receive an AuNP infusion did not show gold labeling (). shows the neuronal layer as revealed by TUJ-1 staining, and the position of the major white matter tracts containing gold (drawing, 6F) beneath the neuronal layer. Some of the AuNPs were also found in the region of the brain surrounding tumor (); however, most of the AuNPs did not penetrate the main tumor mass ().
Figure 6 Directly injected AuNPs are found in the white matter track and peritumor region but not in the main tumor region.
Notes: (A) Left hemisphere, fluorescence; (B) left hemisphere containing a brain tumor, gold enhanced; (B, inset) left hemisphere with no brain tumor, gold enhanced; (C) right hemisphere, fluorescence; (D) right hemisphere, gold enhanced; (D, inset) right hemisphere with no brain tumor; and (E) another animal, left hemisphere, stained for neurons (anti-Tuj 1, red fluorescence). (A and C) mCherry red (tumor, red), anti-albumin (edema, green), DAPI (nuclei, blue), and anti-CD31 (blood vessels, white) and (B, D, and F) gold enhanced (gold stained, black).
Abbreviations: AuNPs, gold nanoparticles; CD31, cluster of differentiation 31.
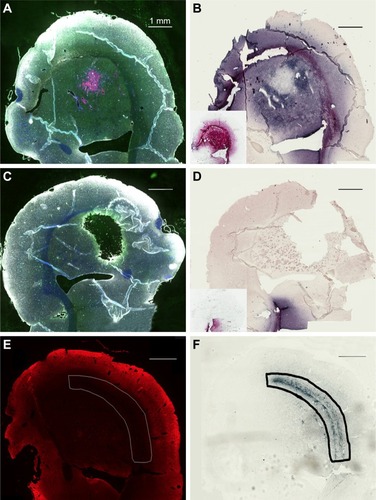
(inset control) shows the infusion of AuNPs into the left hemisphere of a rat brain without a tumor; AuNPs diffuse through the hemisphere. (inset control) shows the corresponding right hemisphere to the (inset).
Most of the directly infused gold does not reach the tumor cells that are surrounding BVs
Interestingly, there was a relative lack of AuNPs in or around the tumor cells surrounding BVs (). The data are summarized in pie charts (). The greatest difference between IV and directly injected AuNPs was seen in the main tumor mass, where the majority of F98 (+) BVs had AuNPs after IV injection but not after direct injection. The peritumor region also showed a higher correlation of AuNP++ with tumor-associated BVs.
Figure 7 Directly injected AuNPs are largely found in the edema surrounding the tumor cells in peritumor region but not in and around the tumor cells themselves.
Notes: (A and C) Left hemisphere and (B and D) left hemisphere, gold enhanced. (A–C) mCherry red (tumor, red), anti-albumin (edema, green), DAPI (nuclei, blue), and anti-CD31 (blood vessels, white) and (B and D) gold enhanced (gold stained, black).
Abbreviations: AuNPs, gold nanoparticles; CD31, cluster of differentiation 31.
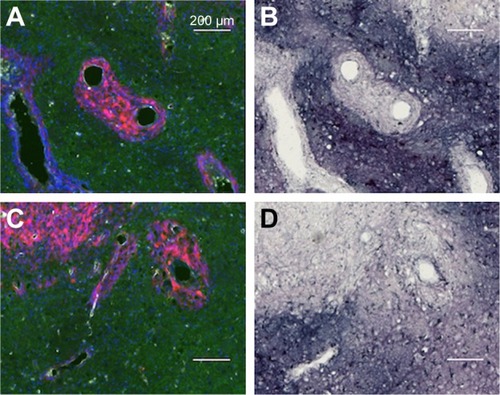
Figure 8 Quantification of the percentage of F98 containing BVs (F98 [+] BV) that also contained either a relatively large amount of AuNPs (AuNP++) or a relatively smaller amount of AuNPs (AuNP+) in the main tumor mass or in the peritumor region after either the direct infusion of AuNPs into the tumors or IV injection of AuNPs.
Abbreviations: AuNPs, gold nanoparticles; BV, blood vessel; IV, intravenous.
![Figure 8 Quantification of the percentage of F98 containing BVs (F98 [+] BV) that also contained either a relatively large amount of AuNPs (AuNP++) or a relatively smaller amount of AuNPs (AuNP+) in the main tumor mass or in the peritumor region after either the direct infusion of AuNPs into the tumors or IV injection of AuNPs.Abbreviations: AuNPs, gold nanoparticles; BV, blood vessel; IV, intravenous.](/cms/asset/fda45c64-81fe-4f95-8907-71e9e237e6cf/dijn_a_12193948_f0008_c.jpg)
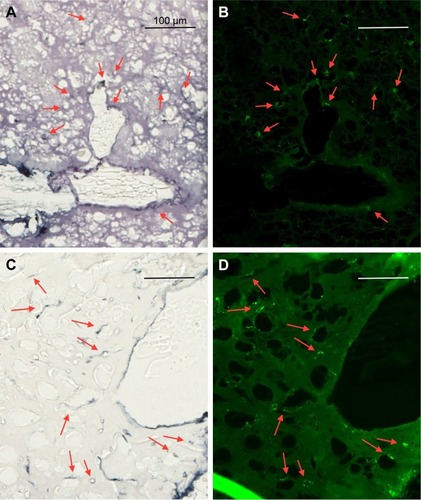
AuNPs that are infused directly into rat brains are taken up by microglia and astrocytes
shows the uptake of AuNPs into brain microglia and astrocytes after the injection of AuNPs directly into control brains (no tumor). Microglia, identified by Iba-1 staining, and astrocytes, identified by GFAP staining, were shown to have taken up the AuNPs identified by gold enhancement. The cells taking up AuNP, as shown in , are identified as microglia by virtue of the Iba-1 staining, as shown in . The cells taking up AuNP in are identified as astrocytes by virtue of the GFAP staining, as shown in . Arrows indicate correspondence between fluorescence and gold staining. The data show that both microglia and astrocytes stain for AuNPs.
Figure 9 Uptake of AuNPs into brain microglia and astrocytes after injection of AuNPs directly into control brains (non-tumor).
Notes: (A and C) Gold enhanced (gold stained, black); (B) fluorescence, anti-Iba-1 (microglia, green); and (D) fluorescence, anti-GFAP (astrocytes, green).
Abbreviations: AuNPs, gold nanoparticles; GFAP, glial fibrillary acidic protein.
Discussion
The F98 gliomaCitation13 is a nonimmunogenic, moderately radiation-insensitive, invasive, aggressive, and rat glioma model that is difficult to treat.Citation18 Its invasive properties are corroborated in in which tumor cells can be seen lining BVs at least one tumor diameter distal to the main tumor mass. This property of high-grade gliomas has been known for many yearsCitation19 and is reproduced by this model. It poses one of the central problems faced by the clinician when treating GBM. High-grade gliomas usually arise as unifocal lesions and are usually discovered when they are large (>3 cm in largest diameter) and occupy ~2% of the human brain. If tumor cells have already migrated one or more tumor diameters away from the main tumor mass, the volume of brain containing tumor is already too large for full surgical removal or highly effective radiation therapy. The standard of care chemotherapy using the best drug known, temozolomide, only provides 1–3 more months of life extension. Other therapies, including immunotherapyCitation20 and cytotoxic viral therapy,Citation21 may have the potential to kill these outlying tumor cells. We hypothesize that radiation therapy could contribute more to the control of the migrated cells if methods were developed, whereby therapeutic radiation doses could be precisely and selectively delivered to the outlying tumor cells.
AuNP-enhanced radiation therapy was first shown to effectively treat subcutaneous tumorsCitation5 and then gliomas in miceCitation8 using nontoxic, well-tolerated AuNPs developed by Nanoprobes, Inc. Theoretical calculations have shown that loading tumors with AuNPs to 2% w/w increases the radiation dose to the tumor by about fourfold.Citation11 Hainfeld et alCitation8 showed that loading advanced gliomas in mice to such levels is feasible due to the leakiness of vessels within these tumors.Citation22,Citation23 Achieving such high AuNP levels in tumors requires injecting a very large amount of AuNPs by the IV route. The AuNPs are designed to circulate for a long time (t1/2 = ~24 h) to maximize leakage into the tumor but ultimately most of the AuNPs are taken up by macrophages, predominantly in the liver and spleen.Citation8 Although AuNP doses >4 g/kg are well tolerated as determined by blood tests (complete blood count [CBC], Chem panel, weight data; Hainfeld et al 2018, Unpublished Results), the long-term persistence of the inert AuNPs in liver and spleen and skin discoloration issues might be drawbacks to their clinical use. For this reason, we have explored the possibility of directly depositing the AuNP into the region of the brain tumor in place of IV injection. Previously, it was shown by Bobyk et alCitation24 that the instillation of 250 μg AuNP (AuroVist) directly into the F98 glioma space by CED followed by a single 15 Gy radiation dose resulted in an 8-day extension of median survival compared to rats that received radiation only.
In this article, a histological examination of the distribution of AuNPs in the brains of rats after either IV injection or CED has been performed. After IV injection, AuNPs localized specifically to the region of the main tumor mass (). This result is similar to that seen with the dye Evans Blue that also localizes to the tumor region due to the leakiness of the vessels in the tumor.Citation9 However, whereas Evans Blue has a molecular weight of 961 Da but binds to albumin (68 kDa) and AuroVist (15 nm) is roughly equivalent to a 150 kDa protein molecule, they appear to leak through the compromised BBB (ie, blood tumor barrier [BTB]) similarly. particularly reveals that the tumor evinces a rim of heightened edema (rim of brighter green) and remarkably the AuNPs extend to the edge of this edematous band. Thus, passive leak, enhanced permeability and retention (EPR)Citation23 is seen to be a very effective mode of AuNP delivery to an F98 brain tumor and to the tumor edema. Tumor cells found around BVs proximal and distal to the main tumor mass () appear to be coated with AuNPs forming a cobblestone-like appearance that could be examined further by electron microscopy. Gold lining of tumor cells was not seen in saline-injected controls () indicating that the visually-enhanced AuNPS surrounding tumor cells are the result of IV AuNP injection. Recent theoretical calculations suggest that gold particles coating the surface of tumor cells could effectively increase the radiation dose to tumor cells, particularly in cases where the tumor cell nucleus is close to the cell membrane.Citation25
shows the distribution of AuNPs in the brain after direct instillation of 2 mg AuNPs in 10 μL by CED. A large arc of gold can be seen along the external capsule and corpus callosum – white matter tracts along which high-grade glioma cells also migrate. Some of the gold, also in the region of the main tumor mass, is concentrated into cell-like structures that have been identified as microglia and astrocytes (). These intensely stained cell-like structures also exist in the white matter tracts. Interestingly, the tumor cells themselves appear to have less gold staining than the surrounding region of brain ( and ). It is possible that the extracellular matrix of the tumorCitation26 prevents the access of AuNPs to the tumor cells when the AuNPs are introduced directly into the brain. Tumor malignancy grade is correlated with an increase in diffusion barriers for small molecules,Citation27 and there is evidence to suggest that collagen may act as a physical barrier to the diffusion of nanoparticles into solid tumors.Citation28
Recently, it has been stated that nanoparticles will not gain access to human tumors via the EPR effect since human tumors grow more slowly than rodent tumors and that the EPR effect is not valid in humans.Citation29 Much literature, however, supports a role for the EPR effect in many human tumors including gliomas. A human trial using radioactive liposomes showed exquisite tumor localization and uptake via the EPR effect.Citation30 Human gliomas are notoriously leaky and produce large amounts of edema, and numerous publications map brain tumors in relation to their edematous surrounding.Citation4,Citation30–Citation35 In fact, human brain tumors are diagnosed on the basis of gadolinium compounds leaking out of tumor BVs through the compromised BBB using MRI.
Our data have shown that after IV injection, AuNPs not only reach the majority of tumor cells in the central tumor mass but also access the peritumor edema, the growing tumor edge, and most of the glioma cells that have migrated far from the central tumor mass. Some migrating tumor cells may not be associated with AuNPs – but most are. This is consistent with the conclusions drawn from other studies not using AuNPs – that migrating tumor cells traveling along BVs alter the permeability from the tight blood–brain barrier to one that passes larger molecules. One study found that “encasing of vessels by gliomas disrupts astrocyte–vascular coupling” and “importantly, single glioma cells are sufficient to locally open the BBB”.Citation9 That would mean that even a single migrating glioma cell could be accessed with dose-enhancing nanoparticles. Why then are IV-injected chemotherapeutic drugs not very effective for humans’ brain tumors as well as the F98 model? Recently, Liu et alCitation36 showed that systemic BCNU + O6-benzylguanine only increased F98 median survival by 6 days but, when delivered in nanoparticles, increased median survival to 17 days. When nanoparticles were modified to contain integrin-binding RGD (Arginyl-Lysyl-Aspartic) motif (iRGD), median survival increased to 40 days. The notion that drugs and nanoparticles are poorly effective for brain tumors due to the intact blood–brain barrier (BBB) is prevalent in the literature, eg, Bhowmik et alCitation37 for BBB review. However, our data suggest that other factors besides an intact BBB need to be invoked to explain the ineffectiveness of systemically delivered drugs, especially for tumors showing extensive disruption of the BBB, such as GBM. For example, drugs typically have a short blood half-life limiting delivery. Nanoparticle constructs may have longer blood half-lives, but they may have other pharmacokinetic problems such as poor release of drug to an active form in the tumor. Other non-BBB factors that impede delivery include brain-to-blood efflux systems, enzymatic activity, plasma protein binding, poor blood flow, and the diffusion blocking tumor microenvironment.Citation38 It would seem that overcoming the BBB would serve to increase problems with therapeutic specificity, as leakage would occur all over the brain. Attempts to make drugs more lipophilic for better BBB penetration often have reduced effectiveness due to their higher uptake in peripheral tissues thus lowering blood concentration.Citation38 Our data suggest that the manifold efforts to increase the access of therapeutics through the BTB would seem to be especially helpful for the development of brain tumor therapies.
What are the implications for radiation therapy? We have already shown that IV-injected AuNPs significantly enhance radiation therapy of advanced gliomas using the Tu2449 glioma model.Citation8 Because of the significantly different gold distributions between IV and CED administration, a comparison of the efficacy of heavy atom-enhanced radiation therapy should be investigated. Since directly instilled AuNPs are concentrated into microglia and astrocytes, one might expect radiation toxicity as microglia and astrocytes would receive large radiation doses. Furthermore, the direct instillation of AuNPs into the brain tumor might be very different from the instillation of AuNPs into the brain after the surgical removal of the tumor. Hence, the potential therapeutic value of directly instilled AuNPs requires further investigation possibly using more therapeutically relevant model systems in which the AuNPs are deposited into the brain after the surgical removal of the tumor, for example. In addition, gadolinium nanoparticles (GdNPs) directly injected into U87 gliomas growing subcutaneously on the legs of mice have been shown to distribute heterogeneously, which might be expected to decrease their overall efficacy.Citation39
Significance and conclusion
The data show that IV-injected AuNP delivery is more specific and reaches migrating tumor cells better than direct infusion into the tumor region. IV-injected AuNPs may thus have a greater therapeutic benefit than the direct infusion of AuNPs suggesting a novel strategy for brain tumor radiation therapy that largely overcomes the main problems faced by radiation oncologists. Currently, it is not possible to irradiate both the main tumor mass and the large portions of brain that contain renegade tumor cells with curative radiation doses. Such irradiations would be too toxic to normal brain structures. However, if enough heavy atom nanoparticles can be loaded into tumor cells that are both in the main tumor mass and throughout the brain surrounding the BVs lined with migrating tumor cells, it might be possible to irradiate larger regions of the brain with lower radiation doses. Tumor cells throughout the brain that have accumulated AuNPs will have their radiation doses augmented. The greater the accumulation of heavy atom nanoparticles into and around the tumor cells, the greater the efficacy of metal-enhanced radiation therapy will be. The study presented here suggests that IV administration of heavy atom nanoparticles could be highly effective in treating gliomas and possibly other cancers, especially as new methods are devised to further increase nanoparticle delivery to tumors.Citation40,Citation41
Acknowledgments
This work was supported by an NIH SBIR Phase I grant 1R43CA192702-01A1 and the Connecticut Brain Tumor Alliance.
Disclosure
JFH is the part owner of Nanoprobes, Inc. The authors report no other conflicts of interest in this work.
References
- LuJCowperthwaiteMCBurnettMGShpakMMolecular predictors of long-term survival in glioblastoma multiforme patientsPLoS One2016114e015431327124395
- SchererHA critical review: the pathology of cerebral gliomasJ Neurol Psych194032147177
- HartmannMJansenOEgelhofTForstingMAlbertFSartorKEinfluß des Hirnödems auf das Rezidivwachstum maligner GliomeRadiologe199838119489539861656
- WatanabeMTanakaRTakedaNMagnetic resonance imaging and histopathology of cerebral gliomasNeuroradiology19923464634691436452
- HainfeldJFSlatkinDNSmilowitzHMThe use of gold nanoparticles to enhance radiotherapy in micePhys Med Biol20044918N309N31515509078
- HainfeldJFDilmanianFASlatkinDNSmilowitzHMRadiotherapy enhancement with gold nanoparticlesJ Pharm Pharmacol200860897798518644191
- HainfeldJFDilmanianFAZhongZSlatkinDNKalef-EzraJASmilowitzHMGold nanoparticles enhance the radiation therapy of a murine squamous cell carcinomaPhys Med Biol201055113045305920463371
- HainfeldJFSmilowitzHMO’ConnorMJDilmanianFASlatkinDNGold nanoparticle imaging and radiotherapy of brain tumors in miceNanomedicine20138101601160923265347
- WatkinsSRobelSKimbroughIFRobertSMEllis-DaviesGSontheimerHDisruption of astrocyte–vascular coupling and the blood–brain barrier by invading glioma cellsNat Comm20145115
- CuddapahVARobelSWatkinsSSontheimerHA neurocentric perspective on glioma invasionNat Rev Neurosci201415745546524946761
- ChoSHEstimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo studyPhys Med Biol20055015N163N17316030374
- LechtmanEChattopadhyayNCaiZMashoufSReillyRPignolJImplications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and locationPhys Med Biol201156154631464721734337
- KobayashiNAllenNClendenonNRKoL-WAn improved rat brain-tumor modelJ Neurosurg19805368088157003068
- AllardEPassiraniCBenoitJ-PConvection-enhanced delivery of nanocarriers for the treatment of brain tumorsBiomaterials200930122302231819168213
- DebinskiWTatterSBConvection-enhanced delivery to achieve widespread distribution of viral vectors: predicting clinical implementationCurr Opin Mol Ther201012664765321154156
- MunsonJMShiehACInterstitial fluid flow in cancer: implications for disease progression and treatmentCancer Manag Res2014196317328
- PietraGJohnsLConfocal- and electron-microscopic localization of FITC-albumin in H2O2-induced pulmonary edemaJ Appl Physiol19968011821908847300
- BarthRFRat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomasJ Neurooncol1998361911029525831
- YamamotoMUenoYHayashiSFukushimaTThe role of proteolysis in tumor invasiveness in glioblastoma and metastatic brain tumorsAnticancer Res2002226C4265426812553067
- ReardonDAFreemanGWuCImmunotherapy advances for glioblastomaNeuro Oncol201416111441145825190673
- CheemaTAWakimotoHFecciPEMultifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell modelProc Natl Acad Sci U S A201311029120061201123754388
- JainRMeasurements of tumor vascular leakiness using DCE in brain tumors: clinical applicationsNMR Biomed20132681042104923832526
- SengerDRGalliSJDvorakAMPerruzziCAHarveyVSDvorakHFTumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluidScience198321945879839856823562
- BobykLEdouardMDemanPPhotoactivation of gold nanoparticles for glioma treatmentNanomedicine2013971089109723643529
- SungWYeSJMcNamaraALDependence of gold nanoparticle radiosensitization on cell geometryNanoscale20179185843585328429022
- WadeARobinsonAEEnglerJRPetritschCJamesCDPhillipsJJProteoglycans and their roles in brain cancerFEBS J2013280102399241723281850
- VargováLHomolaAZámečníkJTichýMBenešVSykováEDiffusion parameters of the extracellular space in human gliomasGlia2003421778812594739
- MattixBMooreTUarovOEffects of polymeric nanoparticle surface properties on interaction with brain tumor environmentNano Life2013304134300325110523
- DanhierFTo exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?J Controlled Release2016244108121
- HarringtonKJMohammadtaghiSUsterPSGlassDPetersAMVileRGStewartJSEffective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomesClin Cancer Res20017224325411234875
- EngelhornTSavaskanNESchwarzMACellular characterization of the peritumoral edema zone in malignant brain tumorsCancer Sci2009100101856186219681905
- MoritaK-iMatsuzawaHFujiiYTanakaRKweeILNakadaTDiffusion tensor analysis of peritumoral edema using lambda chart analysis indicative of the heterogeneity of the microstructure within edemaJ Neurosurg2005102233634115739563
- DuboisLGCampanatiLRighyCD’Andrea-MeiraIGliomas and the vascular fragility of the blood brain barrierFront Cell Neurosci2014841825565956
- GillespieDLFlynnJRRagelBTArce-LarretaMKellyDATrippSRJensenRLSilencing of HIF-1α by RNA interference in human glioma cells in vitro and in vivoMethods Mol Biol200948728330119301653
- RiekenSHabermehlDMohrATargeting α ν β 3 and α ν β 5 inhibits photon-induced hypermigration of malignant glioma cellsRadiat Oncol20116113221978494
- LiuCYaoSLiXWangFJiangYiRGD-mediated core-shell nano-particles loading carmustine and O6-benzylguanine for glioma therapyJ Drug Targeting2017253235246
- BhowmikAKhanRGhoshMKBlood brain barrier: a challenge for effectual therapy of brain tumorsBioMed Res Intl20152015
- BanksWACharacteristics of compounds that cross the blood-brain barrierBMC Neurol20099Suppl1S319534732
- CarmonaARoudeauSL’HomelBPouzouletFBonnet-BoissinotSPrezadoYOrtegaRHeterogeneous intratumoral distribution of gadolinium nanoparticles within U87 human glioblastoma xenografts unveiled by micro-PIXE imagingAnalyt Biochem2017523505728223165
- MaedaHNakamuraHFangJThe EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivoAdvDrug Deliv Rev20136517179
- WilhelmSTavaresAJChanWCWAnalysis of nanoparticle delivery to tumoursNat Rev Mat2016116014