 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The combination of Au-metallic-NPs and CNTs are a new class of hybrid nanomaterials for the development of electrochemical biosensor. Concentration of Au(nanoparticles [NPs]) in the electrochemical biosensor is crucial for the efficient charge transfer between the Au-NPs-MWCNTs modified electrode and electrolytic solution.
Methods
In this work, the charge transfer kinetics in the glassy carbon electrode (GCE) modified with Au(NPs)–multiwalled carbon nanotube (MWCNT) nanohybrid with varied concentrations of Au(NPs) in the range 40–100 nM was studied using electrochemical impedance spectroscopy (EIS). Field emission scanning electron microscopy and transmission electron microscopy confirmed the attachment of Au(NPs) on the surface of MWCNTs.
Results
The cyclic voltammetry and EIS results showed that the charge transfer mechanism was diffusion controlled and the rate of charge transfer was dependent on the concentration of Au(NPs) in the nanohybrid. The formation of spherical diffusion zone, which was dependent on the concentration of Au(NPs) in nanohybrids, was attributed to result in 3 times the increase in the charge transfer rate ks, 5 times increase in mass transfer, and 5% (9%) increase in Ipa (Ipc) observed in cyclic voltammetry in 80 nM Au(NP) nanohybrid-modified GCE from MWCNT-modified GCE. The work was extended to probe the effect of charge transfer rates at various concentrations of Au(NPs) in the nanohybrid-modified electrodes in the presence of Escherichia coli. The cyclic voltammetry results clearly showed the best results for 80 nM Au(NPs) in nanohybrid electrode.
Conclusion
The present study suggested that the formation of spherical diffusion zone in nanohybrid-modified electrodes is critical for the enhanced electrochemical biosensing applications.
Introduction
In recent years, nanomaterials because of specific electrical, optical, magnetic, chemical, and mechanical properties have garnered attention in many upstream fields.Citation1–Citation4 These fields include diagnostics, drug delivery, artificial photosynthesis,Citation5 molecular recognition in therapeutics,Citation6 electrochemical biologic or chemical sensor,Citation7 solar energy harvesting,Citation8 photosynthesis, and biochemical sensing.Citation9 Out of different classes of nanomaterials, the use of metallic nanoparticles (NPs) in electrochemical sensors has received much attention because of their unique role in transfer of free charge to enhance conductivity, and catalytic and photocatalytic activity.Citation10–Citation13 Metallic NPs, for example, Au or Ag, for the detection of biologic molecules have been the center of attraction because of their exceptional biocompatibility. On the other hand, carbon nanotubes (CNTs) since their discovery in 1991Citation14 have gained immense interest as a potential candidate in a wide range of applications in electrochemistry.Citation15 The combination of metallic NPs and CNTs has created a new class of hybrid nanomaterials with multifunctional propertiesCitation16–Citation19 for potential use in optical, catalytic, sensor, solar cells, and most importantly in biosensor applications.Citation20–Citation23 The ability of Au(NPs) to adsorb proteins combined with the catalytic properties of CNTs has encouraged the use of Au(NP)–CNT nanohybrids for the development of electrochemical enzyme-based biosensors.Citation24 It has been demonstrated that several enzymes were able to preserve their enzymatic and electrochemical activity even after they were immobilized on the colloidal Au(NPs).Citation25
The decoration of metallic NPs on multiwalled carbon nanotubes (MWCNTs) has been shown to enhance the conductivity of electrochemical sensors. Hrapovic et alCitation26 used both nafion-coated single-walled carbon nanotubes (SWCNTs) and Pt(NPs) for the detection of low concentration of hydrogen peroxide (H2O2), which was better compared to SWCNTs or Pt(NPs) alone. Au-functionalized MWCNTs have also been employed as a sensing platform in electrochemical sensing of hydroxylamine, paraoxon, laccase from trametes versicolor extracts, and glycan assay on living cancer cells.Citation27–Citation30 Li et alCitation31 reported the facilitated mass transport in acetylene electro-oxidation reaction in Au–MWCNTs, which resulted in low onset potential and high current density compared to either Au(NPs) or Au catalysts. Several works on composites such as Au(NP)/poly(amidoamine)–MWCNT–Chi nanocomposite-modified glassy carbon electrode (GCE),Citation32 Au(NPs)/MWCNT–CS composites,Citation33 CD/Au(NP)/MWCNT composites and MWCNT,Citation34 and Au(NPs)/MWCNTS/reduced graphene oxideCitation35 have shown their use as sensors with great sensitivity and reproducibility.
The development of techniques for efficient and sensitive bacterial detection is the goal of current research in nanomaterial-based electrochemical immunosensors. Microbes in direct contact with the GCE have less efficiency of electron transfer from the bacterial cell to anode in the absence of a mediator. The cytoplasmic membrane in microbes acts as a barrier that blocks electron transfer from the microbes to the neighboring environments. A few strategies have been demonstrated for direct or indirect electron transfer to the working electrode, which include membrane proteins or cell metabolites or through electrically conductive nanowires.Citation36,Citation37 Park and Zeikus suggested the efficient charge transfer from microbes to anode in the presence of mediators such as thionine, methylene blue, and 2-hydroxyl 1, 4-naphthoquinone.Citation38 The use of the above-mentioned mediators overcomes the cytoplasmic membrane barrier and facilitates the electron transfer. Escherichia coli, one of the readily available and easily grown bacterial strains, can transfer electrons from electrochemical active microorganisms to electrode through mediators.Citation39,Citation40 CNTs because of excellent electronic conductivity, easy functionalization, and remarkable chemical stability have been recognized as an ideal electroactive material for their use as a mediator. Recently, dendrimers,Citation41 Au(NPs),Citation42 and quantum dotCitation43-based immunosensors have been developed for the detection of antibodies, peptides, and DNA. The use of Au(NP) and CNT composites has attracted considerable attention because of enhanced loading capability of Au(NPs) with active biomolecules that improves the recognition ratio and binding affinity. The Au(NP)–CNT composite is advantageous because of the biocompatibility of CNTs. The role of the concentration of Au(NPs) is crucial in the sensing efficiency of Au(NP)–MWCNT-modified electrodes used in electrochemical immunosensors. It is important to note that any variation in the concentration of Au(NPs) in the modified electrodes of electrochemical immunosensors affects the rate of charge transfer between the modified electrode and electrolytic solution. The objective of the present study was to explore the kinetics of charge transfer in the Au(NP)–MWCNT-modified GCE in a biologic reaction.
In this paper, different concentrations of Au(NPs) ranging from 40 to 100 nM were functionalized on the surface of acid-treated MWCNTs to form Au(NP)–MWCNT nanohybrids. The GCE was then modified with nanohybrids to measure the electrochemical response. The nature of the reaction processes, rate of charge transfer, and number of electrons transferred were determined by the cyclic voltammetry (CV), which was performed at different scan rates (from 50 to 300 mV s−1) in 0.1 mol L−1 potassium ferricyanide (K4[Fe(CN)6]). The electrochemical impedance spectroscopy (EIS) was employed to determine the charge transfer characteristics for different concentrations of Au(NPs) in nanohybrid-modified GCE. Finally, CV scans at the rate of 50 mV s−1 were performed on nanohybrid-modified electrodes in various concentrations of E. coli (from 104 to 106/mL) to determine the efficiency of the modified electrode.
Experiments
Synthesis of Au(NPs)
By using gold(III) chloride hydrate (Sigma-Aldrich Co., St Louis, MO, USA), 1 mM gold salt solution was prepared and refluxed in a round bottom flask with a continuous stirring for 10 minutes. In 100 mL of deionized (DI) water, 40 mM sodium citrate solution (Sigma-Aldrich) was added separately and the mixture was heated at 65°C±5°C for 30 minutes. The hot solution was then mixed with the Au salt solution followed by reflux for another 45 minutes. The solution was then cooled to room temperature and filtered using Whatman filter paper leading to the formation of citrate-stabilized Au(NPs). The synthesized NPs appeared bright red in color.
Formation of Au(NP)–MWCNT nanohybrids
Pristine MWCNTs (purchased from Beijing DK Nontechnology, Beijing, China) were first oxidized by adding in 40 mL acidic mixture of H2SO4/HNO3 with 3:1 ratio and ultrasonicated for 6 hours. The oxidized MWCNTs were then vacuum filtered, washed by DI water several times to remove the acidic contents, and dried overnight in an oven at 60°C. The dried oxidized MWCNTs were then dispersed in 2 mL of DI water to form four suspension solutions. The synthesized Au(NPs) with concentrations of 40, 60, 80, and 100 nM were added in 2 mL DI water and then mixed in all four oxidize MWCNT suspension solutions. The mixture was then ultrasonicated for 2 hours to form the nanohybrids of Au(NPs) with MWCNTs and afterwards annealed at 150°C to strengthen the attachment of Au(NPs) on the surface of MWCNTs.
Modification of GCE with MWCNTs and Au(NP)–MWCNT nanohybrids
The GCE was first cleaned by 0.3 µm alumina powder followed by ultrasonic bath in ethanol for 10 minutes. Five milligrams of oxidized MWCNTs was added in 5 mL of DI water and then ultrasonicated for 60 minutes to form the homogeneous solution. Ten microliters of the prepared solution was then dropped on the surface of cleaned GCE. The GCE was then dried under the lamp for 30 minutes and rinsed in DI water several times. The same procedure was repeated to modify the GCE with different concentrations of Au(NP)–MWCNT nanohybrids. The electrodes modified with each concentration were also incubated for 30 minutes in different concentrations of E. coli in the range from 104 to 106/mL.
Electrochemical studies were carried out using Metrohm 85695 Autolab – Electrochemical Analyzer with Nova 1.10 software. Platinum sheet was used as a counter electrode while Ag/AgCl electrode was used as a reference electrode. CV was performed at various scan rates in the range from 0.05 to 0.35 V s−1 with an increment of 0.05 V s−1. The pH during the experiment was 7, while all the experiments were performed at room temperature. EIS was carried out in the frequency range of 100 kHz to 100 mHz.
Results and discussion
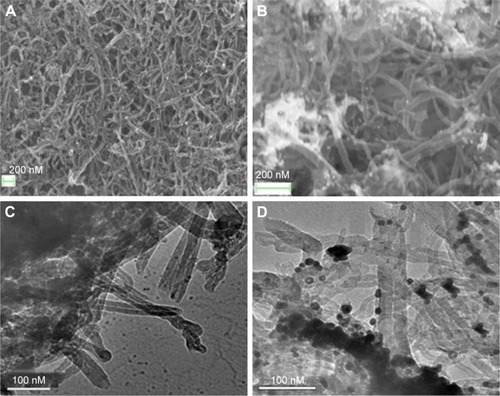
Confirmation of the attachment of the Au(NPs) on the surface of MWCNTs was done by using field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). shows the FESEM images of MWCNTs () and Au(NP)–MWCNT nanohybrids (). The appearance of white spot in in comparison to was due to attachment of Au(NPs) on the surface of MWCNTs. From , it can also be seen that the Au(NPs) were agglomerated on the surface of MWCNTs. For further confirmation of attachment of Au(NPs), TEM was performed, and shows the TEM images of MWNTs and Au(NP)–MWCNT nanohybrids, respectively. Au(NPs) appeared as randomly dispersed sphere on the surface of the MWCNTs as shown in . The average diameter of the MWCNTs determined from the TEM micrographs was 40±5 nm, while that of Au(NPs) was 20±5 nm. FESEM and TEM images confirmed the random immobilization of Au(NPs) on the surface of the MWCNTs and large agglomerated particles were also observed.
Figure 1 FESEM images of (A) MWCNTs, (B) Au(NP)–MWCNT nanohybrids. TEM images of (C) MWCNTs and (D) Au(NP)–MWCNT nanohybrids.
Note: FESEM and TEM confirmed the immobilization of Au(NPs) on the surface of MWCNTs.
Abbreviations: Au(NPs), Au nanoparticles; FESEM, field emission scanning electron microscopy; MWCNTs, multiwalled carbon nanotubes; TEM, transmission electron microscopy.
X-ray photoelectron spectroscopy (XPS) performed on similar hybrids has shown that three peaks at 283.7±0.2, 284.8±0.2, and 290.4±0.2 eV are due to the spCitation2 hybridized graphic carbon atoms, spCitation3 hybridization, and π-plasmon excitations, respectively, of C-1s atom in MWCNTs.Citation44 Decoration of metal catalysts such as Au has introduced new peak in XPS because of Au-4f7/2 at 83.7±0.2 eV.Citation45 We have recently demonstrated the formation of Au(NP)–MWCNT nanohybrids,Citation46 which confirmed that both acid treatment and functionalization of Au(NPs) introduced stresses on the surface of MWCNTs as studied by the X-ray diffraction and Raman spectroscopy (–).Citation46 Linear decrease in D/G ratio in Raman spectra from 1.27 for oxidized MWCNTs to 1.03 for 80 nM Au(NPs) concentrations and then again an increase to 1.21 for 100 nM Au(NPs) on the surface of MWCNTs was the significant signature of Au(NPs) functionalization.
Effect of scan rate
The CV profiles of the bare, MWCNT-, and Au(NP)–MWCNT-modified GCEs in 0.1 mol L−1 K4[Fe(CN)6] taken at various scan rates from 0.05 to 0.3 V s−1 are shown in . Where represents bare, represents MWCNT-modified, and 40–100 nM Au(NP)–MWCNT-modified GCEs. The hysteresis loops showed well-defined redox peaks due to oxidation or reduction of [Fe(CN)Citation6]4− ions. The positive current in was due to the oxidation current Ipc and the peak in current was observed at the corresponding cathodic potential, while negative current represented the reduction current Ipa and an inverted peak in the reduction current was observed at the corresponding anodic potential Epa. It was observed that the Ipc and Ipa increased gradually and Epc and Epa shifted to high and low voltages, respectively, with the increase in scan rates. Emergence of new reduction peak at 0.8 V with increased concentration of Au(NPs) was due to [Fe(CN)6]3− ions reduction.Citation32
Figure 2 CV scans of GCE, MWCNTs-modified GCE, and Au(NP)–MWCNT-modified GCE in 0.1 mol L−1 K4[Fe(CN)6] with scan rates 0.05, 0.1, 0.15, 0.2, 0.25, and 0.3 V s−1.
Note: (A) GCE, (B) MWCNT-modified GCE, (C) 40 nM Au(NP)–MWCNT-modified GCE, (D) 60 nM Au(NP)–MWCNT-modified GCE, (E) 80 nM Au(NP)–MWCNT-modified GCE, and (F) 100 nM Au(NP)–MWCNT-modified GCE.
Abbreviations: Au(NPs), Au nanoparticles; CV, cyclic voltammetery; GCE, glassy carbon electrode; MWCNTs, multiwalled carbon nanotubes.
![Figure 2 CV scans of GCE, MWCNTs-modified GCE, and Au(NP)–MWCNT-modified GCE in 0.1 mol L−1 K4[Fe(CN)6] with scan rates 0.05, 0.1, 0.15, 0.2, 0.25, and 0.3 V s−1.Note: (A) GCE, (B) MWCNT-modified GCE, (C) 40 nM Au(NP)–MWCNT-modified GCE, (D) 60 nM Au(NP)–MWCNT-modified GCE, (E) 80 nM Au(NP)–MWCNT-modified GCE, and (F) 100 nM Au(NP)–MWCNT-modified GCE.Abbreviations: Au(NPs), Au nanoparticles; CV, cyclic voltammetery; GCE, glassy carbon electrode; MWCNTs, multiwalled carbon nanotubes.](/cms/asset/671379db-e463-482e-a107-4e22d39a2ec4/dijn_a_12193955_f0002_b.jpg)
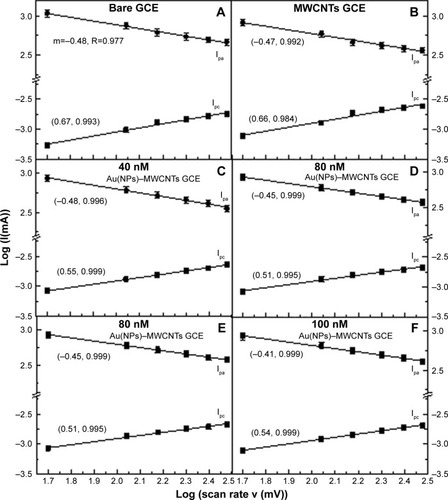
A linear correlation was observed between the log (I) and log (v) with a correlation coefficient® >95% for the bare and modified electrodes as shown in . The slope of log (Ipa) vs log (v) was in the range from −0.41±0.01 to −0.48±0.01, while for log (Ipc) and log (v), the slope was in the range from 0.51±0.02 to 0.67±0.04 for the bare, MWCNT-, and Au(NP)–MWCNT-modified GCEs. The determined slopes were close to the theoretically value of 0.50 for a purely diffusion-controlled current,Citation47 which confirmed that the redox processes at the surface of modified electrode were diffusion controlled. The small values of slope for Ipa represented the slow diffusion of heavier [Fe(CN)Citation6]4− ions with respect to the diffusion of K+ ions. It was important to know if the diffusion-controlled processes involved in the redox reaction were reversible, quasi-reversible, or irreversible, which could give an insight about the charge transfer rate. In a reversible reaction, cathodic peak potential Epa is usually independent of the scan rate “v”. However, in the present case, from , Epa showed dependence on the scan rate for all modified GCEs as given in Laviron’s model EquationEquation 1(1) .Citation48 Laviron’s equation is used for many sensor applications, which claim that the use of modified electrodes produced peaks in CV curves. Laviron’s equation is successfully applied to modified electrode system for determining the electron transfer rate constant where reaction kinetics dominates the system.Citation49–Citation51
Figure 3 Plots of log (Ipc) and log (Ipa) as a function of log of scan rates in mV s−1.
Notes: (A) GCE, (B) MWCNT-modified GCE, and (C–F) 40–100 nM Au(NP)–MWCNT-modified GCE. Linearly varying trend was the indication of diffusion-controlled electrochemical processes. In parentheses, the first term represents the slope while the second term denotes the correlation coefficient (R).
Abbreviations: Au(NPs), Au nanoparticles; GCE, glassy carbon electrode; MWCNTs, multiwalled carbon nanotubes.
According to EquationEquation 1(1) , Epa linearly depends on ln v1/2 as shown in for bare, MWCNT-, and Au(NP)–MWCNT-modified GCEs (only bare GCE and 100 Au(NP)–MWCNT-modified GCEs are shown), and its slope is inversely proportional to the product (αnα) expressed in EquationEquation 2
(2) and used to determine the product αnα.
Figure 4 Plots used for the determination of number of electron transfer (n), energy transfer coefficient α, formal potential Eo and charge transfer rate constant ks.
Notes: (A) Plots of Epa as a function of ln (v1/2) for bare GCE and 100 nM Au(NP)–MWCNT-modified GCE. (B) Plots of Epc as a function of scan rate (V s−1) for bare GCE and 100 nM Au(NP)–MWCNT-modified GCE. The plots were used to determine the formal potential Eo. (C) The plots of ln (Ipc (mA)) as a function of Epc–Eo (V) for bare GCE and 100 nM Au(NP)–MWCNT-modified GC electrodes. The slopes of plots were used to determine the number of electrons transferred. (D) The rate constant ks plotted as a function of Au(NPs) in Au(NP)–MWCNT nanohybrid-modified GCE. Ks for GCE was also added as a reference.
Abbreviations: Au(NPs), Au nanoparticles; GCE, glassy carbon electrode; MWCNTs, multiwalled carbon nanotubes.
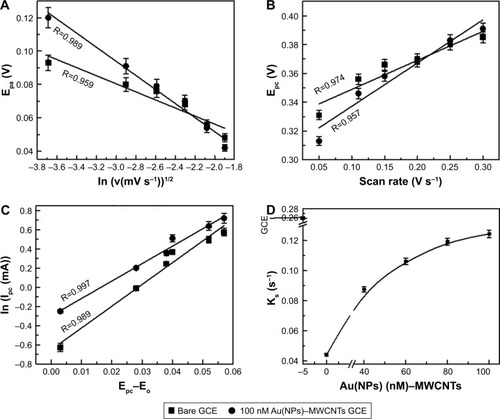
The cathodic peak potential, Epc, also depended linearly on the scan rate as shown in . The y-intercept at zero scan rate of the plot was used to determine built-in or “formal” potential Eo, which was used to determine the corrected potential (Epc−Eo) for all the modified electrodes. The plots of ln (Ipc) as a function of corrected potential (Epc−Eo) are shown in , which showed a linear relation. The slope of the plot was used to determine the tendency of electron loss for bare and modified GCEs. The slope “m” obtained from and the product αnα determined from EquationEquation 2(2) were used to determine the number of electrons (n) participating in the oxidation or reduction of ferricyanide ions at the electrode surface as given in EquationEquation 3
(3) :Citation48
The number of electrons (n) involved in the electrochemical reaction determined from EquationEquation 3(3) was 3, 5, and Equation4
(4) for bare, MWCNT-, and Au(NP)–MWCNT-modified GCEs, respectively. The energy transfer coefficient α for the bare and MWCNT-modified GCE was 0.47 and 0.07, respectively, and for various concentrations of Au(NP)–MWCNT-modified GCEs, it was found in the range from 0.15 to 0.22. Ideally, α varies from 0 to 1 and classifies the type of reaction,Citation52 that is, irreversible if α is close to 0; reversible if it is close to 1; and quasi-reversible in case α is close to 0.5. It was observed that in the present case, the reaction was quasi-reversible for the bare GCE, became irreversible for MWCNT-modified GCE, but for Au(NP)–MWCNT nanohybrid-modified GCE, reaction again became quasi-reversible. The change in the process was affected by the different rates of charge transfer at the electrode surface for different electrodes. It was, thus, pertinent to determine the charge transfer rate constants ks for the modified electrodes by using:Citation53
Thus, the dependence of rate constant ks on the Au concentration in the nanohybrid-modified GCE as determined from EquationEquation 4(4) is shown in , where ks for bare GCE (=0.273±0.001 cm s−1) was taken as a reference. The value of ks was the lowest (0.044±0.001 cm s−1) for MWCNT-modified GCE and then reached to a maximum of 0.124±0.001 cm s−1 for 100 nM Au(NP)–MWCNT-modified GCE. ks was observed to saturate at higher concentrations of Au(NPs) in Au(NP)–MWCNT nanohybrids. The increase in Au(NPs) concentration resulted in the increase in the rate of charge transfer between the electrolytic species and modified electrode.
The choice of proper scan rate is very crucial as it leads to formation of diffusion zone near the vicinity of electrode and can affect the number of charge transferred in the reaction. Low scan rates lead to the formation of thick diffusion layer, thus reducing the flux of charge. However, fast scan rates lead to formation of thin or incomplete diffusion layer, thus resulting in high flux of charge. Also, when potential is scanned, the true potential lags the applied potential according to EquationEquation 5(5) .
The plot of redox currents (Ipa, Ipc) and ΔE (=Epc−Epa) as a function of Au(NPs) concentrations in Au(NP)–MWCNT nanohybrids is shown in , respectively. Ipa(Ipc) increased from −0.90 mA (0.54 mA) to −1.12 mA (0.77 mA) in the MWCNT-modified GCE with reference to the bare GCE as shown in . The increase in the oxidation and the reduction peak currents was due to the presence of carboxylic groups in the MWCNT-modified GCEs. A further linear increase in Ipa (Ipc) of 5% (9%) was observed in Au(NP)–MWCNT-modified GCEs. The increase in both Ipa and Ipc was attributed to the functionalization of Au(NPs) on the surface of MWCNTs, which enhanced the electrochemical reaction for the oxidation and reduction reactions at the surface of modified electrodes. Furthermore, there was a difference in two redox potentials, that is, ΔE increased from 236±3 to 287±3 mV in the MWCNT-modified GCE with reference to bare GCE as shown in . However, ΔE reached its asymptotic value of 191±3 mV from 236±3 mV as the concentration of Au(NPs) was increased from 40 to 100 nM for Au(NP)–MWCNT-modified GCE.
Figure 5 Plots of redox currents (Ipa, Ipc) and ΔE (=Epc–Epa) as a function of Au(NPs) concentrations in Au(NP)–MWCNT nanohybrids for complete understanding about symmetry of CV profile due to formation of diffusion zone around Au (NPs).
Notes: Plots of (A) redox peak currents Ipc and Ipa. Inset of (A) explains the schematic of diffusion zones around NPs. (i) Single NPs, and (ii) two NPs in very close proximity to each other. Overlapping of neighboring diffusion zones has shielded the spherical diffusion of one another. (B) Epc–Epa as a function of different Au(NPs) concentrations on MWCNT nanohybrids used for the GCE modification. Inset graph shows the CV profiles at 0.5 V s−1 for Au(NP)–MWCNT-modified GCE with increasing Au(NPs) concentration.
Abbreviations: Au(NPs), Au nanoparticles; GCE, glassy carbon electrode; MWCNTs, multiwalled carbon nanotubes.
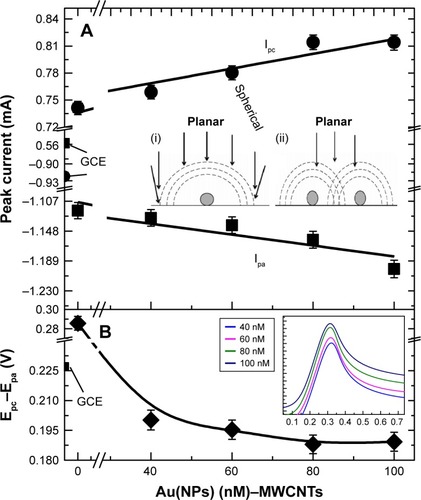
The change in Ipc, Ipa, and ΔE (=Epc−Epa) seen with the change in Au(NPs) concentrations in the nanohybrid-modified GCEs was understood using the concept of formation of diffusion zone around Au(NPs) as explained by Dvies et alCitation54 and is shown in the inset of . Depending on the electrode surface, two diffusion zones can be formed, that is, planar diffusion zone and spherical diffusion zone, where spherical diffusion zone is efficient for the diffusion of ionic species to the electrode surface, which is due to existence of strong electric field in the zone region. Overlap of adjacent diffusion zones is expected to affect the diffusion behavior or mass transport of the ionic species to the modified electrodes. In the present case, for small concentration of Au(NPs) in Au(NP)–MWCNT nanohybrids, spherical diffusion zone (inset of labeled as i) was dominant over planar diffusion zone and was responsible for the electrochemical behavior. The increase in Au(NPs) concentration in the modified electrode caused the overlap of neighboring diffusion zones (inset of labeled as ii) and made planar diffusion dominant over spherical.Citation46 The effect of formation of two different zones was further observed in the CV quarter scans (positive voltage) of the nanohybrid-modified GCEs obtained at 0.5 V s−1 as shown in the inset of . For small concentrations of Au(NPs), that is, 40 and 60 nM, the symmetric CV profiles were due to spherical diffusion. However, with the increase in Au(NPs) concentration, the CV profile showed an asymmetric tail because of dominance of planar diffusion. Asymmetric behavior is just like vestigial microelectrode characteristics in which overlapping of diffusion zone has affected the shape of voltammogram.Citation55
The overlap in the diffusion zones resulted in the saturation of ks at 0.124±0.001 cm s−1 and asymptotic value of Epc−Epa at 191±3 mV for higher concentrations of Au(NPs) in the nanohybrid-modified electrode. This possibly showed that there was a limit of the Au(NPs) concentration in the nanohybrid to achieve optimal performance. The charge transfer characteristics were further probed by the EIS.
EIS analysis of Au(NP)–MWCNT-modified GCE
EIS was also performed for the bare, MWCNT-, and Au(NP)–MWCNT-modified GCEs and the corresponding Nyquist plots are shown in . A linear relation between Z' and −Z" for GCE (bottom Nyquist plot) was observed with a slope of 0.97±0.01, which also confirmed that the reaction kinematics were diffusion-limited processes.Citation56
Figure 6 Electrochemical impedance spectroscopy of GCE, MWCNTs-modified GCE and Au(NP)-MWCNTs -modified GCE.
Notes: (A) Nyquist plots between Z' and −Z" for GCE, MWCNT-modified GCE, 40 nM Au(NP)–MWCNT-modified GCE, 60 nM Au(NP)–MWCNT-modified GCE, 80 nM Au(NP)–MWCNT-modified GCE, and 100 nM Au(NP)–MWCNT-modified GCE in 0.1 mol L−1 K4[Fe(CN)6]. Red line in the bottom Nyquist plot represents the fitted data used to calculate the slope of linear region. Inset is the Randle circuit used for the fitting of Nyquist plots. Rs represents the solution resistance, CPE is the constant-phase element, ZW is the Warburg coefficient, Cad is the double-layer capacitance. (B, C) Plot of W and Rs as a function of Au(NPs) concentration on the surface of MWCNT nanohybrid-modified GCE.
Abbreviations: Au(NPs), Au nanoparticles; GCE, glassy carbon electrode; MWCNTs, multiwalled carbon nanotubes.
![Figure 6 Electrochemical impedance spectroscopy of GCE, MWCNTs-modified GCE and Au(NP)-MWCNTs -modified GCE.Notes: (A) Nyquist plots between Z' and −Z" for GCE, MWCNT-modified GCE, 40 nM Au(NP)–MWCNT-modified GCE, 60 nM Au(NP)–MWCNT-modified GCE, 80 nM Au(NP)–MWCNT-modified GCE, and 100 nM Au(NP)–MWCNT-modified GCE in 0.1 mol L−1 K4[Fe(CN)6]. Red line in the bottom Nyquist plot represents the fitted data used to calculate the slope of linear region. Inset is the Randle circuit used for the fitting of Nyquist plots. Rs represents the solution resistance, CPE is the constant-phase element, ZW is the Warburg coefficient, Cad is the double-layer capacitance. (B, C) Plot of W and Rs as a function of Au(NPs) concentration on the surface of MWCNT nanohybrid-modified GCE.Abbreviations: Au(NPs), Au nanoparticles; GCE, glassy carbon electrode; MWCNTs, multiwalled carbon nanotubes.](/cms/asset/af0e4a70-ae4d-463a-8aec-4481afa48f87/dijn_a_12193955_f0006_c.jpg)
The Nyquist plot was used to draw the equivalent Randle’s circuit and is shown in the inset of . The equivalent circuit contained uncompensated solution resistance Rs, double-layer capacitance Cad, which was related to delamination of coating, mass-transport contribution Zw, that is, ion diffusion, and the constant phase element, CPE. The values of each element, Rs, Cad, and Zw obtained from the fitting of Nyquist plots for each modified electrode are given in . The variation in Warburg resistance Zw is shown in as a function of Au(NPs) concentration in the nanohybrid-modified GCEs. The first point in the plot is for the bare electrode shown for reference. Zw first dropped from 6.32±0.05 mS for bare GCE to 1.10±0.05 mS for MWCNT-modified GCE. It then increased to 5.67±0.05 mS for 80 nM Au(NPs) in the nanohybrid-modified electrode. The drop in Zw for the MWCNT-modified electrode suggested reduced ionic diffusion to reach the electrode surface. The increase in the Zw for Au(NP)–MWCNT nanohybrids suggested efficient ionic diffusion, which resulted in increased charge transfer. The solution resistance Rs, which is the resistance offered by electrolytic solution, is plotted as a function of Au(NPs) and is shown in . Rs increased to a maximum of 82.5 Ω for MWCNT-modified GCE. However, Rs dropped to 50.0±0.5 Ω for 80 nM Au(NPs) nanohybrid-modified GCE. An increase in Zw to 5.67±0.05 mS and decrease in Rs to 50.0±0.5 Ω with the increase in the concentration of Au(NPs) was due to enhanced charge transfer characteristics for Au(NP)–MWCNT-modified GCE.
Table 1 Value of different variables such as Rs, CPE, Cad, and Zw used for the fitting of Nyquist plots of GCE, MWCNT GCE, and Au(NP)–MWCNT GCE
From the above discussion, it can be deduced that the charge transfer characteristics can be controlled by varying the concentration of Au(NPs) in Au(NP)–MWCNT nanohybrid to a certain limit. The CV and EIS studies confirmed the diffusion-controlled processes in the charge transfer between the modified electrode and electrolytic solution. The diffusion processes were quasi-reversible (α=0.22) in Au(NP)–MWCNT nanohybrid-modified electrodes with a charge transfer rate ks of 0.124±0.001 cm s−1. An increase in the value of ks with the increase in concentration of Au(NPs) in nanohybrid-modified electrodes was due to enhanced charge transfer rate, which led to increase in Ipc(Ipa). The characteristics of charge transfer in the modified electrodes were further studied with the attachment of different concentrations of E. coli.
Electrochemical response of modified electrodes against E. coli
The electrochemical behavior of the modified electrodes was determined for different concentrations of E. coli (104, 105, and 106/mL in 0.1 mol L−1 K4[Fe(CN)6]). shows the CV scans at the rate of 0.05 V s−1 for various concentrations of E. coli attached to 40 nM and 80 nM Au(NPs) in nanohybrid-modified GCEs. The CV profiles are displaced vertically for clarity and the horizontal line in each graph is at y=0. The dotted vertical lines were drawn to demonstrate the change in the position of Epc and Epa. The bottom three rows in each part of are the CV profiles for the bare, MWCNTs, and Au(NPs)–MWCNTs in the nanohybrid-modified GCEs. The top three scans in each part of show the CV profiles of the nanohybrid-modified GCEs exposed to various concentrations of E. coli in the range from 104 to 106/mL. It is shown in that the attachment of E. coli resulted in increase of redox current in nanohybrid-modified electrodes because of transfer of electrons through two glutathione enzymes from E. coli.Citation57
Figure 7 CV plots of the Au(NP)–MWCNT-modified GCE in 0.1 mol L−1 K4[Fe(CN)6] at 0.05 V s−1 for different concentrations of Escherichia coli 104, 105, and 106/mL.
Note: (A) 40 nM Au(NP)–MWCNT-modified GCE, and (B) 80 nM Au(NP)–MWCNT-modified GCE.
Abbreviations: Au(NPs), Au nanoparticles; GCE, glassy carbon electrode; CV, cyclic voltammetery; MWCNTs, multiwalled carbon nanotubes.
![Figure 7 CV plots of the Au(NP)–MWCNT-modified GCE in 0.1 mol L−1 K4[Fe(CN)6] at 0.05 V s−1 for different concentrations of Escherichia coli 104, 105, and 106/mL.Note: (A) 40 nM Au(NP)–MWCNT-modified GCE, and (B) 80 nM Au(NP)–MWCNT-modified GCE.Abbreviations: Au(NPs), Au nanoparticles; GCE, glassy carbon electrode; CV, cyclic voltammetery; MWCNTs, multiwalled carbon nanotubes.](/cms/asset/d1c7d129-5f32-4d11-8199-d7d51e52be2d/dijn_a_12193955_f0007_b.jpg)
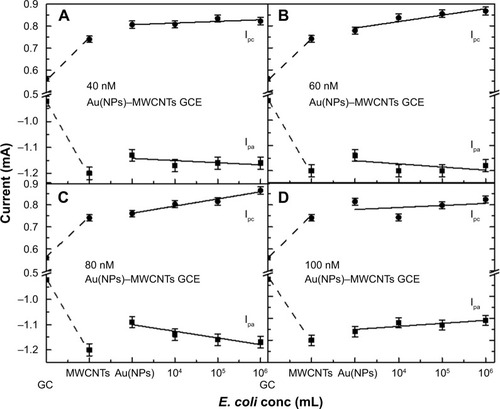
The increase in both Ipa and Ipc as a function of E. coli was used to estimate the dependence of sensitivity of the nanohybrid-modified GCEs on Au(NPs) concentration to detect E. coli as shown in . In , the first two points on x-axis in each plot are for bare and MWCNT-modified GCEs as reference, respectively. The increase in Ipc(Ipa) observed was 2% (2%), 9% (3%), 13% (7%), and 2% (5%) for 40, 60, 80, and 100 nM Au(NPs) concentrations in nanohybrid-modified electrodes, respectively. The difference in the rate of increase of both Ipc and Ipa peak current values with E. coli concentrations demonstrated that the conductivity and electrochemical catalytic properties of nanohybrid had a synergistic effect on the detection of E. coli. This could be attributed to the varied concentrations of Au(NPs) on the surface of the MWCNTs. In case of low concentrations of Au(NPs) such as 40 and 60 nM, the charge transfer rate was low, that is, ks =0.08 s−1, which delayed the charge transfer between E. coli and nanohybrid-modified electrodes. For 80 nM Au(NP)–MWCNT-modified GCE, high value of ks was 0.11 s−1, which resulted in efficient charge transfer between Au(NP)–MWCNT-modified GCE and ferrocyanide ions in the presence of E. coli. For 100 nM concentration of Au(NPs), the observed drop in the redox current was attributed to the dominance of planar diffusion, which reduced the diffusion of ions. This behavior revealed that a suitable concentration of Au(NPs) had an important role for the charge transfer characteristics between the modified electrode and electrolytic solution. On the basis of these results, it is concluded that Au(NPs) functionalized on MWCNTs facilitate enhanced charge transfer with increasing concentrations of Au(NPs).
Figure 8 Plots of Ipc and Ipa as a function of different Escherichia coli concentrations on Au(NP)–MWCNT-modified GCE.
Notes: Ipc and Ipa for GCE and MWCNT-modified GCE were also shown for the reference (first two points on x-axis in each graph). (A) 40 nM Au(NP)–MWCNT-modified GCE, (B) 60 nM Au(NP)–MWCNT-modified GCE, (C) 80 nM Au(NP)–MWCNT-modified GCE, and (D) 100 nM Au(NP)–MWCNT-modified GCE. Ipc has changed linearly for 40, 60, and 80 nM Au(NP)–MWCNT nanohybrids with increased concentration of E. coli.
Abbreviations: Au(NPs), Au nanoparticles; GCE, glassy carbon electrode; MWCNTs, multiwalled carbon nanotubes.
The limit of detection (LoD) and limit of quantitation (LoQ) values were also determined for each Au concentration of modified GCE in E. coli surroundings by using EquationEquation 6(6) :
Table 2 Comparison of results obtained in the present work with the literature for the LoD
Conclusion
The present study demonstrated the critical role of concentration of Au(NPs) in the electrochemical response of Au(NP)–MWCNT-modified electrodes. The increase in the number of electrons in the electrochemical reaction was significant in the Au(NP)–MWCNT nanohybrids as compared to bare GCE. The rate of charge transfer was also dependent on the concentration of Au(NPs), which increased from 0.044±0.001 cm s−1 for MWCNT-modified GCE to 0.124±0.001 cm s−1 for 80 nM Au(NP)–MWCNT-modified GCE. This was due to enhanced redox reaction in the presence of nanohybrid electrodes. EIS measurements also confirmed that increase in mass transfer by 5 times was the highest when 80 nM concentration of Au(NPs) was present in the nanohybrid-modified electrodes. Electrochemical behavior of Au(NP)-modified electrodes to the E. coli was also maximum for 80 nM concentration of Au(NPs) in the nanohybrid-modified electrode and a maximum increase of 13% in Ipc was observed. The increased number of charge transfer and enhanced rate of charge transfer were attributed to the formation of spherical diffusion zones around the modified electrodes, which showed strong dependence on the concentration of Au(NPs) in the nanohybrids. High concentration of Au(NPs) in nanohybrids led to formation of planar diffusion zones, which led to reduced number of charge transfer and transfer rates.
Acknowledgments
Research work was funded by HEC NRPU grant 261 and 1770. SM is thankful to Higher Education Commission for a PhD scholarship. Authors are thankful to Dr H Ismail and Prof Dr B Mirza from Department of Biochemistry, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, Pakistan, for providing Escherichia coli and also to Dr Sana from Department of Physics, COMSATS Institute of Information Technology, Islamabad, Pakistan, for synthesis of Au(NPs).
Disclosure
The authors report no conflicts of interest in this work.
References
- ŞahinBDemirEAygunAInvestigation of the effect of pomegranate extract and monodisperse silver nanoparticle combination on MCF-7 cell lineJ Biotechnol20172601798328923716
- OliveiraMDCorreiaMTDinizFBConcanavalin A and polyvinyl butyral use as a potential dengue electrochemical biosensorBiosens Bioelectron200925472873219747814
- IversonNMBaronePWShandellMIn vivo biosensing via tissue-localizable near-infrared-fluorescent single-walled carbon nanotubesNat Nanotechnol201381187388024185942
- KimJHPatraCRArkalgudJRSingle-molecule detection of H2O2 mediating angiogenic redox signaling on fluorescent single-walled carbon nanotube arrayACS Nano20115107848785721899329
- ParveenSMisraRSahooSKNanoparticles: a boon to drug delivery, therapeutics, diagnostics and imagingNanomed Nanotechnol201282147166
- ZhangJLandryMPBaronePWMolecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubesNat Nanotechnol201381295996824270641
- BozkurtSTosunBSenBA hydrogen peroxide sensor based on TNM functionalized reduced graphene oxide grafted with highly monodisperse Pd nanoparticlesAnal Chim Acta20179891889428915946
- BoghossianAASenFGibbonsBMApplication of nanoparticle antioxidants to enable hyperstable chloroplasts for solar energy harvestingAdva Energy Mat201337881893
- GiraldoJPLandryMPFaltermeierSMPlant nanobionics approach to augment photosynthesis and biochemical sensingNat Mater201413440040824633343
- ChenSWMurrayRWElectrochemical quantized capacitance charging of surface ensembles of gold nanoparticlesJ Phys Chem B199910345999610000
- LiJYamadaYMurakoshiKNakatoYSustainable metal nano-contacts showing quantized conductance prepared at a gap of thin metal wires in solutionChem Commun200112121702171
- HarutaMSize- and support-dependency in the catalysis of goldCatal Today1997361153166
- SubramanianVWolfEEKamatPVGreen emission to probe photoinduced charging events in ZnO-Au nanoparticles. Charge distribution and fermi-level equilibrationJ Phys Chem B2003107174797485
- IijimaSHelical microtubules of graphitic carbonNature199135463485658
- WangYRPingHLiangQLGuoLWangYMApplication of carbon nanotube modified electrode in bioelectroanalysisChina J Anal Chem200836810111016
- GaoCLiWJinYZKongHFacile and large-scale synthesis and characterization of carbon nanotube/silver nanocrystal nanohybridsNanotechnology2006171228822890
- LiuXSHuFZhuDROne-step synthesis of carbon nanotubes with Ni nanoparticles as a catalyst by the microwave-assisted polyol methodJ Alloy Compd2011509628292832
- SowichaiKSupothinaSNimittrakoolchaiOUSetoTOtaniYCharinpanitkulTFacile method to prepare magnetic multi-walled carbon nanotubes by in situ co-precipitation routeJ Ind Eng Chem201218515681571
- ZhengRXianSongLFengHOne-step preparation of carbon nanotubes with nickel as the coreSci China Technol Sci20115417680
- EderDCarbon nanotube–inorganic hybridsChem Rev201011031348138520108978
- JeongHYKimJYKimJWGraphene oxide thin films for flexible nonvolatile memory applicationsNano Lett201010114381438620919689
- YadavSKMadeshwaranSRChoJWSynthesis of a hybrid assembly composed of titanium dioxide nanoparticles and thin multi-walled carbon nanotubes using “click chemistry”J Colloid Interface Sci2011358247147621463867
- GuptaVKotnalaRKMultifunctional ferromagnetic carbon-nanotube arrays prepared by pulse-injection chemical vapor depositionAngew Chem Int Ed Engl201251122916291922318942
- HrapovicSLiuYMaleKBElectrochemical biosensing platforms using platinum nanoparticles and carbon nanotubesAnal Chem20047641083108814961742
- WuHYHuSSThe fabrication of a colloidal gold–carbon nanotubes composite film on a gold electrode and its application for the determination of cytochrome cColloid Surf B Biointerfaces200541429930415748825
- HuangXJLiCCGuBKimJHChoSOChoiYKControlled molecularly mediated assembly of gold nanooctahedra for a glucose biosensorJ Phys Chem C20081121036053611
- BuiMPNPhamXHHanKNElectrochemical sensing of hydroxylamine by gold nanoparticles on single-walled carbon nanotube filmsElectrochem Commun2010122250253
- JhaNRamaprabhuSDevelopment of Au nanoparticles dispersed carbon nanotube-based biosensor for the detection of paraoxonNanoscale20102580681020648328
- FaveroGFuscoGMazzeiFTascaFAntiochiaRElectrochemical characterization of graphene and MWCNTs screen-printed electrodes modified with aunps for laccase biosensor developmentNanomaterials (Basel)2015541995200628347108
- ZhangXHuangCJiangYAn electrochemical glycan biosensor based on a thionine-bridged multiwalled carbon nanotube/gold nanoparticle composite-modified electrodeRSC Adv20166114112981112987
- LiCSuYLvXXiaHWangYElectrochemical acetylene sensor based on Au/MWCNTsSensor Actuat B Chem20101492427431
- DongJZhaoHXuMMaQAiSA label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-Chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milkFood Chem201314131980198623870918
- BaiJZhangXPengYUltrasensitive sensing of diethylstilbestrol based on AuNPs/MWCNTs-CS composites coupling with sol-gel molecularly imprinted polymer as a recognition element of an electrochemical sensorSens Actuat B Chem20172381420426
- KanXZhangTZhongMLuXCD/AuNPs/MWCNTs based electrochemical sensor for quercetin dual-signal detectionBiosens Bioelectron201677163864326485178
- HaoYUXiaoFENGXiao-XiaCHENElectrochemical determination of bisphenol a on a glassy carbon electrode modified with gold nanoparticles loaded on reduced graphene oxide-multi walled carbon nanotubes compositeChinese J Anal Chem2017455713720
- GorbyYAYaninaSMcLeanJSElectrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganismsProc Natl Acad Sci U S A200610330113581136316849424
- ZhangTCuiCZChenSYangHShenPThe direct electrocatalysis of Escherichia coli through electroactivated excretion in microbial fuel cellElectrochem Commun2008102293297
- ParkDHZeikusJGImproved fuel cell and electrode designs for producing electricity from microbial degradationBiotechnol Bioeng200381334835512474258
- RingeisenBRHendersonEWuPKHigh power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10Environ Sci Technol20064082629263416683602
- YiaHNevinKPKimBCSelection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cellsBiosens Bioelectron200924123498350319487117
- GuLLuoPGWangHSingle-walled carbon nanotube as a unique scaffold for the multivalent display of sugarsBiomacromolecules2008992408241818712920
- LiRWuKLiuC4-Amino-1-(3-mercapto-propyl)-pyridine hexafluorophosphate ionic liquid functionalized gold nanoparticles for IgG immunosensing enhancementAnal Chem201486115300530724803006
- LiuHXuSHeZSupersandwich cytosensor for selective and ultrasensitive detection of cancer cells using aptamer-DNA concatamer-quantum dots probesAnal Chem20138563385339223418929
- DilonardoEPenzaMAlvisiMElectrophoretic deposition of Au NPs on MWCNT-based gas sensor for tailored gas detection with enhanced sensing propertiesSens Actuat B Chem20162231417428
- DilonardoEPenzaMAlvisiMElectrophoretic deposition of Au NPs on CNT networks for sensitive NO2 detectionJ Sens Sens Syst201432245252
- MehmoodSNaeemASabahatSModified structural and optical characteristics of Au-NPs–MWCNTs nanohybridsSuperlattice Microst2015811248264
- HajianRYusofNAFaragiTShamsNFabrication of an electrochemical sensor based on gold nanoparticles/carbon nanotubes as nanocomposite materials: determination of myricetin in some drinksPLoS One20149517
- YueDJiaYYaoYSunJJingYStructure and electrochemical behavior of ionic liquid analogue based on choline chloride and ureaElectrochim Acta20126513036
- ZhaoYDBiYHZhangWDLuoQMThe interface behavior of hemoglobin at carbon nanotube and the detection for H2O2Talanta200565248949418969824
- ZhaoGCYinZZZhangLWeiXWDirect electrochemistry of cytochrome c on a multi-walled carbon nanotubes modified electrode and its electrocatalytic activity for the reduction of H2O2Electrochem Commun20057318871890
- LiuXShiLNiuWLiHXuGAmperometric glucose biosensor based on single-walled carbon nanohornsBiosens Bioelectron200823121887189018387291
- RafieeBFakhariARGhaffarzadehMImpedimetric and stripping voltammetric determination of methamphetamine at gold nanoparticles-multiwalled carbon nanotubes modified screen printed electrodeSensor Actuat B20152181271279
- LavironEGeneral expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systemsJ Electroanal Chem197910111928
- DviesTJBanksCEComptonRGVoltammetry at spatially heterogeneous electrodesJ Solid State Electr2005912797808
- DaviesTJComptonRGThe cyclic and linear sweep voltammetry of regular and random arrays of microdisc electrodes: theoryJ Electroanal Chem200558516382
- SuniIIImpedance methods for electrochemical sensors using nanomaterialsTrAC Trend Anal Chem2008277604611
- ChalenkoYShumyantsevVErmolaevSArchakovAElectrochemistry of Escherichia coli JM109: direct electron transfer and antibiotic resistanceBiosens Bioelectron201232121923322209070
- PangBZhaoCLiLDevelopment of a low-cost paper-based ELISA method for rapid Escherichia coli O157: H7 detectionAnal Biochem20185421586229158131
- SongCLiuJLiJLiuQDual FITC lateralflow immunoassay for sensitive detection of Escherichia coli O157: H7 in food samplesBiosens Bioelctron2016851734739
- SongCLiJLiuJSimple sensitive rapid detection of Escherichia coli O157: H7 in food samples by label-free immunofluorescence strip sensorTalanta20161561424727260433
- HassanARHAAMunizADLEMerkoçiAHighly sensitive and rapid determination of Escherichia coli O157: H7 in minced beef and water using electrocatalytic gold nanoparticle tagsBiosens Bioelectron201567151151525241123
- AjishJKKumarKARuhelaASubramanianMBallalADKumarMAIE based fluorescent self assembled glycoacrylamides for E.coli detection and cell imagingSens Actuat B Chem2018255117261734
