 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Gold nanoshells (NSs) have already shown great promise as photothermal actuators for cancer therapy. Integrin αvβ3 is a marker that is specifically and preferentially overexpressed on multiple tumor types and on angiogenic tumor neovasculature. Active targeting of NSs to integrin αvβ3 offers the potential to increase accumulation preferentially in tumors and thereby enhance therapy efficacy.
Methods
Enzyme-linked immunosorbent assay (ELISA) and cell binding assay were used to study the in vitro binding affinities of the targeted nanoconjugate NS–RGDfK. In vivo biodistribution and tumor specificity were analyzed using 64Cu-radiolabeled untargeted and targeted NSs in live nude rats bearing head and neck squamous cell carcinoma (HNSCC) xenografts. The potential thermal therapy applications of NS–RGDfK were evaluated by subablative thermal therapy of tumor xenografts using untargeted and targeted NSs.
Results
ELISA and cell binding assay confirmed the binding affinity of NS–RGDfK to integrin αvβ3. Positron emission tomography/computed tomography imaging suggested that tumor targeting is improved by conjugation of NSs to cyclo(RGDfK) and peaks at ~20 hours postinjection. In the subablative thermal therapy study, greater biological effectiveness of targeted NSs was implied by the greater degree of tumor necrosis.
Conclusion
The results presented in this paper set the stage for the advancement of integrin αvβ3-targeted NSs as therapeutic nanoconstructs for effective cancer therapy.
Introduction
Gold nanoshells (NSs) are core/shell particles comprising a gold shell and a dielectric silica core with peak plasmon resonances tunable to desired wavelengths by adjusting the relative core and shell thicknesses. At near infrared (NIR) wavelengths, light penetrates deep within the tissue (up to several centimeters), making this an optimal wavelength for biomedical applications. Indeed, NSs that absorb maximally in the NIR wavelength and efficiently convert incident light to heat can be readily synthesized (eg, a 120-nm core diameter and a 14-nm-thick shell result in an absorption peak between 780 nm and 800 nm). Biodistribution studies indicate that untargeted NSs passively accumulate in solid tumors through the enhanced permeability and retention (EPR) effect, do not enter healthy tissues to the same extent, and appear to be safe and well tolerated.Citation1,Citation2 This tumor-specific accumulation and NIR activation have been exploited for thermal ablation of solid tumors using NIR illumination.Citation3,Citation4 Studies have suggested that gold NSs are suitable candidates for clinical use in thermal therapy applications in cancer. To further enhance tumor selectivity, active targeting strategies have been pursued by many investigators.Citation5,Citation6 Given the relatively large size of NSs,Citation7 they are unlikely to penetrate deep into tumor parenchyma but are readily sequestered within the perivascular space. Therefore, active targeting to antigens specific to tumor cells is not expected to achieve substantially higher tumor penetration.
In contrast to tumor antigen-targeting strategies, tumor vascular targeting has some unique advantages. Integrins are a family of cell adhesion molecules consisting of two noncovalently bound transmembrane subunits (α and β) that form heterodimers.Citation8 The integrin αvβ3 binds to arginine– glycine–aspartic acid (RGD)-containing components of the extracellular matrix, blood, and cell surface proteins. Multiple lines of evidence suggest that this integrin heterodimer can serve as a target for tumor neovascular imaging and image-guided cancer therapies. First, integrin αvβ3 is significantly upregulated on endothelium during angiogenesis and on fast-growing solid tumor cells but not on quiescent endothelium and normal tissues.Citation9–Citation12 Second, RGD molecular probes have been developed for imaging integrin expression using different modalities, such as magnetic resonance imaging,Citation13 ultrasound,Citation14,Citation15 optical imaging,Citation16–Citation19 positron emission tomography (PET),Citation20–Citation22 and single-photon emission computed tomography (SPECT).Citation22,Citation23 Third, reagents that bind selectively to integrin αvβ3 can be designed by cyclizing peptides with selected sequences around the RGD and by synthesizing RGD mimics.Citation8 Fourth, in many cancers, higher numbers of tumor-associated vessels express integrin αvβ3 than vessels in normal tissue. Citation24 Lastly, inhibition of integrin αvβ3 using monoclonal antibodies, cyclic RGD peptide antagonists (), and peptidomimetics has been shown to induce endothelial cell apoptosis, inhibit angiogenesis, and increase endothelial monolayer permeability.Citation25,Citation26 Collectively, these studies suggest that integrin αvβ3 can serve as a selective, but not specific, target for imaging and therapy of cancer. Further, cyclic RGD peptides can serve as conduits to anchor probes on these integrins, and when combined with the EPR effect mediated by leaky vasculature and insufficient lymphatic drainage of tumors, additional accumulations of these conjugated probes may enhance this selectivity. In addition to RGD peptides, some research groups have synthesized small molecule antagonists based on RGD motifs to achieve comparable affinity with integrin αvβ3.Citation27–Citation29
Figure 1 Structures of cyclo (Arg-Gly-Asp-D-Phe-Lys) (RGDfK) (A) and the antagonist, 4-[2-(3,4,5,6-tetrahydro-pyrimidin-2-ylamino)-ethyloxy]benzoyl-2-(S)-[N-(3-aminoneopenta- 1-carbamyl)]-aminoethylsulfonylamino-h-alanine hydrochloride (IAC) (B).
![Figure 1 Structures of cyclo (Arg-Gly-Asp-D-Phe-Lys) (RGDfK) (A) and the antagonist, 4-[2-(3,4,5,6-tetrahydro-pyrimidin-2-ylamino)-ethyloxy]benzoyl-2-(S)-[N-(3-aminoneopenta- 1-carbamyl)]-aminoethylsulfonylamino-h-alanine hydrochloride (IAC) (B).](/cms/asset/3bfda11a-f64e-4350-93a9-92127267ebcb/dijn_a_15479_f0001_b.jpg)
In this study we compared the binding affinities to integrin αvβ3 of the cyclo(RGDfK) (cyclo(Arg-Gly-Asp-D-Phe-Lys)) peptide and the synthesized antagonist 4-[2-(3,4,5,6-tetrahydropyrimidin- 2-ylamino)-ethyloxy]benzoyl-2-(S)-[N-(3-aminoneopenta- 1-carbamyl)]-aminoethylsulfonylamino- h-alanine hydrochloride (IAC) ()Citation27 and their NS conjugates by three different (ELISAs). The binding specificity of NS– RGDfK to integrin αvβ3-expressing U87 tumor cells was then validated by silver staining assays. In vivo biodistribution and tumor specificity were analyzed using 64Cu-radiolabeled untargeted NSs and targeted NSs in live rats bearing head and neck squamous cell carcinoma (HNSCC) xenografts. Lastly, the relative ability of untargeted and targeted NSs to induce tissue damage after induction of subablative temperatures was assessed in a tumor xenograft model.
Material and methods
NS synthesis and conjugation
Gold–silica NSs were synthesized as previously described.Citation30 NS formation was assessed using an ultraviolet-visible (UV-VIS) spectrophotometer (U-0080D, Hitachi) and Zetasizer (Nano-ZS, Malvern Instruments, Malvern, UK). Integrin αvβ3-targeting peptide cyclo-(RGDfK) (molecular weight [MW] = 617.71) and control peptide cyclo-(Arg-Ala-Asp- D-Phe-Lys) (cyclo-(RADfK), MW = 603.68) were purchased from Peptides International (Louisville, KY, USA). Integrin αvβ3 antagonist IAC (MW = 622.14) was provided by Dr Narasimhan Danthi from the Molecular Imaging Laboratory at National Institutes of Health. Bifunctional ortho-pyridyldisulfide-polyethylene glycol 2000-N-hydroxysuccinimide ester (OPSS-PEG2k-NHS) was purchased from Nektar (Huntsville, AL, USA). Chelating agent S-2-(4- aminobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (DOTA) was purchased from Macrocyclics (Dallas, TX, USA). The free amine groups of RGDfK, RADfK, IAC, and DOTA were conjugated to OPSS–PEG–NHS by mixing 1:1 molar ratios overnight at pH 7.4 at room temperature (RT). The resulting OPSS–PEG conjugates were then mixed with NS solution (in 10 mM phosphate buffer, pH7) at 10,000:1 molar ratio overnight at RT on a shaker, allowing the OPSS group to conjugate to the gold surface of the particles. The mixture was centrifuged, and the supernatant with unconjugated OPSS– PEG was removed. The pellets of NS–peptide, NS–IAC, or NS–DOTA were resuspended in phosphate buffer and analyzed by a spectrophotometer and Zetasizer to determine the NS concentration and size, respectively, for further conjugation. To prepare NS with both RGDfK and DOTA on the surface, OPSS–PEG–RGDfK and OPSS–PEG–DOTA were mixed with NS at a 5000:1 molar ratio followed by the incubation and separation steps.
In vitro binding affinity assays
Competitive ELISA for RGDfK and IAC
Purified integrin αvβ3 protein (Chemicon International, Temecula, CA, USA) was coated on 96-well plates at 0.1 μg/well. After overnight incubation at 4°C, the plates were washed (washing buffer: 1X phosphate buffered saline [PBS] with 0.05% Tween 20) and then blocked with blocking buffer (1X PBS with 2% bovine serum albumin [BSA]) at RT for 2 hours. The blocking buffer was removed, and the plates were inoculated in triplicate with both RGDfK and IAC at a typical starting concentration of 0.125 μg/mL. Serial dilutions were prepared in the 96-well plates with blocking buffer supplemented with 0.9 mM of Ca2+ and 0.5 mM of Mg2+. After 30 minutes of incubation, 0.2 μg of biotinylated vitronectin solution (Molecular Innovations, MI, USA) was added to each well as a standard competitor. The plates were incubated at RT for 3 hours then washed, and the bound vitronectin was detected using 0.01 μg/well of avidin–horse radish peroxidase (HRP) conjugate (Pierce, Rockford, IL, USA). After washing, 100 μL of substrate solution was added to each well and then the luminescence was read (). The resulting luminescence signal intensity was plotted against RGDfK and IAC concentration and fitted by SigmaPlot 10.0 (SYSTAT Software, Inc., San Jose, CA, USA), and the concentrations of inhibitor producing 50% inhibition (IC50) of vitronectin binding to integrin αvβ3 were calculated based on the curve fittings.
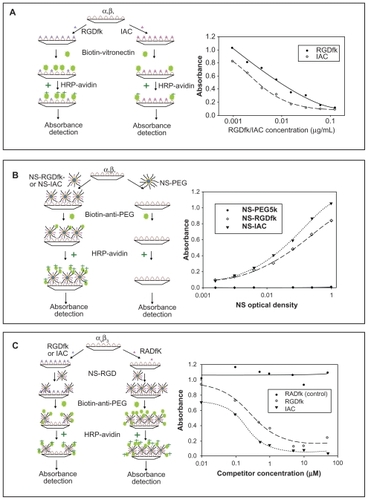
Figure 2 Schematic drawings (left) and results (right) of the three ELISAs. (a) Competitive ELISA of vitronectin against RGDfK and the antagonist IAC on an integrin αvβ3 coated plate. The absorbance of HRP-avidin is proportional to the amount of the biotinylated vitronectin on the plate. (b) Sandwich ELISA of NS-PEG5K (control), NS-RGDfK and NSIAC on an integrin αvβ3 coated plates. The absorbance of HR P-avidin is proportional to the amount of the blocker PEG 5K on NS and therefore, to the number of NS binding to the integrin αvβ3. (c) Competitive ELISA of NS-RGDfK against RGDfK and IAC on an integrin αvβ3 coated plates. The absorbance of HR P-avidin is proportional to the amount of the blocker PEG5K on NS and therefore, to the number of NS binding to the integrin αvβ3. All values are means of duplicate assays in the three ELISA experiments.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HRP, horse radish peroxidase; IAC, 4-[2-(3,4,5,6-tetrahydro-pyrimidin-2-ylamino)-ethyloxy]benzoyl-2-(S)- [N-(3-amino-neopenta-1-carbamyl)]-aminoethylsulfonylamino-h-alanine hydrochloride; NS, nanoshell; PEG, polyethylene glycol; RADfK, Arg-Ala-Asp-D-Phe-Lys; RGDfK, Arg-Gly-Asp-D-Phe-Lys.
![Figure 2 Schematic drawings (left) and results (right) of the three ELISAs. (a) Competitive ELISA of vitronectin against RGDfK and the antagonist IAC on an integrin αvβ3 coated plate. The absorbance of HRP-avidin is proportional to the amount of the biotinylated vitronectin on the plate. (b) Sandwich ELISA of NS-PEG5K (control), NS-RGDfK and NSIAC on an integrin αvβ3 coated plates. The absorbance of HR P-avidin is proportional to the amount of the blocker PEG 5K on NS and therefore, to the number of NS binding to the integrin αvβ3. (c) Competitive ELISA of NS-RGDfK against RGDfK and IAC on an integrin αvβ3 coated plates. The absorbance of HR P-avidin is proportional to the amount of the blocker PEG5K on NS and therefore, to the number of NS binding to the integrin αvβ3. All values are means of duplicate assays in the three ELISA experiments.Abbreviations: ELISA, enzyme-linked immunosorbent assay; HRP, horse radish peroxidase; IAC, 4-[2-(3,4,5,6-tetrahydro-pyrimidin-2-ylamino)-ethyloxy]benzoyl-2-(S)- [N-(3-amino-neopenta-1-carbamyl)]-aminoethylsulfonylamino-h-alanine hydrochloride; NS, nanoshell; PEG, polyethylene glycol; RADfK, Arg-Ala-Asp-D-Phe-Lys; RGDfK, Arg-Gly-Asp-D-Phe-Lys.](/cms/asset/9bb38dc4-b928-4ae0-9dde-942b572e6b74/dijn_a_15479_f0002_b.jpg)
Sandwich ELISA for NS–RGDfK and NS–IAC
Purified integrin αvβ3 protein was coated on 96-well plates as described previously. The plates were inoculated in triplicate with NS–PEG5K (control), NS–RGDfK, and NS–IAC, with a typical starting NS concentration of 1 optical density (OD). 1 OD NS is approximately 3~4 pM. Serial dilutions were prepared in the 96-well plates using blocking buffer containing Ca2+ and Mg2+ and then incubated at RT for 1 hour. The detection antibody biotin-anti-PEG (Epitomics, Burlingame, CA, USA), which recognizes the methoxy group of the PEG5K, was added to each well at 0.05 μg/well. The plates were incubated at RT for 1 hour then washed, and the bound biotin–anti–PEG was detected using avidin–HRP conjugate at 0.005 μg/well. After washing, 100 μL of substrate solution was added to each well and then the luminescence was read (). The resulting luminescence signal intensity was plotted against NS concentration and fitted by SigmaPlot 10.0. The half-maximal binding values (Kd) were calculated based on the curve fittings.
Competitive ELISA for NS–RGDfK and NS–IAC
Purified integrin αvβ3 protein was coated on 96-well plates as described previously. The plates were inoculated in triplicate with RADfK (control), RGDfK, and IAC with a typical starting concentration of 50 μM (100 μL/well). Serial dilutions were prepared in the 96-well plates using blocking buffer containing Ca2+ and Mg2+ and then incubated at RT for 0.5 hour, followed by addition of NS–RGDfK (0.5 OD, 100 μL/well) as competitor, and incubation for 2.5 hours. After extensive washing, the detection antibody biotin–anti-PEG (0.05 μg/well) was added to each well. The plates were incubated at RT for 1 hour then washed, and the bound biotin–anti-PEG was detected using avidin–HRP conjugate at 0.005 μg/well with 1-hour incubation. After washing, 100 μL of substrate solution was added to each well and then the luminescence was read (). The resulting luminescence signal intensity was plotted against NS concentration and fitted by SigmaPlot 10.0, and the IC50 values were calculated based on the curve fittings.
Cell adhesion assay
U87 human glioblastoma cell lines (ATCC, Manassas, VA, USA) were cultured under standard conditions and grown to 80% confluence in 48-well plates. Cells were rinsed three times in PBS (pH 7.4). NS–RGDfK and NS–PEG5K were mixed in the cell growth medium to obtain samples with 1.25 OD, which were then added to the U87-coated plate with 300 μL/well and incubated for 45 minutes at 37°C. Cells in PBS served as negative control. Cells were rinsed three times in PBS and fixed with 2% glutaraldehyde for 15 minutes and then rinsed three times with deionized water. A silver staining kit (Amersham Biosciences, Piscataway, NJ, USA) was used to stain cells, depositing silver on the NSs surfaces, to allow visualization of NS binding to cells via light microscopy with 40× magnification.
Radiolabeling and imaging
Radiolabeling procedure
All the radioisotope work was performed at the Department of Radiology, University of Texas Health Science Center (UTHSC)-San Antonio, TX, USA. 64CuCl2 (Washington University, St Louis, MO, USA) was diluted in 30 mM ammonia citrate buffer (pH 6.5). Then, ~1000 μCi of 64Cu was added to 200 μL of the solutions of NS–DOTA and NS with both RGDfK and DOTA (concentrations of NSs were about 1 nM) and incubated at 37°C for 90 minutes. Then the blocking agent PEG5K-SH (Nektar) was added to NS solution at 300,000:1 molar ratio and incubated at RT on a shaker for 1 hour. The mixtures were then centrifuged to remove unconjugated 64Cu and PEG5K. The 64Cu activity of the supernatant and pellet was measured in a dose calibrator (Atomlab 100, Biodex, Shirley, NY, USA). Labeling efficiencies were calculated by using the equation [activity in pellet/ (activity in supernatant + activity in pellet)] × 100.
Animal model and in vivo PET imaging
An HNSCC xenograft model in nude rats was established via subcutaneous inoculation of the HNSCC cell line SCC-4.Citation31 Animal experiments with radioactive agents were carried out at UTHSC according to the NIH Animal Use Guidelines. On the day of imaging, the average tumor volume of the nude rats was 1.2 cm3. During all procedures, the nude rats (~150 g) were anesthetized with intraperitoneal Avertin® (400 mg/kg) by intraperitoneal injection. 64Cu-NS (~800 μCi of 64Cu) and 64Cu-NS–RGDfK (~600 μCi of 64Cu) were injected manually into the rats’ tail veins at about 0.5 mL per minute. PET imaging was performed at 1 hour, 4 hours, 20 hours, and 44 hours postinjection of radiolabeled NS with the FLEX X-PET/CT/SPECT scanner (Gamma Medica-Ideas, Inc., Northridge, CA, USA) followed by CT image acquisition (80 kVp, 0.25 mA, 256 projections).
In vivo analysis of thermoablation efficacy
In a subcutaneous colorectal cancer xenograft model using HCT116 human tumor cells in nude mice, 100 μL (~0.4 nM) of NS–PEG5K and NS–RGDfK solutions were injected via the tail vein of the animals (two mice in each group). After 24 hours, thermocouple probes were inserted into the center and the base of the tumor at about 5 mm depth from the surface. T-type (copper-constantan) needle thermocouple probes (HYP1-30-1/2-T-G-60-SMP-M, Omega Engineering) were used. The probe alone did not generate heat as tested by irradiating the probe with the laser. The NIR laser power was set at values predicted to result in subablative temperatures based on an Arrhenius modelCitation32 of thermal injury (47°C for 10 minutes was predicted to achieve 50% cell kill with untargeted NSs, a level that would allow comparison with agents that potentially increased the efficacy of thermoablation). With a 1.2 W 75% duty cycle, 808 nm NIR laser and a spot size of 1 cm, consistent temperature elevation to 47°C was achieved within 5 minutes at the center of the tumor and was maintained steadily for 10 minutes in the untargeted NS group. Tumors were excised 2 hours after thermal therapy following pretreatment with NS–PEG5K and conjugated NS–RGDfK and stained with hematoxylin and eosin. Tissues were observed by Olympus DP 70 camera fitted on a Nikon Microphot-FXA microscope, and images captured using Olympus DP software.
Results and discussion
NSs used in this study were manufactured to comprise a silica core (~120 nm in diameter) and a gold shell (8~10 nm) to absorb light at NIR wavelengths. The UV–VIS spectrum confirmed the NS absorbance peak at ~760 nm. Zetasizer measurements confirmed the NS diameter of 140 nm and zeta potential of −50 mV. By adapting a method that has been developed for conjugation of molecules with amine groups,Citation2,Citation4 NSs were conjugated to cyclic RGD peptides, IAC, or DOTA through bifunctional PEG. Dynamic lightscattering measurements showed that after surface modification the NS size increased to ~170 nm in diameter, and zeta potential changed to around −5 mV.
The binding affinities and specificities of RGDfK and IAC for integrin αvβ3 receptor were measured by a competitive ELISA. Serial dilutions of these peptides were assayed for their ability to compete with biotinylated vitronectin for the immobilized integrin αvβ3 receptor. After obtaining the curve fittings by SigmaPlot (), the calculated IC50 concentration of inhibitors RGDfK and IAC for vitronectin binding to integrin αvβ3 were 4.05 nM and 3.38 nM, respectively. These results confirmed the relatively comparable binding affinities of IAC and RGDfK to integrin αvβ3 protein and closely matched the reported IC50 of 2.94 ± 0.19 nM for IAC by Burnett et al.Citation27 The lower IC50 of RGDfK than that noted by Burnett et al for cyclo-(RGDfV) (6.41 ± 0.49 nM) might be due to the different amino acids in the two peptides valine (V) and lysine (K), with the amine group in lysine potentially affording greater protein binding.
Subsequently, a sandwich ELISA was used to compare the binding affinities of NS–RGDfK, NS–IAC, and NS– PEG5K (control, no targeting molecule). Serial dilutions of these agents were added to an integrin αvβ3-coated plate prior to washing and detection of bound NSs. As NSs are not readily detected on ELISA plates, the PEG coating was used as a surrogate for their presence. This sandwich ELISA therefore employed a biotinylated anti-PEG antibody that recognizes the methoxy group of the PEG5K. The antibody was subsequently detected by HRP–avidin. shows that control NS–PEG5K had no detectable binding to the integrin αvβ3, whereas NS– RGDfK and NS–IAC had increasing binding to the integrin αvβ3 with increasing concentration. The Kd values for NS–RGDfK and NS–IAC were 0.267 OD and 0.288 OD, respectively, calculated based on the SigmaPlot curve fitting.
In the third competitive ELISA, serial dilutions of the competitors RGDfK, IAC, and RADfK (negative control) were pre-incubated in separate wells of an integrin αvβ3- coated plate. The targeted NS–RGDfK (concentrated to give 0.5 OD at 800 nm) particles were then added to all the wells to compete with RGDfK or IAC for binding. Biotin-labeled anti-PEG antibody and HRP–avidin was used for detection as before. shows that negative control RADfK did not have any detectable competition with NS–RGDfK, whereas RGDfK and IAC did compete with NS–RGDfK for binding to the integrin. The IC50s of RGDfK and IAC were 0.267 and 0.197 μM, respectively. Because of the relatively small difference in binding affinity between RGDfK and IAC for all three ELISAs, further in vitro and in vivo experiments were performed with NS–RGDfK.
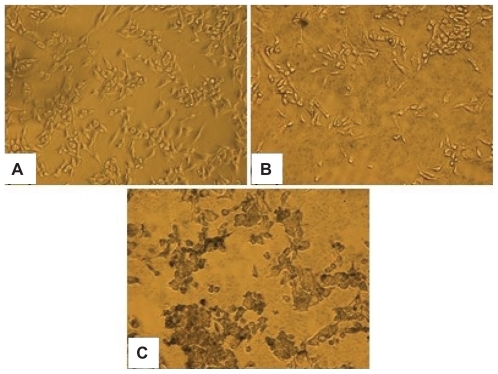
The in vitro binding affinity and specificity of NS–RGDfK was tested using U87 glioblastoma cells that express abundant integrin αvβ3. Cells were incubated with PBS, NS–PEG5K, and NS–RGDfK and treated with a silver staining kit. shows that the two controls (PBS and NS–PEG5K) had no detectable binding to cells, and NS–RGDfK bound specifically and avidly to integrin αvβ3-overexpressing cells. For all the in vitro affinity studies, we found that a small amount of Ca2+ (0.9 mM) and Mg2+ (0.5 mM) must be added to the buffer or cell growth medium for RGDfK to bind to the integrin αvβ3. Hu et al have reported how Ca2 and Mg2+ regulate ligand binding to β3 integrins.Citation33 They concluded that low concentrations of Ca2+ (μM) promoted the binding of the ligand, whereas higher concentrations of Ca2+ (mM) blocked the binding. This study revealed that there are two classes of cation binding sites on the β3-containing integrins. The first class of sites promotes ligand binding to integrin when occupied by divalent cations, and the second class of sites is allosteric to the ligand binding site and highly specific for Ca2+ and when occupied increases the dissociation rate between RGD and integrin.Citation34
Figure 3 Silver staining of integrin αvβ3 overexpressing U87 glioma cancer cells with PBS (A), NS–PEG5K (B), and NS–RGDfK (C) solutions. Images were taken by light microscope with 40× magnification.
Abbreviations: NS, nanoshell; PBS, phosphate buffered saline; PEG, polyethylene glycol; RGDfK, Arg-Gly-Asp-D-Phe-Lys.
After confirming biochemical and in vitro binding affinities of NS–RGDfK, we evaluated its stability, biodistribution, and efficacy in imaging integrin αvβ3 in a solid tumor model in vivo by dual labeling NSs with a radionuclide (64Cu) and the RGDfK peptide. We have used both 64Cu and 111In for radiolabeling of gold NSs.Citation35,Citation36 The PET and SPECT images of the rats showed that both 64Cu and 111In appeared to be useful for tracking the in vivo distribution of NSs. The biodistribution data determined with 111In were very close to those of 64Cu, but image quality was better with the 64Cu. This is likely due to the more efficient detection of the 64Cu with the PET ring coincidence imaging system than with the SPECT rotating camera system. Therefore, PET imaging with 64Cu was used for the NS imaging studies described in this paper.
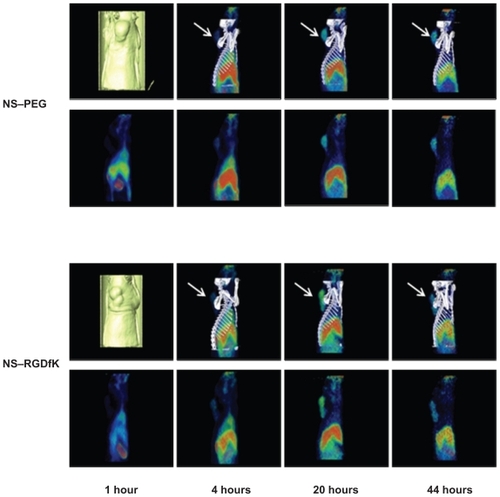
The 64Cu radiolabeling efficiencies were 81.3% and 84.2% for NS and NS–RGDfK, respectively. Using PET/CT imaging, we monitored the in vivo distribution of radiolabeled NS– PEG and NS–RGDfK at various time points after intravenous injection through the tail vein into nude rats with HNSCC xenografts. shows PET/CT fusion images (top) and PET sagittal images (bottom) of rats at 1 hour, 4 hours, 20 hours, and 44 hours postinjection. Our previous studies suggested that the radioisotope was not cleaved from the NSs, so we believe that our images and biodistribution data truly reflect the location of NSs.Citation2 Neither NS–PEG nor NS–RGDfK showed obvious accumulation within tumors at 1 hour postinjection. However, NS–RGDfKs progressively accumulated in the tumor, beginning at 4 hours postinjection and reaching an apparent maximum accumulation at approximatly 20 hours postinjection, followed by gradual decline in tumor-specific accumulation by 44 hours postinjection, whereas untargeted NS accumulation in tumors was less pronounced during each of these time points. These results suggest that RGDfK conjugation improves NS accumulation within tumors and that laser treatment for photothermal applications should optimally occur at ~20–44 hours postinjection. The animals were sacrificed at 46 hours postinjection, and major organs were collected for analysis of specific tissue accumulations. The amount of NSs in the tissue samples as estimated by gamma well counter was expressed as a percentage of injected dose per organ (see ). The gold content per gram of tissue was also estimated by neutron activation analysis (NAA) (see ). These results confirm the expected accumulation of NSs within the liver and spleen and clearance from the tumor in both targeted and untargeted NS-treated animals at a late time point.
Figure 4 PET/CT fusion and PET sagittal images of two nude rats bearing HNSC xenograft at 1 hour, 4 hours, 20 hours, and 44 hours post tail-vein injection of 64Cu-NS and 64Cu-NS–RGDfK. The arrows are pointing at the tumor.
Abbreviations: CT, computed tomography; HNSC, head and neck squamous cell carcinoma; NS, nanoshell; PEG, polyethylene glycol; PET, positron emission tomography; RGDfK, Arg-Gly-Asp-D-Phe-Lys.
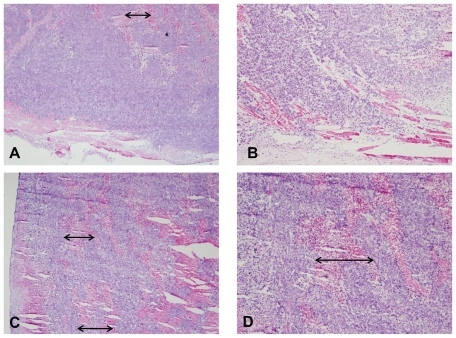
In addition to potential imaging applications of NS– RGDfK, the feasibility of using these targeted probes for improved in vivo photothermal efficiency was also investigated. Assuming the targeted NSs were more biologically effective as thermal therapy conduits, we expected to observe more necrosis in tumors laden with these particles than the untargeted particles if a subablative thermal treatment was delivered. First, an in vivo model for subablative thermal treatment was created. This was based on an Arrhenius mathematical prediction modelCitation32 of thermal injury, which predicted that a sustained stable temperature of 47°C for 10 minutes would result in ~50% cell death. Next, in a cohort of nude mice harboring ~1 cm subcutaneous HCT116 colorectal cancers on their right thighs, laser settings that would result in these temperatures were established. The NSs were injected intravenously and subablative thermal treatments were conducted 24 hours after the injection. Finally, the effect of such treatment was compared between two mice injected with similar particle concentrations (~40 fmol) of untargeted and targeted NSs. The targeted NSs treatment group had more profound hemorrhage during treatment than the untargeted NSs treatment group. Greater amounts of hemorrhagic and necrotic debris were evident within the tumor core upon sectioning of the tumors from the targeted treatment group than the untargeted treatment group. As noted in , the lower magnification images (A and C, 4×) demonstrate greater necrosis in the vascular-targeted group, suggestive of more significant vascular disruption. The results suggest that greater tumor and tumor vascular specificity via the active targeting technique improves the efficacy of thermal therapy. The observed enhancement in therapeutic efficacy is attributed to the higher tumor concentrations of targeted NSs as well as the possibly more intense focal temperature hot spots generated in the vicinity of vascular- targeted NSs, resulting in more vascular disruption.
Figure 5 Hematoxylin and eosin stain of tumors excised immediately following sub-ablative thermal therapy using untargeted (A and B) NSs and vascular-targeted (C and D) NSs. Images on the left are lower magnification images (A and C, 4×), whereas images on the right are higher magnification images (B and D, 10×). Arrows represent the width of areas of necrosis observed.
Abbreviation: NSs, gold nanoshells.
Conclusion
In this work we investigated the binding affinity of cyclo- (RGDfK) peptide and the antagonist IAC, as well as their NS conjugates to the integrin αvβ3 by ELISA. We found that IAC has similar affinity to integrin αvβ3 as RGDfK in the competitive ELISA and the sandwich ELISA and that the difference is not as significant as previous reports.Citation27 Because of the relatively small difference in the binding affinity to integrin αvβ3 between NS–RGDfK and NS–IAC, we pursued further experiments with NS–RGDfK. We confirmed potent, selective, and specific NS–RGDfK adhesion to integrin αvβ3-expressing U87 tumor cells by silver staining. We also documented in vivo tumor-specific accumulation of conjugated NSs and nonspecific accumulation of probe within the liver and spleen at four different time points. Biodistribution analysis of untargeted and targeted NSs by measurement of residual radioactivity and NAA confirmed that both targeted and untargeted NSs are cleared from the circulation by the liver and spleen and suggested that integrin αvβ3 targeting using RGDfK improved NS accumulation in tumors. Lastly, we confirmed increased biological efficacy of conjugated NSs for potential thermoablation applications.
As demonstrated in multiple cancers, integrin αvβ3 is a crucial cell adhesion and signaling molecule that promotes the proliferation, survival, drug-resistant properties, and metastatic potential of cancer cells. This specific and preferential overexpression noted across multiple tumor types makes targeting of integrin αvβ3 for imaging and therapy particularly appealing, as it offers a class solution to the challenge posed by heterogeneity of tumor-specific antigens across diverse tumor types. Furthermore, integrin αvβ3 localization to angiogenic neovascular endothelia offers greater targeting potential for larger biomolecules and nanoparticles that are less capable of penetrating deep into tumor parenchyma across interstitial spaces. We demonstrate here that integrin αvβ3-targeted NSs serve as potent theranostic agents that facilitate enhanced imaging and treatment of tumors.
Supplementary data
Biodistribution studies
All the animals were used in the imaging studies were anesthetized and then sacrificed at 46 hours postinjection. The organs of interest were removed and wet weighed. The radioactivity in the tissues was measured using a γ-counter (Wallac 1480, Perkin Elmer Life Sciences, Boston, MA, USA). The radioactivity of the tissue samples was calibrated against a known aliquot of the injectate. The percentage injected dose per organ (%ID/organ) values were calculated using the following equation
.
Major organs that showed high concentrations of other types of nanoparticles in previous biodistribution studiesCitation1–Citation4 were also subjected to neutron activation analysis (NAA). Portions of tumor, spleen, liver, lung, and kidney tissue were collected, wet weighed, and allowed to decay for 2 weeks. The samples were placed into precleaned and labeled polyethylene irradiation vials. After the wet sample weight was calculated and recorded, the samples were covered and dried under a heat lamp for 48 hours before delivery into the high temperature nuclear reactor core for NAA gold measurements. The procedure of NAA for trace gold quantification in animal tissues has been described elsewhere.Citation5 The data were reported as gold mass versus the original wet sample mass.
Table S1 Biodistribution data of 64Cu-NS and 64Cu-NS–RGDfK in HNSC xenograft-bearing nude rats at 46 hours postinjection.
Figure S1 Neutron activation analysis for concentration of gold in tumor, spleen, liver, lung, and kidney at 46 hours postinjection of NS–PEG (control) and NS–RGDfK.
Acknowledgments
This work was supported by NIST ATP Cooperative Agreement Number 70NANB4H3040 and NIH 1R21CA133691. We thank Pharmaceutical Research and Manufacturers of America Foundation for their support of Dr Xie’s work. We thank Dr Narasimhan Danthi at Molecular Imaging Laboratory of National Institutes of Allergies and Infectious Diseases for kindly providing us with the IAC. We also thank Dr Ting Tung A Chang for his imaging technical support.
References
- JamesWDHirschLRWestJLO’NealPDPayneJDApplication of INAA to the build-up and clearance of gold nanoshells in clinical studies in miceJ Radioanal Nucl Chem20072712455459
- XieHWangZJBaoAGoinsBPhillipsWTIn vivo PET imaging and biodistribution of radiolabeled gold nanoshells in rats with tumor xenograftsInt J Pharm201039532433020540999
- O’NealDPHirschLRHalasNJPayneJDWestJLPhoto-thermal tumor ablation in mice using near infrared-absorbing nanoparticlesCancer Lett2004209217117615159019
- HirschLRStaffordRJBanksonJANanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidanceProc Natl Acad Sci U S A200310023135491355414597719
- CaiWShinDWChenKPeptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjectsNano Lett20066466967616608262
- HuangYFChangHTTanWCancer cell targeting using multiple aptamers conjugated on nanorodsAnal Chem200880356757218166023
- DiagaradjanePShettyAWangJCModulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: characterizing an integrated antihypoxic and localized vascular disrupting targeting strategyNano Lett2008851492150018412402
- RuoslahtiERGD and other recognition sequences for integrinsAnnu Rev Cell Dev Biol1996126977158970741
- HoodJDChereshDARole of integrins in cell invasion and migrationNat Rev Cancer2002229110012635172
- XiongJPStehleTDiefenbachBCrystal structure of the extracellular segment of integrin alpha Vbeta3Science2001294554133934511546839
- CairnsRAKhokhaRHillRPMolecular mechanisms of tumor invasion and metastasis: an integrated viewCurr Mol Med20033765967114601640
- Felding-HabermannBIntegrin adhesion receptors in tumor metastasisClin Exp Metastasis200320320321312741679
- SchmiederAHWinterPMCaruthersSDMolecular MR imaging of melanoma angiogenesis with alphanubeta3-targeted paramagnetic nanoparticlesMagn Reson Med200553362162715723405
- EllegalaDBLeong-PoiHCarpenterJEImaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v) beta3Circulation2003108333634112835208
- DaytonPAPearsonDClarkJUltrasonic analysis of peptide- and antibody-targeted microbubble contrast agents for molecular imaging of alphavbeta3-expressing cellsMol Imaging20043212513415296677
- TkachenkoAGXieHLiuYCellular trajectories of peptidemodified gold particle complexes: comparison of nuclear localization signals and peptide transduction domainsBioconjug Chem200415348249015149175
- WangWKeSWuQNear-infrared optical imaging of integrin alphavbeta3 in human tumor xenograftsMol Imaging20043434335115802051
- AchilefuSBlochSMarkiewiczMASynergistic effects of light-emitting probes and peptides for targeting and monitoring integrin expressionProc Natl Acad Sci U S A2005102227976798115911748
- ChengZWuYXiongZGambhirSSChenXNear-infrared fluorescent RGD peptides for optical imaging of integrin alphavbeta3 expression in living miceBioconjug Chem20051661433144116287239
- ChenXContiPSMoatsRAIn vivo near-infrared fluorescence imaging of integrin alphavbeta3 in brain tumor xenograftsCancer Res200464218009801415520209
- HaubnerRKuhnastBMangC[18F]Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimatesBioconjug Chem2004151616914733584
- JanssenMLOyenWJDijkgraafITumor targeting with radiolabeled alpha(v)beta(3) integrin binding peptides in a nude mouse modelCancer Res200262216146615112414640
- SadeghiMMKrassilnikovaSZhangJDetection of injury-induced vascular remodeling by targeting activated alphavbeta3 integrin in vivoCirculation20041101849015210600
- MaxRGerritsenRRNooijenPTImmunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomasInt J Cancer19977133203249139861
- CaiWChenXAnti-angiogenic cancer therapy based on integrin alphavbeta3 antagonismAnticancer Agents Med Chem20066540742817017851
- FriessHLangrehrJMOettleHA randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancerBMC Cancer2006628517156477
- BurnettCAXieJQuijanoJSynthesis, in vitro, and in vivo characterization of an integrin alpha(v)beta(3)-targeted molecular probe for optical imaging of tumorBioorg Med Chem200513113763377115863003
- HaubnerRWesterHJBurkhartFGlycosylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokineticsJ Nucl Med200142232633611216533
- HutchinsonJHHalczenkoWBrashearKMNonpeptide alphavbeta3 antagonists. 8. In vitro and in vivo evaluation of a potent alphavbeta3 antagonist for the prevention and treatment of osteoporosisJ Med Chem200346224790479814561098
- OldenburgSJJacksonJBWestcottSLHalasNJInfrared extinction properties of gold nanoshellsAppl Phys Lett19991112897
- BaoAPhillipsWTGoinsBSetup and characterization of a human head and neck squamous cell carcinoma xenograft model in nude ratsOtolaryngol Head Neck Surg2006135685385717141073
- WelschAJvan GemertMJCOptical-Thermal Response of Laser-Irradiated TissueNew YorkPlenum Press1995
- RamosOHKauskotACominettiMRA novel alpha(v)beta (3)-blocking disintegrin containing the RGD motive, DisBa-01, inhibits bFGF-induced angiogenesis and melanoma metastasisClin Exp Metastasis2008251536417952617
- HuDDBarbasCFSmithJWAn allosteric Ca2+ binding site on the beta3-integrins that regulates the dissociation rate for RGD ligandsJ Biol Chem19962713621745217518702970
- XieHWangZBaoAGoinsBPhillipsWTRadiolabeled Gold Nanoshells for In Vivo Imaging: Example of Methodology for Initial Evaluation of Biodistribution of a Novel NanoparticleNanoimaging-Biomedical NanotechnologyPan Stanford Publishing Pte Ltd in press2010
- XieHWangZBaoAGoinsBPhillipsWTColemanCLRadiolabeling of Gold Nanoshells with Cu-64 and In-111 for PET and SPECT Imaging in RatsProceedings of the Nanotech Conference and Expo2009 May 3–7Houston Texas2009214
References
- AkiyamaYMoriTKatayamaYNiidomeTThe effects of PEG grafting level and injection dose on gold nanorod biodistribution in the tumor-bearing miceJ Control Release20091391818419538994
- CaiWShinDWChenKPeptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjectsNano Lett20066466967616608262
- LiuZCaiWHeLIn vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in miceNat Nanotechnol200721475218654207
- XieHWangZJBaoAGoinsBPhillipsWTIn vivo PET imaging and biodistribution of radiolabeled gold nanoshells in rats with tumor xenograftsInt J Pharm201039532433020540999
- JamesWDHirschLRWestJLO’NealPDPayneJDApplication of INAA to the build-up and clearance of gold nanoshells in clinical studies in miceJ Radioanal Nucl Chem20072712455459
Disclosure
The authors report no conflicts of interest in this work.